Selective IgM Deficiency: Evidence, Controversies, and Gaps
Abstract
1. Introduction
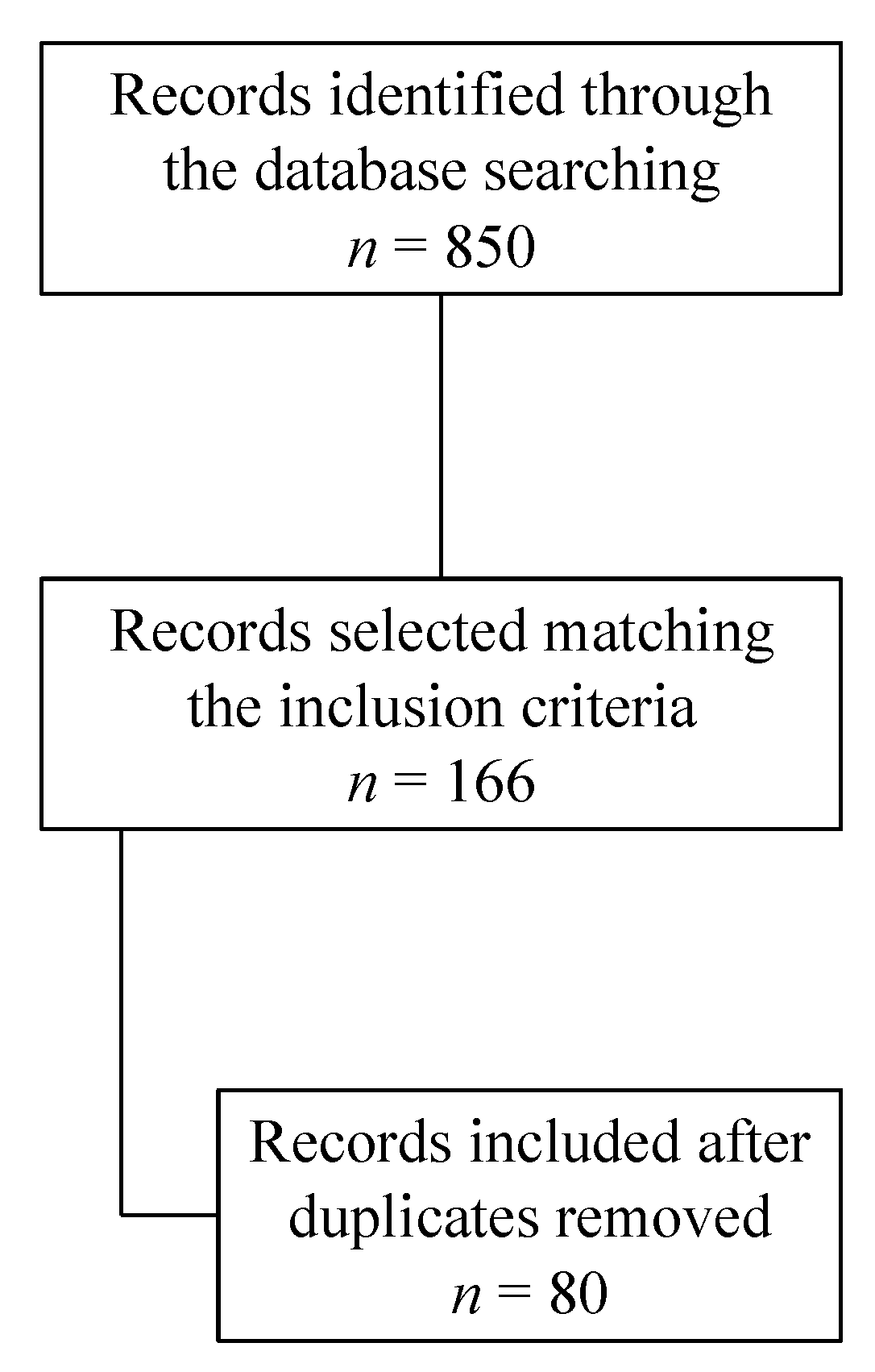
2. Materials and Methods
3. Results
3.1. Definition (Clinical and Laboratory)
| Predominant Antibody Deficiencies | ||||
|---|---|---|---|---|
| Isotype, Light Chain, or Functional Deficiencies with Generally Normal Numbers of B Cells | ||||
| Disease | Genetic Defect | Inheritance | Immunoglobulin | Associated Features |
| Selective IgM deficiency | Unknown | Not established | Low/absent IgM | Pneumococcal/ Bacterial infection |
3.2. Epidemiology
3.3. Etiopathogenesis and Pathophysiology
3.3.1. Evidence from Genetic Studies (Table 2)
| Author, Year [Ref] | Type of Study | Genetic Defects | Notes |
|---|---|---|---|
| Seidel et al., 2014 [24] | Case report | Partial trisomy 19p13 | Clear reduction in IgM and IgG1 and IgG3 subclasses in a patient with organ malformation. |
| Inoue CN et al., 2017 [25] | Case report | Trisomy 13 | Multi-year history of extensive acne conglobata with abscesses on the face and neck. |
| Al-Herz et al., 2004 [26] | Case report | De novo chromosome 22q11.2 deletion | A 15-year-old female with velopharyngeal incompetence and developmental and speech delay but no heart defects. |
| Kung et al., 2007 [27] | Case series | 22q11.2 deletion | A 6-year-old boy with recurrent otitis media, sinopulmonary infections, wheezing, velopharyngeal insufficiency, and speech delay. IgM ↓, IgA, and IgG N. Protective antibody titers to protein and carbohydrate antigens.A 14-year-old girl with neonatal seizures, atrial and ventricular septal defects, recurrent otitis media, intellectual disability, and asthma. IgM ↓, IgA, and IgG N. Protective antibody titers to protein and carbohydrate antigens. |
| Celmeli et al., 2014 [23] | Case report | De novo mosaic ring chromosome 18 | |
| Lim et al., 2013 [29] | Case report | c.347C > T (p.P116L) BTK gene mutation | - Six-year-old patient.- X-linked inheritance. |
| Geier et al., 2018 [30] | Case series | (1) BTK E206D mutation(2) biallelic missense mutations in BLNK, Pro110Ala, and Ala158Ser | (1) A 15-year-old male with recurrent aphthous stomatitis and recurrent respiratory tract infections (sinusitis, pneumonia, and bronchitis).(2) A 37-year-old male with asymptomatic renal insufficiency (cirrhosis of the left kidney and mild hydronephrosis of the right kidney found at 28 years of age) with no increased susceptibility to infections. |
| Smulsky et al., 2018 [31] | Case report | Inactivation of the TNFRSF13C gene | BAFFR deficiency. |
3.3.2. Evidence from Immunological Studies (Table 3)
| Author, Year [Ref] | Type of Study | B-Cells Defects | T-cells Defects | Notes |
|---|---|---|---|---|
| Karsh et al., 1982 [32] | Case report | Intrinsic B cell defect. | ||
| Kondo et al., 1992 [33] | Case reports | Defective secretion of Igμ messenger RNA. | ||
| Ohno et al., 1987 [34] Inoue T et al., 1986 [39] Matsushita et al., 1984 [40] | Case reports | Increased isotype-specific suppressor T cells. | ||
| Yamasaki et al., 1992 [35] | Case-control study | Intrinsic B cell defect. | Decreased T helper cell activity. | |
| De la Concha et al., 1982 [36] | Case reports | Decreased T helper cell activity. | ||
| Louis et al., 2016 [37] | Case–control study | Increased B-reg. | Increased CD8 T-reg cells. | |
| Kasahara et al., 2020 [38] | Case–control study | Lower percentage of follicular regulatory T (TFR) cells. A higher percentage of circulating follicular helper T (cTFH) cells in SIgMD patients with specific antibody response deficiency than in SIgMD patients with normal specific antibody response. | The role is not established. | |
| Gupta et al., 2016 [41] | Case–control study | Decreased FcμR expression on marginal zone B cells. |
3.4. Clinical Manifestations (Table 4)
| Infectious Manifestations | |
| Upper respiratory tract infections Recurrent otitis media Sinusitis (recurrent, chronic) Bronchitis Pneumonia (also recurrent) Bronchiectasis [15,44] Urinary tract infections Diarrhea, gastroenteric infections, hepatitis, and cholangitis Lymphadenopathy | Severe infections (meningitis, osteomyelitis, septic arthritis, and deep tissue and liver abscesses) and sepsis (mainly meningococcal and pneumococcal infections; Pseudomonas). Mycobacteria infections (also miliar tuberculosis [53] and atypical mycobacterial adenitis [54]). Soft tissue infections and skin infections (also herpes infections, acne conglobate [25], disseminated molluscum contagiosum in a 16-year-old girl [55], recurrent Staphylococcal pyoderma in two adult men [56], and recurrent impetigo in a 6.5-year-old boy [57]. Multiple recurrent hordeola (reported in a 10-year-old boy [58]). |
| Allergic Manifestations | |
| Allergic rhinitis Asthma and recurrent wheezing in the infancy | Idiopathic angioedema and anaphylaxis (reported in adulthood) Atopic dermatitis |
| Autoimmune Manifestations | |
| Addison’s disease Autoimmune glomerulonephritis Autoimmune hemolytic anemia Autoimmune thrombocytopenia Celiac disease Crohn’s disease Hashimoto’s thyroiditis Rheumatic heart disease (reported [15]) | Myasthenia gravis Polymyositis Idiopathic Juvenile Arthritis and Rheumatoid Arthritis Sjogren’s syndrome Systemic lupus erythematosus Vitiligo Psoriasis and scleroderma (reported [15]) |
| Neoplastic Manifestations | |
| Acute myeloid leukemia, tubular adenoma in the sigmoid colon, and neuroblastoma [20]. Multiple myeloma, non-Hodgkin lymphoma, thyroid cancer, and oropharyngeal carcinoma [68]. Gastric cancer [68]; EBV+ gastric adenocarcinoma in a 53-year-old male with collagenous gastritis and a history of asthma, allergic rhinitis, recurrent upper respiratory tract infections, multiple cases of pneumonia, acute sinusitis, and meningitis [69]. | MGUS [68]; IgAλ MGUS in a 21-year-old female with a history of recurrent urinary tract infections [70]. Primary cutaneous anaplastic large-cell lymphoma in a 13-year-old boy [67]. |
3.5. Immunological Characterization (Table 5)
| Immunoglobulins | IgG Subclasses | Lymphocyte Subsets | ||||||||||
| IgM | IgA | IgG | IgG1 | IgG2 | IgG3 | IgG4 | CD3 | CD4 | CD8 | CD19 | ||
| ↓ or absent | N | N | N or ↓ | N or ↓ | N or ↓ | N or ↓ | N or ↓ | N or ↓ | N or ↓ | N; ↓ or absent | ||
| B-cell Subsets | In Vitro IgProduction | Lymphocyte Stimulation | Response to Vaccines | |||||||||
| Naïve (CD27-, IgM+, IgD+) | Non-switched Memory (CD27+, IgM+, IgD+) | Class Switched Memory (CD27+, IgD-, IgG+ or IgA+ or IgE+) | IgA | IgM | IgG | Mitogen | Antigen | Polysaccharide | Protein | |||
| N | N or ↓ | N or ↓ | N | N or ↓ | N | N | N | N or impaired | N or impaired | |||
3.6. Therapeutic Interventions (Table 6)
| Intervention | Notes |
|---|---|
| Vaccination | Before the administration of attenuated vaccines, an evaluation of T cell function is advised. |
| Prompt treatment of febrile illness | |
| Immunoglobulin replacement therapy | Patients with significant antibody deficiency, particularly in the presence of impaired pneumococcal antibody responses, recurrent or severe infections and/or bronchiectasis. |
| Prophylactic antibiotics | Particularly in patients with other associated immunological defects. |
| Management of atopic diseases | May be helpful in reducing the incidence of complicating sinopulmonary infections. |
3.7. Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Picard, C.; Bobby Gaspar, H.; Al-Herz, W.; Bousfiha, A.; Casanova, J.-L.; Chatila, T.; Crow, Y.J.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J. Clin. Immunol. 2018, 38, 96–128. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Bousfiha, A.; Moundir, A.; Tangye, S.G.; Picard, C.; Jeddane, L.; Al-Herz, W.; Rundles, C.C.; Franco, J.L.; Holland, S.M.; Klein, C.; et al. The 2022 Update of IUIS Phenotypical Classification for Human Inborn Errors of Immunity. J. Clin. Immunol. 2022, 42, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- ESID—European Society for Immunodeficiencies. Available online: https://esid.org/Working-Parties/Registry-Working-Party/Diagnosis-criteria (accessed on 25 March 2023).
- Gupta, S.; Gupta, A. Selective IgM Deficiency—An Underestimated Primary Immunodeficiency. Front. Immunol. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Lozano, R.; Marin, R.; Santacruz, M.-J.; Pascual, A. Selective Immunoglobulin M Deficiency Among Clozapine-Treated Patients: A Nested Case-Control Study. Prim. Care Companion CNS Disord. 2015, 17. [Google Scholar] [CrossRef]
- Janssen, L.M.A.; Macken, T.; Creemers, M.C.W.; Pruijt, J.F.M.; Eijk, J.J.J.; de Vries, E. Truly selective primary IgM deficiency is probably very rare. Clin. Exp. Immunol. 2018, 191, 203–211. [Google Scholar] [CrossRef]
- Arani, M.H.; Razavizadeh, M.; ArefNezhad, R.; Motedayyen, H. Selective immunoglobulin M deficiency in a patient with celiac disease and recurrent pneumonia. Clin. Case Rep. 2021, 9, 158–163. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, A. Defining Primary Selective IgM Deficiency. J. Clin. Immunol. 2019, 39, 350–352. [Google Scholar] [CrossRef]
- Hobbs, J.R.; Milner, R.D.; Watt, P.J. Gamma-M deficiency predisposing to meningococcal septicaemia. Br. Med. J. 1967, 4, 583–586. [Google Scholar] [CrossRef][Green Version]
- Thong, Y.H.; Maxwell, G.M. Primary selective deficiency of immunoglobulin M. Aust. N. Z. J. Med. 1978, 8, 436–438. [Google Scholar] [CrossRef]
- Kutukculer, N.; Gulez, N. The outcome of patients with unclassified hypogammaglobulinemia in early childhood. Pediatr. Allergy Immunol. 2009, 20, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Núñez, R.M. Primary immunodeficiency in Colombian children. Allergol. Immunopathol. 1988, 16, 273–275. [Google Scholar]
- Datta, U.; Kaur, K.J.; Kumar, L.; Walia, B.N.; Mehta, S.; Mehta, S.K.; Sehgal, S. Immunoglobulin deficiency. Indian Pediatr. 1993, 30, 461–467. [Google Scholar] [PubMed]
- Ni, J.; Zhang, J.; Chen, Q.; Chen, Y.; Liu, J. The epidemiology and clinical features of selective immunoglobulin M deficiency: A single-center study in China. J. Clin. Lab. Anal. 2020, 34, e23289. [Google Scholar] [CrossRef] [PubMed]
- Louis, A.G.; Gupta, S. Primary Selective IgM Deficiency: An Ignored Immunodeficiency. Clinic Rev. Allergy Immunol. 2014, 46, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.T.; Nordby, G.L. Human serum immunoglobulin concentrations: Prevalence of immunoglobulin deficiencies. J. Allergy Clin. Immunol. 1975, 55, 35–48. [Google Scholar] [CrossRef]
- Entezari, N.; Adab, Z.; Zeydi, M.; Saghafi, S.; Jamali, M.; Kardar, G.A.; Pourpak, Z. The prevalence of Selective Immunoglobulin M Deficiency (SIgMD) in Iranian volunteer blood donors. Hum. Immunol. 2016, 77, 7–11. [Google Scholar] [CrossRef]
- Goldstein, M.F.; Goldstein, A.L.; Dunsky, E.H.; Dvorin, D.J.; Belecanech, G.A.; Shamir, K. Selective IgM immunodeficiency: Retrospective analysis of 36 adult patients with review of the literature. Ann. Allergy Asthma Immunol. 2006, 97, 717–730. [Google Scholar] [CrossRef]
- Caka, C.; Cimen, O.; Kahyaoğlu, P.; Tezcan, İ.; Cagdas, D. Selective IgM deficiency: Follow-up and outcome. Pediatr. Allergy Immunol. 2021, 32, 1327–1334. [Google Scholar] [CrossRef]
- Goldstein, M.F.; Goldstein, A.L.; Dunsky, E.H.; Dvorin, D.J.; Belecanech, G.A.; Shamir, K. Pediatric Selective IgM Immunodeficiency. J. Immunol. Res. 2008, 2008, e624850. [Google Scholar] [CrossRef]
- Janssen, L.M.A.; Reijnen, I.C.G.M.; Milito, C.; Edgar, D.; Chapel, H.; de Vries, E. Protocol for the unclassified primary antibody deficiency (unPAD) study: Characterization and classification of patients using the ESID online Registry. PLoS ONE 2022, 17, e0266083. [Google Scholar] [CrossRef]
- Celmeli, F.; Turkkahraman, D.; Cetin, Z.; Mihci, E.; Yegin, O. Selective IgM deficiency in a boy with ring chromosome 18. J. Investig. Allergol. Clin. Immunol. 2014, 24, 442–444. [Google Scholar] [PubMed]
- Seidel, M.G.; Duerr, C.; Woutsas, S.; Schwerin-Nagel, A.; Sadeghi, K.; Neesen, J.; Uhrig, S.; Santos-Valente, E.; Pickl, W.F.; Schwinger, W.; et al. A novel immunodeficiency syndrome associated with partial trisomy 19p13. J. Med. Genet. 2014, 51, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Inoue, C.N.; Tanaka, Y.; Tabata, N. Acne conglobata in a long-term survivor with trisomy 13, accompanied by selective IgM deficiency. Am. J. Med. Genet. Part A 2017, 173, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Al-Herz, W.; McGeady, S.J.; Gripp, K.W. 22q11.2 deletion syndrome and selective igm deficiency: An association of a common chromosomal abnormality with a rare immunodeficiency. Am. J. Med. Genet. Part A 2004, 127A, 99–100. [Google Scholar] [CrossRef]
- Kung, S.-J.; Gripp, K.W.; Stephan, M.J.; Fairchok, M.P.; McGeady, S.J. Selective IgM deficiency and 22q11.2 deletion syndrome. Ann. Allergy Asthma Immunol. 2007, 99, 87–92. [Google Scholar] [CrossRef]
- Ozawa, T.; Kondo, N.; Motoyoshi, F.; Kasahara, K.; Orii, T. Dna Mutation Induced in the Sequence Upstream of the Secreted Myu C-Terminal Coding Sequence by Ultraviolet Irradiation in the Cell Line of Bloom’s Syndrome. Int. J. Immunogenet. 1995, 22, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-M.; Chang, J.-M.; Wang, I.-F.; Chang, W.-C.; Hwang, D.-Y.; Chen, H.-C. Atypical X-linked agammaglobulinaemia caused by a novel BTK mutation in a selective immunoglobulin M deficiency patient. BMC Pediatr. 2013, 13, 150. [Google Scholar] [CrossRef]
- Geier, C.B.; Sauerwein, K.M.T.; Leiss-Piller, A.; Zmek, I.; Fischer, M.B.; Eibl, M.M.; Wolf, H.M. Hypomorphic Mutations in the BCR Signalosome Lead to Selective Immunoglobulin M Deficiency and Impaired B-cell Homeostasis. Front. Immunol. 2018, 9, 2984. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02984 (accessed on 30 May 2023). [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02285 (accessed on 20 May 2023). [CrossRef]
- Karsh, J.; Watts, C.S.; Osterland, C.K. Selective immunoglobulin M deficiency in an adult: Assessment of immunoglobulin production by peripheral blood lymphocytes in vitro. Clin. Immunol. Immunopathol. 1982, 25, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Ozawa, T.; Kato, Y.; Motoyoshi, F.; Kasahara, K.; Kameyama, T.; Orii, T. Reduced secreted μ mRNA synthesis in selective IgM deficiency of Bloom’s syndrome. Clin. Exp. Immunol. 1992, 88, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Inaba, M.; Kuribayashi, K.; Masuda, T.; Kanoh, T.; Uchino, H. Selective IgM deficiency in adults: Phenotypically and functionally altered profiles of peripheral blood lymphocytes. Clin. Exp. Immunol. 1987, 68, 630–637. [Google Scholar] [PubMed]
- Yamasaki, T. Selective IgM Deficiency: Functional Assessment of Peripheral Blood Lymphocytes in vitro. Intern. Med. 1992, 31, 866–870. [Google Scholar] [CrossRef]
- De la Concha, E.G.; Garcia-Rodriguez, M.C.; Zabay, J.M.; Laso, M.T.; Alonso, F.; Bootello, A.; Fontan, G. Functional assessment of T and B lymphocytes in patients with selective IgM deficiency. Clin. Exp. Immunol. 1982, 49, 670–676. [Google Scholar]
- Louis, A.G.; Agrawal, S.; Gupta, S. Analysis of subsets of B cells, Breg, CD4Treg and CD8Treg cells in adult patients with primary selective IgM deficiency. Am. J. Clin. Exp. Immunol. 2016, 5, 21–32. [Google Scholar]
- Kasahara, T.D.M.; Bento, C.A.D.M.; Gupta, S. Phenotypic analysis of T follicular helper and T follicular regulatory cells in primary selective IgM deficiency. Hum. Immunol. 2020, 81, 625–633. [Google Scholar] [CrossRef]
- Inoue, T.; Okumura, Y.; Shirahama, M.; Ishibashi, H.; Kashiwagi, S.; Okubo, H. Selective partial IgM deficiency: Functional assessment of T and B lymphocytesin vitro. J. Clin. Immunol. 1986, 6, 130–135. [Google Scholar] [CrossRef]
- Matsushita, S.; Inoue, T.; Okubo, H. A Case of Selective IgM Deficiency: Isotype-specific Suppressor T Lymphocytes. Jpn. J. Med. 1984, 23, 149–151. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, S.; Gollapudi, S.; Kubagawa, H. FcμR in human B cell subsets in primary selective IgM deficiency, and regulation of FcμR and production of natural IgM antibodies by IGIV. Hum. Immunol. 2016, 77, 1194–1201. [Google Scholar] [CrossRef]
- Fallon, K.E. Inability to train, recurrent infection, and selective IgM deficiency. Clin. J. Sport. Med. 2004, 14, 357–359. [Google Scholar] [CrossRef]
- Ozen, A.; Baris, S.; Karakoc-Aydiner, E.; Ozdemir, C.; Bahceciler, N.N.; Barlan, I.B. Outcome of hypogammaglobulinemia in children: Immunoglobulin levels as predictors. Clin. Immunol. 2010, 137, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.F.; Craig, S.; Bardana, E.J. Humoral immunity in bronchiectasis. Ann. Allergy 1987, 59, 179–182. [Google Scholar] [PubMed]
- Dhir, V.; Sagar, V.; Aggarwal, A.; Rawat, A.; Singhal, M. An unusual cause of recurrent pneumonia in adults. Lung Indian 2014, 31, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.M.A.; van Hout, R.W.N.M.; de Vries, E.; Pignata, C.; Cirillo, E.; Arkwright, P.D.; Lougaris, V.; Buckland, M.; Garcia-Prat, M.; D’Hebron, V.; et al. Challenges in investigating patients with isolated decreased serum IgM: The SIMcal study. Scand. J. Immunol. 2019, 89, e12763. [Google Scholar] [CrossRef]
- Phuphuakrat, A.; Ngamjanyaporn, P.; Nantiruj, K.; Luangwedchakarn, V.; Malathum, K. Selective IgM deficiency in an adult presenting with Streptococcus pneumoniae septic arthritis. J. Microbiol. Immunol. Infect. 2016, 49, 150–153. [Google Scholar] [CrossRef]
- Indian Pediatrics-Editorial. Available online: https://www.indianpediatrics.net/sep2005/sep-961-962.htm (accessed on 22 February 2023).
- Ross, I.N.; Thompson, R.A. Severe selective IgM deficiency. J. Clin. Pathol. 1976, 29, 773–777. [Google Scholar] [CrossRef]
- Hong, R.; Gupta, S. Selective immunoglobulin M deficiency in an adult with Streptococcus pneumoniae sepsis and invasive aspergillosis. J. Investig. Allergol. Clin. Immunol. 2008, 18, 214–218. [Google Scholar]
- Kampitak, T. Selective immunoglobulin M deficiency in a patient with refractory giardiasis. J. Investig. Allergol. Clin. Immunol. 2010, 20, 358–360. [Google Scholar]
- Moise, A.; Nedelcu, F.; Toader, M.; Sora, S.; Tica, A.; Ferastraoaru, D.; Constantinescu, I. Primary immunodeficiencies of the B Lymphocyte. J. Med. Life 2010, 3, 60–63. [Google Scholar]
- Hassanein, H.A.; Elbadry, M.I. Selective immunoglobulin M deficiency in an adult with miliary tuberculosis: A clinically interesting coexistence. A case report and review of the literature. Int. J. Mycobacteriol. 2016, 5, 106–110. [Google Scholar] [CrossRef]
- Consonni, F.; Chiti, N.; Ricci, S.; Venturini, E.; Canessa, C.; Bianchi, L.; Lippi, F.; Montagnani, C.; Giovannini, M.; Chiappini, E.; et al. Unbalanced serum immunoglobulins in clinical subtypes of pediatric tuberculosis disease. Front. Pediatr. 2022, 10, 908963. [Google Scholar] [CrossRef]
- Mayumi, M.; Yamaoka, K.; Tsutsui, T.; Mizue, H.; Doi, A.; Matsuyama, M.; Ito, S.; Shinomiya, K.; Mikawa, H. Selective immunoglobulin M deficiency associated with disseminated molluscum contagiosum. Eur. J. Pediatr. 1986, 145, 99–103. [Google Scholar] [CrossRef]
- Yocum, M.W.; Strong, D.M.; Chusid, M.J.; Lakin, J.D. Selective immunoglobulin M (IgM) deficiency in two immunodeficient adults with recurrent staphylococcal pyoderma. Am. J. Med. 1976, 60, 486–494. [Google Scholar] [CrossRef]
- Belgemen, T.; Suskan, E.; Dogu, F.; Ikinciogullari, A. Selective Immunoglobulin M Deficiency Presenting with Recurrent Impetigo: A Case Report and Review of the Literature. Int. Arch. Allergy Immunol. 2009, 149, 283–288. [Google Scholar] [CrossRef]
- Kıratlı, H.K.; Akar, Y. Multiple recurrent hordeola associated with selective IgM deficiency. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2001, 5, 60–61. [Google Scholar] [CrossRef]
- Campochiaro, C.; Atay, S.; Clark, K.E.N.; Ong, V.; Denton, C.P. Autoimmunity and immunodeficiency at the crossroad: Autoimmune disorders as the presenting feature of selective IgM deficiency. BMJ Case Rep. CP 2019, 12, e223180. [Google Scholar] [CrossRef]
- Takeuchi, T.; Nakagawa, T.; Maeda, Y.; Hirano, S.; Sasaki-Hayashi, M.; Makino, S.; Shimizu, A. Functional Defect of B Lymphocytes in a Patient with Selective IgM Deficiency Associated with Systemic Lupus Erythematosus. Autoimmunity 2001, 34, 115–122. [Google Scholar] [CrossRef]
- Kimura, S.; Tanigawa, M.; Nakahashi, Y.; Inoue, M.; Yamamura, Y.; Kato, H.; Sugino, S.; Kondo, M. Selective IgM Deficiency in a Patient with Hashimoto’s Disease. Intern. Med. 1993, 32, 302–307. [Google Scholar] [CrossRef]
- Antar, M.; Lamarche, J.; Peguero, A.; Reiss, A.; Cole, S. A case of selective immunoglobulin M deficiency and autoimmune glomerulonephritis. Clin. Exp. Nephrol. 2008, 12, 300–304. [Google Scholar] [CrossRef]
- Sano, A.; Inoue, J.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Morosawa, T.; Takai, S.; Nakamura, T.; Masamune, A. Acute-onset Autoimmune Hepatitis in a Patient with Selective Immunoglobulin M Deficiency. Intern. Med. 2019, 58, 2185–2190. [Google Scholar] [CrossRef]
- Oh, J.; McGarry, D.; Peppers, B.; Hostoffer, R. Selective IgM deficiency associated with adult-onset Still disease. Ann. Allergy Asthma Immunol. 2018, 120, 444–446. [Google Scholar] [CrossRef]
- Makay, B.; Ünsal, E.; Anal, Ö.; Güneş, D.; Men, S.; Çakmakçı, H.; Özer, E. Chronic recurrent multifocal osteomyelitis in a patient with selective immunoglobulin M deficiency. Rheumatol. Int. 2009, 29, 811–815. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, S.; Airun, M. Symptomatic Primary Selective IgM Immunodeficiency—B Lymphoid Cell Defect in Adult Man with Secondary HLH Syndrome. J. Assoc. Physicians India 2016, 64, 91–93. [Google Scholar]
- Saini, S.; Dettore, A.J.; Bhambhani, K.J.; Buck, S.; Poulik, J.; Savasan, S. Selective IgM Deficiency in CD30+ Cutaneous Lymphoproliferative Disorder. J. Pediatr. Hematol./Oncol. 2011, 33, e156. [Google Scholar] [CrossRef]
- Lucuab-Fegurgur, D.L.; Gupta, S. Comprehensive clinical and immunological features of 62 adult patients with selective primary IgM deficiency. Am. J. Clin. Exp. Immunol. 2019, 8, 55–67. [Google Scholar] [PubMed]
- Narsai, T.; Su, H.; Braxton, D.; Gupta, S. Collagenous Gastritis in Primary Selective IgM Deficiency: Transition to EBV+ Gastric Adenocarcinoma. Case Rep. Immunol. 2021, 2021, 5574944. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, S. IgAλ monoclonal gammopathy of undetermined significance (MGUS) associated with primary selective IgM deficiency. Am. J. Clin. Exp. Immunol. 2019, 8, 37–46. [Google Scholar]
- Chovancova, Z.; Kralickova, P.; Pejchalova, A.; Bloomfield, M.; Nechvatalova, J.; Vlkova, M.; Litzman, J. Selective IgM Deficiency: Clinical and Laboratory Features of 17 Patients and a Review of the Literature. J. Clin. Immunol. 2017, 37, 559–574. [Google Scholar] [CrossRef]
- Bolia, R.; Misra, D.P.; Aggarwal, A.; Srivastava, A. Paediatric selective IgM deficiency and IgG4 deficiency: An extremely unusual association. Case Rep. 2014, 2014, bcr2014204769. [Google Scholar] [CrossRef]
- Ideura, G.; Agematsu, K.; Komatsu, Y.; Hatayama, O.; Yasuo, M.; Tsushima, K.; Hanaoka, M.; Koizumi, T.; Fujimoto, K.; Kubo, K. Selective IgM deficiency accompanied with IgG4 deficiency, dermal complications and a bronchial polyp. Allergol. Int. 2008, 57, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Endoh, M.; Kaneshige, H.; Tomino, Y.; Nomoto, Y.; Sakai, H.; Arimori, S. Selective IgM deficiency: A case study. Tokai J. Exp. Clin. Med. 1981, 6, 327–331. [Google Scholar] [PubMed]
- Celiksoy, M.H.; Yildiran, A. A comparison of B cell subsets in primary immune deficiencies that progress with antibody deficiency and age-matched healthy children. Allergol. Immunopathol. 2016, 44, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Cipe, F.E.; Doğu, F.; Güloğlu, D.; Aytekin, C.; Polat, M.; Biyikli, Z.; Ikincioğullari, A. B-cell subsets in patients with transient hypogammaglobulinemia of infancy, partial IgA deficiency, and selective IgM deficiency. J. Investig. Allergol. Clin. Immunol. 2013, 23, 94–100. [Google Scholar]
- Mensen, A.; Krause, T.; Hanitsch, L.G.; Meisel, C.; Kleint, M.E.; Volk, H.-D.; Na, I.-K.; Scheibenbogen, C. Altered B-cell subsets and functional B-cell defects in selective IgM deficiency. Clin. Immunol. 2015, 161, 96–102. [Google Scholar] [CrossRef]
- Moffitt, J.E.; Guill, M.F.; Wray, B.B.; Brown, D.A.; Peacocke, N.W.; Ades, E.W. Effect of interleukin-2 and mitogen on in vitro immunoglobulin production by peripheral blood lymphocytes from patients with selective IgM deficiency. Ann. Allergy 1988, 61, 424–427. [Google Scholar]
- Raziuddin, S.; Bilal, N.; Benjamin, B. Transient T-cell abnormality in a selective IgM-immunodeficient patient with Brucella infection. Clin. Immunol. Immunopathol. 1988, 46, 360–367. [Google Scholar] [CrossRef]
- Patel, S.S.; Fergeson, J.E.; Glaum, M.C.; Lockey, R.F. Symptomatic Primary Selective Immunoglobulin M Deficiency with Nonprotective Pneumococcal Titers Responsive to Subcutaneous Immunoglobulin Treatment. Int. Arch. Allergy Immunol. 2016, 170, 138–140. [Google Scholar] [CrossRef]
- Goldstein, M.F.; Hilditch, G.J.; Dvorin, D.J.; Belecanech, G.A. Immunoglobulin replacement for selective IgM immunodeficiency, bronchiectasis, and asthma. Ann. Allergy Asthma Immunol. 2016, 116, 172–173. [Google Scholar] [CrossRef]
- Langereis, J.D.; van der Flier, M.; de Jonge, M.I. Limited Innovations After More Than 65 Years of Immunoglobulin Replacement Therapy: Potential of IgA- and IgM-Enriched Formulations to Prevent Bacterial Respiratory Tract Infections. Front. Immunol. 2018, 9, 1925. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.01925 (accessed on 9 May 2023). [CrossRef]
- Narsai, T.; Gupta, S. Progression of primary selective immunoglobulin M deficiency to common variable immunodeficiency. Ann. Allergy Asthma Immunol. 2021, 126, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Educational Materials/Library/JMF. Available online: https://info4pi.org/library/educational-materials/ (accessed on 22 June 2023).
- Guill, M.F.; Brown, D.A.; Ochs, H.D.; Pyun, K.H.; Moffitt, J.E. IgM deficiency: Clinical spectrum and immunologic assessment. Ann. Allergy 1989, 62, 547–552. [Google Scholar] [PubMed]
- Kaufman, H.; Hobbs, J. Immunoglobulin deficiencies in an atopic population. Lancet 1970, 296, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, A. Chapter 6—Autoimmunity and autoimmune diseases in primary selective IgM deficiency. In Translational Autoimmunity; Rezaei, N., Ed.; Translational Immunology; Academic Press: Cambridge, MA, USA, 2022; Volume 3, pp. 129–139. ISBN 978-0-323-85415-3. [Google Scholar]
- Gaspar, H.B.; Conley, M.E. Early B cell defects. Clin. Exp. Immunol. 2000, 119, 383–389. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Barlan, I.; Chapel, H.; Costa-Carvalho, B.T.; Cunningham-Rundles, C.; de la Morena, M.T.; Espinosa-Rosales, F.J.; Hammarström, L.; Nonoyama, S.; Quinti, I.; et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2016, 4, 38–59. [Google Scholar] [CrossRef]
- Seidel, M.G.; Kindle, G.; Gathmann, B.; Quinti, I.; Buckland, M.; van Montfrans, J.; Scheible, R.; Rusch, S.; Gasteiger, L.M.; Grimbacher, B.; et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J. Allergy Clin. Immunol. Pract. 2019, 7, 1763–1770. [Google Scholar] [CrossRef]
- Martire, B.; Azzari, C.; Badolato, R.; Canessa, C.; Cirillo, E.; Gallo, V.; Graziani, S.; Lorenzini, T.; Milito, C.; Panza, R.; et al. Vaccination in immunocompromised host: Recommendations of Italian Primary Immunodeficiency Network Centers (IPINET). Vaccine 2018, 36, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Radigan, L.; Cunningham-Rundles, C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin. Immunol. 2005, 116, 37–41. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Infectious Diseases. Red Book: 2015 Report of the Committee on Infectious Diseases; American Academy of Pediatrics: Itasca, IL, USA, 2015. [Google Scholar]
- Cunningham-Rundles, C. Common variable immune deficiency: Case studies. Hematology 2019, 2019, 449–456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taietti, I.; Votto, M.; De Filippo, M.; Naso, M.; Montagna, L.; Montagna, D.; Licari, A.; Marseglia, G.L.; Castagnoli, R. Selective IgM Deficiency: Evidence, Controversies, and Gaps. Diagnostics 2023, 13, 2861. https://doi.org/10.3390/diagnostics13172861
Taietti I, Votto M, De Filippo M, Naso M, Montagna L, Montagna D, Licari A, Marseglia GL, Castagnoli R. Selective IgM Deficiency: Evidence, Controversies, and Gaps. Diagnostics. 2023; 13(17):2861. https://doi.org/10.3390/diagnostics13172861
Chicago/Turabian StyleTaietti, Ivan, Martina Votto, Maria De Filippo, Matteo Naso, Lorenza Montagna, Daniela Montagna, Amelia Licari, Gian Luigi Marseglia, and Riccardo Castagnoli. 2023. "Selective IgM Deficiency: Evidence, Controversies, and Gaps" Diagnostics 13, no. 17: 2861. https://doi.org/10.3390/diagnostics13172861
APA StyleTaietti, I., Votto, M., De Filippo, M., Naso, M., Montagna, L., Montagna, D., Licari, A., Marseglia, G. L., & Castagnoli, R. (2023). Selective IgM Deficiency: Evidence, Controversies, and Gaps. Diagnostics, 13(17), 2861. https://doi.org/10.3390/diagnostics13172861








