A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases
Abstract
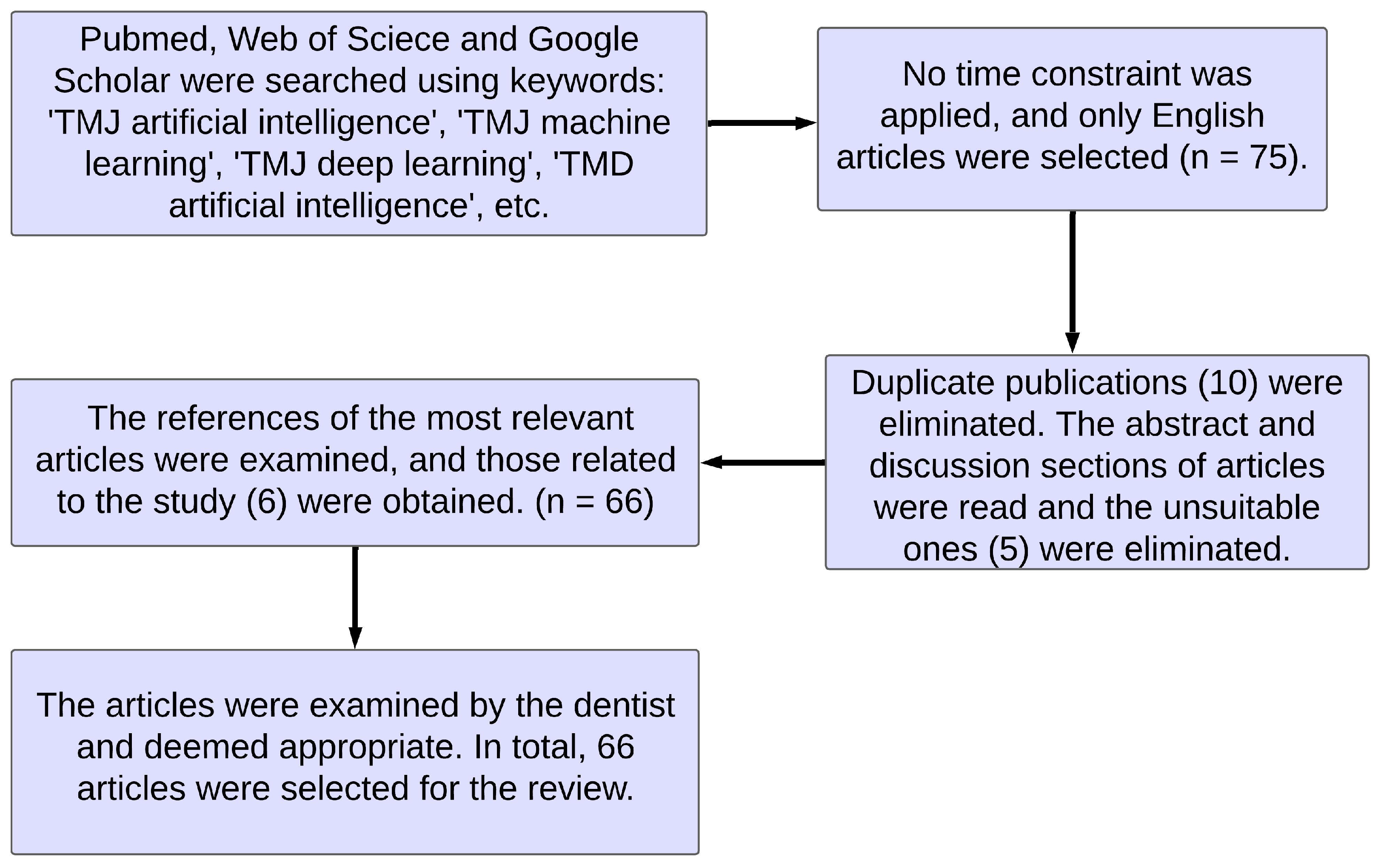
:1. Introduction
- Fundamentals of TMJ (Section 2): Provides information about TMJ, data acquisition, data utilized in diagnosis, and automatic diagnosis.
- Diseases and Diagnostics
- Independent Variables and Data Capturing
- Fundamentals of AI (Section 3): Foundational knowledge about AI, ML, and DL.
- ML
- DL
- Literature on TMJ (Section 4): Computer-based studies conducted on TMJ.
- Segmentation of TMJ
- Audio and Image-Based Diagnostic
- Decision Support Systems
- Review studies on TMJ
- Discussion (Section 5)
- Conclusion (Section 6)
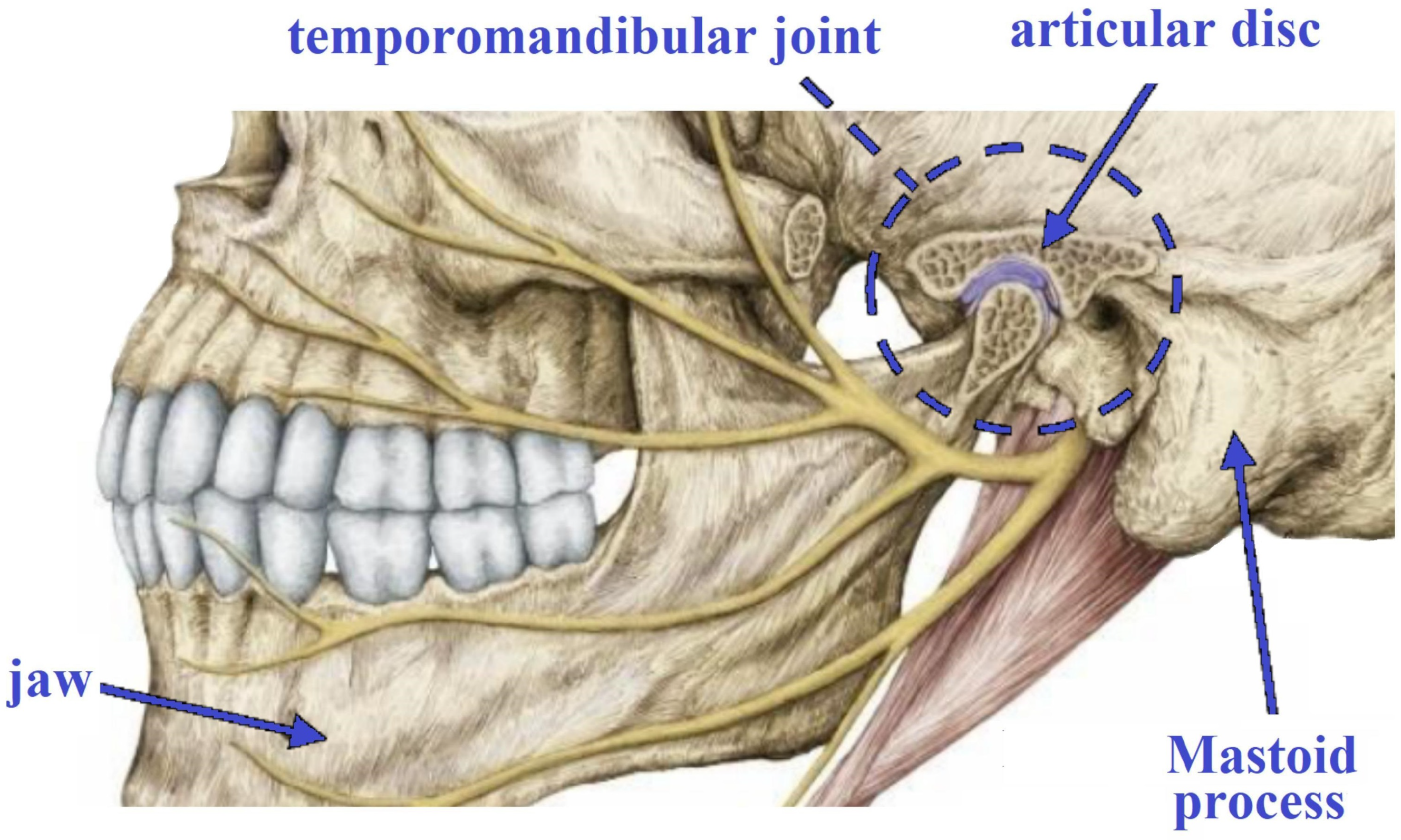
2. Fundamentals of TMJ
2.1. Diseases and Diagnostics
- Gathering Medical History: the healthcare professional will start by obtaining a comprehensive medical history from the patient, including any past jaw-related problems, dental procedures, or recent injuries that could be pertinent to the current symptoms.
- Comprehensive Clinical Assessment: a detailed examination of the jaw, face, head, and neck will be performed to evaluate the extent of movement, joint sounds, tenderness, and any indications of inflammation or swelling.
- Diagnostic Imaging: X-rays, Computed Tomography (CT) scans, or Magnetic Resonance Imaging (MRI) can be employed to capture detailed images of the TMJ and adjacent structures. These imaging modalities have the capability to detect any irregularities, such as joint degeneration, disc displacement, or fractures [15].
- Occlusion Assessment: the dentist will examine how the upper and lower teeth fit together (occlusion) to identify any malocclusion or teeth alignment issues that may contribute to TMJ problems.
- TMJ Function Tests: specific tests will be conducted to assess the functioning of the TMJ and jaw muscles. These tests aid in detecting any limitations or irregularities in jaw movement.
- Palpation: a gentle examination of the jaw area will be performed to locate tender points or areas of muscle tension.
- Pain and Symptom Evaluation: the patient will be asked about the location, intensity, and duration of pain, as well as any accompanying symptoms such as headaches or earaches.
- Exclusion of Alternative Causes: due to the potential overlap of TMJ-related symptoms with other conditions, the healthcare provider will carefully eliminate other potential causes of jaw pain or dysfunction.
2.2. Independent Variables and Data Capturing
3. Fundamentals of AI
3.1. ML
3.2. DL
4. Literature on TMJ
4.1. Segmentation of TMJ
4.2. Audio and Image-Based Diagnostic
4.2.1. Juvenile Idiopathic Arthritis
4.2.2. TMJ Osteoarthritis
4.2.3. TMD
4.2.4. Studies Using Audio Data
4.3. Decision Support Systems
4.4. Review Studies on TMJ
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 2018, 172, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Woerl, A.C.; Eckstein, M.; Geiger, J.; Wagner, D.C.; Daher, T.; Stenzel, P.; Fernandez, A.; Hartmann, A.; Wand, M.; Roth, W.; et al. Deep learning predicts molecular subtype of muscle-invasive bladder cancer from conventional histopathological slides. Eur. Urol. 2020, 78, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Savitha, G.; Jidesh, P. A holistic deep learning approach for identification and classification of sub-solid lung nodules in computed tomographic scans. Comput. Electr. Eng. 2020, 84, 106626. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, J.; Du, H.; Qiu, B. A bone age assessment system for real-world X-ray images based on convolutional neural networks. Comput. Electr. Eng. 2020, 81, 106529. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z. The risk prediction of Alzheimer’s disease based on the deep learning model of brain 18F-FDG positron emission tomography. Saudi J. Biol. Sci. 2020, 27, 659–665. [Google Scholar] [CrossRef]
- Saha, S.; Pagnozzi, A.; Bourgeat, P.; George, J.M.; Bradford, D.; Colditz, P.B.; Boyd, R.N.; Rose, S.E.; Fripp, J.; Pannek, K. Predicting motor outcome in preterm infants from very early brain diffusion MRI using a deep learning convolutional neural network (CNN) model. Neuroimage 2020, 215, 116807. [Google Scholar] [CrossRef]
- Mahesh, B. Machine learning algorithms-a review. Int. J. Sci. Res. 2020, 9, 381–386. [Google Scholar]
- Shrestha, A.; Mahmood, A. Review of deep learning algorithms and architectures. IEEE Access 2019, 7, 53040–53065. [Google Scholar] [CrossRef]
- Ingawale, S.; Goswami, T. Temporomandibular joint: Disorders, treatments, and biomechanics. Ann. Biomed. Eng. 2009, 37, 976–996. [Google Scholar] [CrossRef]
- Kulesa-Mrowiecka, M.; Barański, R.; Kłaczyński, M. sEMG and Vibration System Monitoring for Differential Diagnosis in Temporomandibular Joint Disorders. Sensors 2022, 22, 3811. [Google Scholar] [CrossRef]
- Detamore, M.S.; Athanasiou, K.A. Structure and function of the temporomandibular joint disc: Implications for tissue engineering. J. Maxillofac. Surg. 2003, 61, 494–506. [Google Scholar] [CrossRef]
- Detamore, M.S.; Athanasiou, K.A.; Mao, J. A call to action for bioengineers and dental professionals: Directives for the future of TMJ bioengineering. Ann. Biomed. Eng. 2007, 35, 1301–1311. [Google Scholar] [CrossRef]
- Van Loon, J.P.; De Bont, L.; Stegenga, B.; Spijkervet, F.; Verkerke, G. Groningen temporomandibular joint prosthesis. Development and first clinical application. J. Maxillofac. Surg. 2002, 31, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P.; Fried, J.L. Temporomandibular disorders and hormones in women. Cells Tissues Organs 2001, 169, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J.P. Management of Temporomandibular Disorders and Occlusion-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Farook, T.H.; Rashid, F.; Alam, M.K.; Dudley, J. Variables influencing the device-dependent approaches in digitally analysing jaw movement—A systematic review. Clin. Oral Investig. 2023, 27, 489–504. [Google Scholar] [CrossRef]
- Larheim, T.A.; Westesson, P.L.; Hicks, D.G.; Eriksson, L.; Brown, D.A. Osteonecrosis of the temporomandibular joint: Correlation of magnetic resonance imaging and histology. J. Oral Maxillofac. Surg. 1999, 57, 888–898. [Google Scholar] [CrossRef]
- Yılmaz, D.; Kamburoğlu, K. Comparison of the effectiveness of high resolution ultrasound with MRI in patients with temporomandibular joint dısorders. Dentomaxillofacial Radiol. 2019, 48, 20180349. [Google Scholar] [CrossRef]
- Yılmaz, D.; Kamburoğlu, K.; Arslan, R. Quantitative volume and area assessment of masticatory muscles through magnetic resonance imaging in patients with temporomandibular joint disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Tsiklakis, K.; Syriopoulos, K.; Stamatakis, H. Radiographic examination of the temporomandibular joint using cone beam computed tomography. Dentomaxillofacial Radiol. 2004, 33, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Schiffman, E.L. Temporomandibular joint disorders and orofacial pain. Dent. Clin. 2016, 60, 105–124. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Mine, Y.; Yoshimi, Y.; Takeda, S.; Tanaka, A.; Onishi, A.; Peng, T.Y.; Nakamoto, T.; Nagasaki, T.; Kakimoto, N.; et al. Automated segmentation of articular disc of the temporomandibular joint on magnetic resonance images using deep learning. Sci. Rep. 2022, 12, 221. [Google Scholar] [CrossRef]
- Brosset, S.; Dumont, M.; Cevidanes, L.; Soroushmehr, R.; Bianchi, J.; Gurgel, M.L.; Deleat-Besson, R.; Le, C.; Ruellas, A.; Yatabe, M.; et al. Web infrastructure for data management, storage and computation. In Proceedings of the Medical Imaging 2021: Biomedical Applications in Molecular, Structural, and Functional Imaging, Online, 15–19 February 2021; Volume 11600, p. 116001N. [Google Scholar]
- Vinayahalingam, S.; Berends, B.; Baan, F.; Moin, D.A.; van Luijn, R.; Bergé, S.; Xi, T. Deep learning for automated segmentation of the temporomandibular joint. J. Dent. 2023, 132, 104475. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Tanaka, K.; Mori, H. An Artificial Intelligence-Based Cosmesis Evaluation for Temporomandibular Joint Reconstruction. Laryngoscope 2023, 133, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Brosset, S.; Dumont, M.; Bianchi, J.; Ruellas, A.; Cevidanes, L.; Yatabe, M.; Goncalves, J.; Benavides, E.; Soki, F.; Paniagua, B.; et al. 3D Auto-Segmentation of Mandibular Condyles. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 1270–1273. [Google Scholar]
- Fan, Y.; Beare, R.; Matthews, H.; Schneider, P.; Kilpatrick, N.; Clement, J.; Claes, P.; Penington, A.; Adamson, C. Marker-based watershed transform method for fully automatic mandibular segmentation from CBCT images. Dentomaxillofacial Radiol. 2019, 48, 20180261. [Google Scholar] [CrossRef]
- Nozawa, M.; Ito, H.; Ariji, Y.; Fukuda, M.; Igarashi, C.; Nishiyama, M.; Ogi, N.; Katsumata, A.; Kobayashi, K.; Ariji, E. Automatic segmentation of the temporomandibular joint disc on magnetic resonance images using a deep learning technique. Dentomaxillofacial Radiol. 2022, 51, 20210185. [Google Scholar] [CrossRef] [PubMed]
- Burget, R.; Cika, P.; Zukal, M.; Masek, J. Automated localization of temporomandibular joint disc in mri images. In Proceedings of the 2011 34th International Conference on Telecommunications and Signal Processing (TSP), Budapest, Hungary, 18–20 August 2011; pp. 413–416. [Google Scholar]
- Liu, Y.; Lu, Y.; Fan, Y.; Mao, L. Tracking-based deep learning method for temporomandibular joint segmentation. Ann. Transl. Med. 2021, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Shin, J.Y.; Lee, A.; Park, S.; Han, S.S.; Hwang, H.J. Automated cortical thickness measurement of the mandibular condyle head on CBCT images using a deep learning method. Sci. Rep. 2021, 11, 14852. [Google Scholar] [CrossRef]
- Kwak, G.H.; Kwak, E.J.; Song, J.M.; Park, H.R.; Jung, Y.H.; Cho, B.H.; Hui, P.; Hwang, J.J. Automatic mandibular canal detection using a deep convolutional neural network. Sci. Rep. 2020, 10, 5711. [Google Scholar] [CrossRef]
- Li, M.; Punithakumar, K.; Major, P.W.; Le, L.H.; Nguyen, K.C.T.; Pacheco-Pereira, C.; Kaipatur, N.R.; Nebbe, B.; Jaremko, J.L.; Almeida, F.T. Temporomandibular joint segmentation in MRI images using deep learning. J. Dent. 2022, 127, 104345. [Google Scholar] [CrossRef]
- Larheim, T.A.; Doria, A.S.; Kirkhus, E.; Parra, D.A.; Kellenberger, C.J.; Arvidsson, L.Z. TMJ imaging in JIA patients—An overview. In Seminars in Orthodontics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 21, pp. 102–110. [Google Scholar]
- Perpetuini, D.; Trippetti, N.; Cardone, D.; Breda, L.; D’Attilio, M.; Merla, A. Detection of temporomandibular joint disfunction in juvenile idiopathic arthritis through infrared thermal imaging and a machine learning procedure. In 8th European Medical and Biological Engineering Conference: Proceedings of the EMBEC 2020, Portorož, Slovenia, 29 November–3 December 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 372–381. [Google Scholar]
- Eng, S.W.; Yeung, R.S.; Morris, Q. The promise of machine learning to inform the management of juvenile idiopathic arthritis. Expert Rev. Clin. Immunol. 2021, 17, 1–3. [Google Scholar] [CrossRef]
- Van Nieuwenhove, E.; Lagou, V.; Van Eyck, L.; Dooley, J.; Bodenhofer, U.; Roca, C.; Vandebergh, M.; Goris, A.; Humblet-Baron, S.; Wouters, C.; et al. Machine learning identifies an immunological pattern associated with multiple juvenile idiopathic arthritis subtypes. Ann. Rheum. Dis. 2019, 78, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Gan, Y.; Zhou, Y. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.; de Oliveira Ruellas, A.C.; Gonçalves, J.R.; Paniagua, B.; Prieto, J.C.; Styner, M.; Li, T.; Zhu, H.; Sugai, J.; Giannobile, W.; et al. Osteoarthritis of the Temporomandibular Joint can be diagnosed earlier using biomarkers and machine learning. Sci. Rep. 2020, 10, 8012. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kwak, H.; Oh, J.; Jha, N.; Kim, Y.; Kim, W.; Baik, U.; Ryu, J. Automated detection of TMJ osteoarthritis based on artificial intelligence. J. Dent. Res. 2020, 99, 1363–1367. [Google Scholar] [CrossRef]
- Choi, E.; Kim, D.; Lee, J.Y.; Park, H.K. Artificial intelligence in detecting temporomandibular joint osteoarthritis on orthopantomogram. Sci. Rep. 2021, 11, 10246. [Google Scholar] [CrossRef]
- Jung, W.; Lee, K.E.; Suh, B.J.; Seok, H.; Lee, D.W. Deep learning for osteoarthritis classification in temporomandibular joint. Oral Dis. 2021, 29, 1050–1059. [Google Scholar] [CrossRef]
- Shoukri, B.; Prieto, J.; Ruellas, A.; Yatabe, M.; Sugai, J.; Styner, M.; Zhu, H.; Huang, C.; Paniagua, B.; Aronovich, S.; et al. Minimally invasive approach for diagnosing TMJ osteoarthritis. J. Dent. Res. 2019, 98, 1103–1111. [Google Scholar] [CrossRef]
- Ribera, N.T.; De Dumast, P.; Yatabe, M.; Ruellas, A.; Ioshida, M.; Paniagua, B.; Styner, M.; Gonçalves, J.R.; Bianchi, J.; Cevidanes, L.; et al. Shape variation analyzer: A classifier for temporomandibular joint damaged by osteoarthritis. In Proceedings of the Medical Imaging 2019: Computer-Aided Diagnosis, San Diego, CA, USA, 16–21 February 2019; Volume 10950, pp. 517–523. [Google Scholar]
- Le, C.; Deleat-Besson, R.; Turkestani, N.A.; Cevidanes, L.; Bianchi, J.; Zhang, W.; Gurgel, M.; Shah, H.; Prieto, J.; Li, T. TMJOAI: An Artificial Web-Based Intelligence Tool for Early Diagnosis of the Temporomandibular Joint Osteoarthritis. In Clinical Image-Based Procedures, Distributed and Collaborative Learning, Artificial Intelligence for Combating COVID-19 and Secure and Privacy-Preserving Machine Learning; Springer: Berlin/Heidelberg, Germany, 2021; pp. 78–87. [Google Scholar]
- Kim, D.; Choi, E.; Jeong, H.G.; Chang, J.; Youm, S. Expert system for mandibular condyle detection and osteoarthritis classification in panoramic imaging using r-cnn and cnn. Appl. Sci. 2020, 10, 7464. [Google Scholar] [CrossRef]
- de Dumast, P.; Mirabel, C.; Cevidanes, L.; Ruellas, A.; Yatabe, M.; Ioshida, M.; Ribera, N.T.; Michoud, L.; Gomes, L.; Huang, C.; et al. A web-based system for neural network based classification in temporomandibular joint osteoarthritis. Comput. Med. Imaging Graph. 2018, 67, 45–54. [Google Scholar] [CrossRef]
- Eşer, G.; Duman, Ş.B.; Bayrakdar, İ.Ş.; Çelik, Ö. Classification of Temporomandibular Joint Osteoarthritis on Cone-Beam Computed Tomography Images Using Artificial Intelligence System. J. Oral Rehabil. 2023, 50, 758–766. [Google Scholar] [CrossRef]
- Lin, B.; Cheng, M.; Wang, S.; Li, F.; Zhou, Q. Automatic detection of anteriorly displaced temporomandibular joint discs on magnetic resonance images using a deep learning algorithm. Dentomaxillofacial Radiol. 2021, 50, 20210341. [Google Scholar] [CrossRef] [PubMed]
- Orhan, K.; Driesen, L.; Shujaat, S.; Jacobs, R.; Chai, X. Development and Validation of a Magnetic Resonance Imaging-Based Machine Learning Model for TMJ Pathologies. Biomed Res. Int. 2021, 2021, 6656773. [Google Scholar] [CrossRef] [PubMed]
- Diniz de Lima, E.; Souza Paulino, J.A.; Lira de Farias Freitas, A.P.; Viana Ferreira, J.E.; Barbosa, J.d.S.; Bezerra Silva, D.F.; Bento, P.M.; Araújo Maia Amorim, A.M.; Melo, D.P. Artificial intelligence and infrared thermography as auxiliary tools in the diagnosis of temporomandibular disorder. Dentomaxillofacial Radiol. 2022, 51, 20210318. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.; Jeon, K.J.; Kim, H.; Huh, J.K. Using deep learning to predict temporomandibular joint disc perforation based on magnetic resonance imaging. Sci. Rep. 2021, 11, 6680. [Google Scholar] [CrossRef]
- Lee, K.S.; Jha, N.; Kim, Y.J. Risk factor assessments of temporomandibular disorders via machine learning. Sci. Rep. 2021, 11, 19802. [Google Scholar] [CrossRef] [PubMed]
- Taşkıran, U.; Çunkaş, M. A deep learning based decision support system for diagnosis of Temporomandibular joint disorder. Appl. Acoust. 2021, 182, 108292. [Google Scholar] [CrossRef]
- Sharma, N.; Dar, I.G.; Kumar, J.; Khan, A.; Thakur, A. Temporomandibular Joint Syndrome Prediction Using Neural Network. In Engineering Vibration, Communication and Information Processing; Springer: Singapore, 2019; pp. 1–7. [Google Scholar]
- Ebadian, B.; Abbasi, M.; Nazarifar, A.M. Frequency distribution of temporomandibular disorders according to occlusal factors: A Cross-Sectional Study. Dent. Res. J. 2020, 17, 186. [Google Scholar]
- Magalhães, B.G.; de Sousa, S.T.; de Mello, V.V.C.; da Silva-Barbosa, A.C.; de Assis-Morais, M.P.L.; Barbosa-Vasconcelos, M.M.V.; Caldas-Júnior, A.F. Risk factors for temporomandibular disorder: Binary logistic regression analysis. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e232. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H. Bayesian belief network analysis applied to determine the progression of temporomandibular disorders using MRI. Dentomaxillofacial Radiol. 2015, 44, 20140279. [Google Scholar] [CrossRef]
- Ashraf, J.; Närhi, M.; Suominen, A.L.; Saxlin, T. Association of temporomandibular disorder-related pain with severe Headaches—A Bayesian view. Clin. Oral Investig. 2022, 26, 729–738. [Google Scholar] [CrossRef]
- Lee, Y.H.; Won, J.H.; Kim, S.; Auh, Q.S.; Noh, Y.K. Advantages of Deep Learning with Convolutional Neural Network in Detecting Disc Displacement of the Temporomandibular Joint in Magnetic Resonance Imaging. Sci. Rep. 2022, 12, 11352. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.W.; Park, S.H.; On, S.W.; Song, S.I. Correlation between clinical symptoms and magnetic resonance imaging findings in patients with temporomandibular joint internal derangement. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 125. [Google Scholar] [CrossRef]
- Troka, M.; Wojnicz, W.; Szepietowska, K.; Podlasiński, M.; Walerzak, S.; Walerzak, K.; Lubowiecka, I. Towards classification of patients based on surface EMG data of temporomandibular joint muscles using self-organising maps. Biomed. Signal Process. Control 2022, 72, 103322. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, H.G.; Kho, H.S. Differential diagnosis of jaw pain using informatics technology. J. Oral Rehabil. 2018, 45, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Nocera, L.; Vistoso, A.; Yoshida, Y.; Abe, Y.; Nwoji, C.; Clark, G.T. Building an automated orofacial pain, headache and temporomandibular disorder diagnosis system. In AMIA Annual Symposium Proceedings; American Medical Informatics Association: Bethesda, MD, USA, 2020; Volume 2020, p. 943. [Google Scholar]
- Jeon, K.J.; Kim, Y.H.; Ha, E.G.; Choi, H.S.; Ahn, H.J.; Lee, J.R.; Hwang, D.; Han, S.S. Quantitative analysis of the mouth opening movement of temporomandibular joint disorder patients according to disc position using computer vision: A pilot study. Quant. Imaging Med. Surg. 2022, 12, 1909. [Google Scholar] [CrossRef]
- Kreiner, M.; Viloria, J. A novel artificial neural network for the diagnosis of orofacial pain and temporomandibular disorders. J. Oral Rehabil. 2022, 49, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Kao, Z.K.; Chiu, N.T.; Wu, H.T.H.; Chang, W.C.; Wang, D.H.; Kung, Y.Y.; Tu, P.C.; Lo, W.L.; Wu, Y.T. Classifying temporomandibular disorder with artificial intelligent architecture using magnetic resonance imaging. Ann. Biomed. Eng. 2023, 51, 517–526. [Google Scholar] [CrossRef]
- Ozsari, S.; Yapıcıoğlu, F.R.; Yılmaz, D.; Kamburoglu, K.; Guzel, M.S.; Bostanci, G.E.; Acici, K.; Asuroglu, T. Interpretation of Magnetic Resonance Images of Temporomandibular Joint Disorders by Using Deep Learning. IEEE Access 2023, 11, 49102–49113. [Google Scholar] [CrossRef]
- Akan, A.; Ergin, A.; Yildirim, M.; Öztaş, E. Analysis of temporomandibular joint sounds in orthodontic patients. Comput. Electr. Eng. 2006, 32, 312–321. [Google Scholar] [CrossRef]
- Djurdjanovic, D.; Widmalm, S.E.; Williams, W.J.; Koh, C.K.; Yang, K.P. Computerized classification of temporomandibular joint sounds. IEEE Trans. Biomed. Eng. 2000, 47, 977–984. [Google Scholar] [CrossRef]
- Ghodsi, M.; Hassani, H.; Sanei, S.; Hicks, Y. The use of noise information for detection of temporomandibular disorder. Biomed. Signal Process. Control 2009, 4, 79–85. [Google Scholar] [CrossRef]
- Kaymak, D.; Karakis, D.; Dogan, A. Evolutionary spectral analysis of temporomandibular joint sounds before and after anterior repositioning splint therapy in patients with internal derangement. Int. J. Prosthodont. 2019, 32, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.P.; Djurdjanovic, D.; Koh, K.H.; Williams, W.; Widmalm, S. Automatic classification of the temporomandibular joint sounds using scale and time-shift invariant representation of their time-frequency distributions. In Proceedings of the IEEE-SP International Symposium on Time-Frequency and Time-Scale Analysis (Cat. No.98TH8380), Pittsburgh, PA, USA, 9 October 1998; pp. 265–268. [Google Scholar]
- Al Turkestani, N.; Bianchi, J.; Deleat-Besson, R.; Le, C.; Tengfei, L.; Prieto, J.C.; Gurgel, M.; Ruellas, A.C.; Massaro, C.; Aliaga Del Castillo, A.; et al. Clinical decision support systems in orthodontics: A narrative review of data science approaches. Orthod. Craniofacial Res. 2021, 24, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Machoy, M.E.; Szyszka-Sommerfeld, L.; Vegh, A.; Gedrange, T.; Woźniak, K. The ways of using machine learning in dentistry. Adv. Clin. Exp. Med. 2020, 29, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Waked, J.P.; Canuto, M.P.d.A.; Gueiros, M.C.S.; Aroucha, J.M.C.; Farias, C.G.; Caldas, A.D.F., Jr. Model for predicting temporomandibular dysfunction: Use of classification tree analysis. Braz. Dent. J. 2020, 31, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Mago, V.K.; Bhatia, N.; Bhatia, A.; Mago, A. Clinical decision support system for dental treatment. J. Comput. Sci. 2012, 3, 254–261. [Google Scholar] [CrossRef]
- Polášková, A.; Feberová, J.; Dostálová, T.; Kříž, P.; Seydlová, M. Clinical decision support system in dental implantology. MEFANET J. 2013, 1, 11–14. [Google Scholar]
- Mendonça, E.A. Clinical decision support systems: Perspectives in dentistry. J. Dent. Educ. 2004, 68, 589–597. [Google Scholar] [CrossRef]
- Farook, T.H.; Jamayet, N.B.; Abdullah, J.Y.; Alam, M.K. Machine learning and intelligent diagnostics in dental and orofacial pain management: A systematic review. Pain Res. Manag. 2021, 2021, 6659133. [Google Scholar] [CrossRef]
- Bianchi, J.; Ruellas, A.; Prieto, J.C.; Li, T.; Soroushmehr, R.; Najarian, K.; Gryak, J.; Deleat-Besson, R.; Le, C.; Yatabe, M.; et al. Decision Support Systems in Temporomandibular Joint Osteoarthritis: A review of Data Science and Artificial Intelligence Applications. Semin. Orthod. 2021, 27, 78–86. [Google Scholar] [CrossRef]
- Chen, Y.W.; Stanley, K.; Att, W. Artificial intelligence in dentistry: Current applications and future perspectives. Quintessence Int. 2020, 51, 248–257. [Google Scholar] [PubMed]
- Shan, T.; Tay, F.; Gu, L. Application of artificial intelligence in dentistry. J. Dent. Res. 2021, 100, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Almăsan, O.; Leucuta, D.C.; Hedesiu, M.; Muresanu, S.; Popa, S.L. Temporomandibular Joint Osteoarthritis Diagnosis Employing Artificial Intelligence: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Johannsmeier, L.; Eich, L.; Griga, L.; Haddadin, S. Dentronics: Towards robotics and artificial intelligence in dentistry. Dent. Mater. 2020, 36, 765–778. [Google Scholar] [CrossRef]
- Corbella, S.; Srinivas, S.; Cabitza, F. Applications of deep learning in dentistry. Oral Surg. Oral Med. Oral Radiol. 2021, 132, 225–238. [Google Scholar] [CrossRef]
- Brickley, M.; Shepherd, J.; Armstrong, R. Neural networks: A new technique for development of decision support systems in dentistry. J. Dent. 1998, 26, 305–309. [Google Scholar] [CrossRef]
- Jha, N.; Lee, K.S.; Kim, Y.J. Diagnosis of temporomandibular disorders using artificial intelligence technologies: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272715. [Google Scholar] [CrossRef]
- Farook, T.H.; Dudley, J. Automation and deep (machine) learning in temporomandibular joint disorder radiomics: A systematic review. J. Oral Rehabil. 2023, 50, 501–521. [Google Scholar] [CrossRef]
- Chollet, F. Xception: Deep learning with depthwise separable convolutions. In Proceedings of the CVPR, Online, 4 August 2017; pp. 1251–1258. [Google Scholar]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the inception architecture for computer vision. In Proceedings of the CVPR, Online, 5 July 2016; pp. 2818–2826. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Volume 30. [Google Scholar]
- Chlap, P.; Min, H.; Vandenberg, N.; Dowling, J.; Holloway, L.; Haworth, A. A review of medical image data augmentation techniques for deep learning applications. J. Med. Imaging Radiat. Oncol. 2021, 65, 545–563. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Grochowski, M. Data augmentation for improving deep learning in image classification problem. In Proceedings of the 2018 International Interdisciplinary PhD Workshop (IIPhDW), Swinoujscie, Poland, 9–12 May 2018; pp. 117–122. [Google Scholar]

| Segmentation | JIA | TMJ OA | TMD | Decision Support Systems | Review | Sound |
|---|---|---|---|---|---|---|
| [22] | [37] | [39] | [49] | [74] | [80] | [69] |
| [23] | [36] | [40] | [50] | [75] | [81] | [70] |
| [26] | [35] | [41] | [51] | [77] | [82] | [71] |
| [27] | [42] | [52] | [78] | [83] | [72] | |
| [28] | [43] | [53] | [79] | [86] | [73] | |
| [29] | [44] | [62] | [76] | [84] | ||
| [24] | [45] | [54] | [85] | |||
| [25] | [46] | [55] | [87] | |||
| [30] | [47] | [56] | [88] | |||
| [31] | [48] | [57] | [89] | |||
| [32] | [58] | |||||
| [59] | ||||||
| [60] | ||||||
| [61] | ||||||
| [10] | ||||||
| [63] | ||||||
| [64] | ||||||
| [65] | ||||||
| [66] | ||||||
| [67] | ||||||
| [68] |
| Author | Data Set | Method | Outcome | Category |
|---|---|---|---|---|
| [22] | 217 MRIs (106 from the 10 patients (between 19 and 39 years) and 111 from the 10 control subjects (between 18 and 41 years)). | 3DiscNet, U-Net, Seg-Net Basic. No preprocessing, no augmentation. For 3DiscNet, ADAM optimizer, 1.0 × 10 learning rate. For all algorithms, 2000 epoch. | For normal, 3DiscNet had (0.76 ± 0.08) dice, (0.73 ± 0.12) sensitivity, and (0.81 ± 0.11) PPV For displacement, 3DiscNet had (0.70 ± 0.17) dice, (0.72 ± 0.19) sensitivity. SegNet-Basic had (0.76 ± 0.16) PPV | Seg. |
| [23] | CBCT scans with small FOV and large FOV. %20 for test, 10-fold cross validation. | U-Net, RUNET (UNET with residual connections similar to RESNET). Contrast adjustment, Canny edge detection, and contour techniques. No augmentation, 60 epochs, batch size of 8, and learning rate. | Average 0.95 Dice coefficient | Seg. |
| [26] | 187 3D CBCTs scans of 95 patients. 94 left condyles, 93 right condyles | Denoising, 3D active contour and morphological operations. | A Dice coefficient of 0.9461 accompanied by a standard deviation of 0.0888 | Seg. |
| [27] | CBCT images of 20 adolescent subjects. | Marker-based watershed transform. | The Dice coefficient was 0.97 ± 0.01 | Seg. |
| [28] | 1200 MRIs (600 TMJs of 357 patients). 1000 MRIs from Hospital A (800 of them for training, 200 of them for internal validity test). 200 MRIs from Hospital B (All of them were used for external validaiton). | U-Net (from a Neural Network Console) No preprocessing, no augmentation. 300 epochs, 0.001 initial learning rate, and ADAM solver. | The evaluation of effectiveness utilizing data from Hospital B revealed reduced sensitivity compared with Hospital A, with both achieving accuracy rates exceeding 80%. Precision was diminished in utilizing Hospital A’s test data for ascertaining the anterior disc displacement position. In terms of classifying intra-articular TMD, a greater ratio of accurately categorized TMJs was observed with the utilization of images from Hospital A, surpassing those obtained from Hospital B. | Seg. |
| [29] | 160 training MRIs of 4 persons (80 positive and 80 negative). 10-fold cross validation | 19 different approaches (Viola-Jones, Auto Color Correlogram measure, CEDD, Color Layout, Edge histogram, FCTH, Fuzzy Colour Histogram, Gabor, General Color Layout, IPEG Coefficient Histogram, Simple Color Histogram, Tamura, Scalable Color, SVM comb-radial kernel, SVM comb-dot kernel, SVM comb -linear kernel, k-NN (k = 1, Canberra measure), Decision trees, SVM comb. -Gaussian kernel, SVM comb.-Neural network, SVMcomb.-Anova). No augmentation | The highest accuracy was attained by employing a Gaussian kernel-based SVM method (accuracy: 98.16 ± 2.81%). | Seg. |
| [30] | A total of 206 CT scans with reduced radiation dosage were employed. Of these, 123 3D images were utilized for training, 42 for validation, and 41 for the test dataset. Subsequently, the images were divided into individual 2D slices, resulting in 10,313 2D images for train, 3502 for val., and rest 3351 for the test. | U-Net+tracking-based algorithm. Normalization, horizontal flip and random rotation were applied. ADAM algorithm, 0.01 initial learning rate, discard rate is 0.5 for dropout layer, and model was obtained after 43 iteration. | = 0.92 ± 0.03 (condyles) and 0.90 ± 0.04 (glenoid fossae), and = 0.20 ± 0.19 mm (condyles) and 0.19 ± 0.08 mm (glenoid fossae). | Seg. |
| [31] | 12,800
CBCTs from 25 subjects, 18 subjects (9216 slices) for the training, 5 subjects (2560 slices) for the validation, 2 subjects (1024 slices) for the test set. | U-Net-based model. Adam optimizer with a fixed momentum (0.9, 0.999), weight decay of , a batch size of 0.1, learning rate of . 50 experiments using Monte Carlo cross validation with 30 epochs. | The IoU and Hausdorff distance accuracy were 0.870 and 0.928 for marrow bone and 0.734 and 0.247 for cortical bone, respectively. | Seg. |
| [32] | 49,094 CBCTs of 102 patients (aged 18–90 years). 6:2:2 ratio for train:validation:test. | U-Net, SegNet. For 2D network, 600 epochs, ADAM optimizer, learning rate and decay (0.01, 0.005) for SegNet, and (0.0001, 5 × 10) and a momentum of 0.9 for U-Net. For 3D network, 100 epochs, ADAM optimizer, learning rate 5 × 10(decay factor 5), batch size of 8. No augmentation, preprocess was applied. | The accuracy achieved by the 2D U-Net utilizing neighboring images was 0.82. The 2D SegNet exhibited an accuracy of 0.96, while the 3D U-Net demonstrated the highest accuracy of 0.99. | Seg. |
| [24] | 130 manually segmented CBCTs for train 24 for validation, and 8 CBCTs for test. | 3D U-Net model. ADAM optimizer, decaying triangular cyclic learning rate, 5 × 10 the base learning rate, 2 × 10 the max learning rate, 2000 step size. Batch size of 8 for the first 3D U-Net, batch size of 4 for the other two 3D U-Nets. Batch normalization with a momentum of 0.9, a dropout rate of 0.2. | The condyles and glenoid fossa achieved IoU scores of 0.955 and 0.935, respectively. | Seg. |
| [25] | Patient’s frontal face photograph | AI-based method | - | Seg. |
| [35] | EMG data, 23 healthy participants and 9 TMD patients (18 males, age range 5–17 years, mean 12.4 years) | Multivariate data-driven approach based on General Linear Model (supervised ML). Bootstrap (1000 iterations) was applied. No augmentation, no preprocessing | AUC of 0.71 | JIA |
| [37] | Immune signature 85 patients diagnosed with JIA and 43 controls matched for age. 10-fold cross validation | Random forest, ANN, SVM Hyperparameters for the algorithms were determined by experiment. No preprocessing, no augmentation. | A ∼90% accuracy was achieved in distinguishing patients with JIA from healthy controls. | JIA |
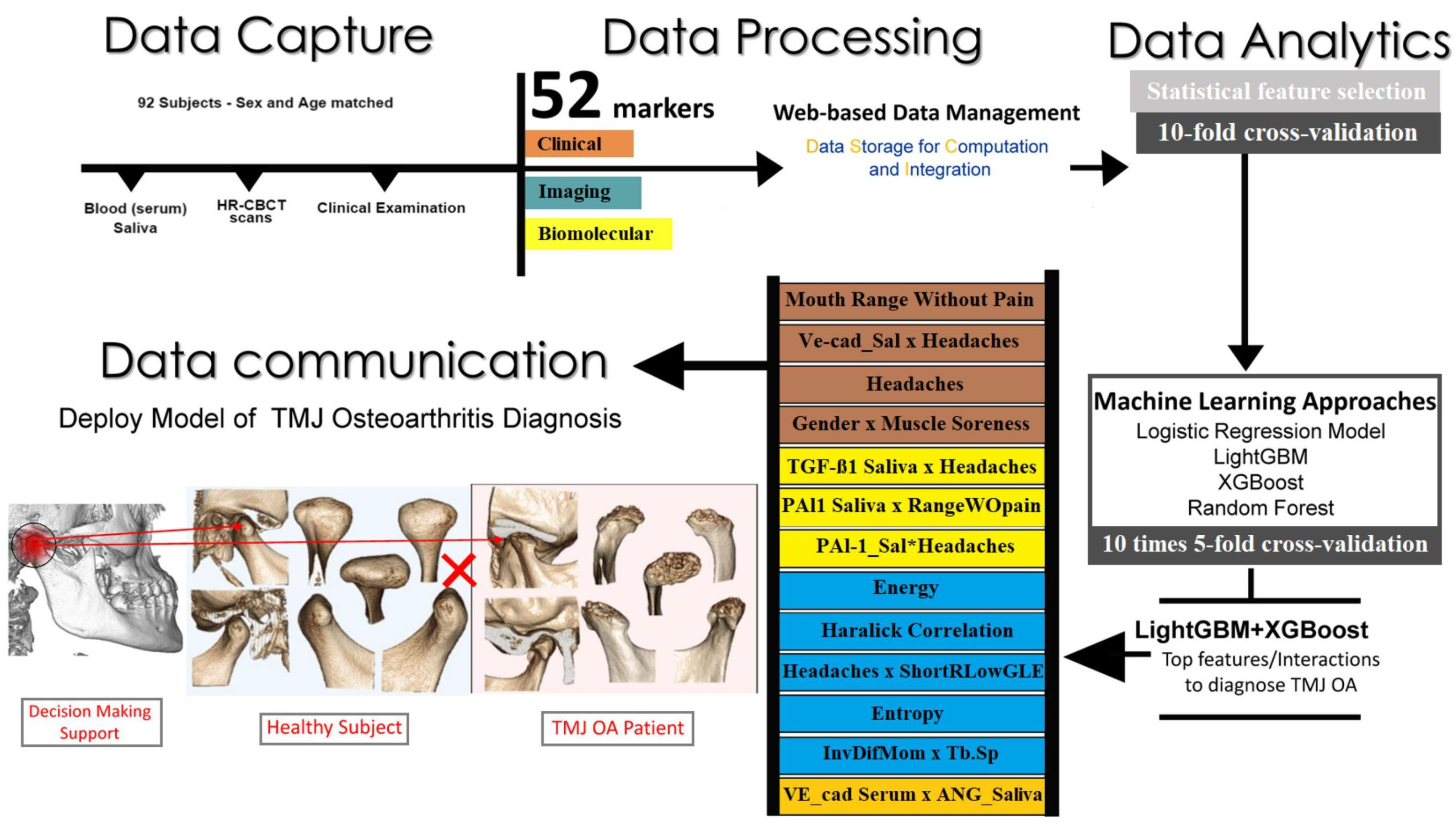
| [39] | CBCT 92 patients, 46 TMJ OA and 46 control subjects matched for age and gender 5-fold cross (10 times) validation | Logistic regression, random forest, LightGBM, XGBoost. No augmentation | The XGBoost and LightGBM ensemble model attained an accuracy of 0.823, an AUC of 0.870, and an F1-score of 0.823 for the diagnosis of TMJ OA condition. | TMJ OA |
| [40] | 314 patients, 3514 sagital CBCTs for train 300 images in total (2 sets) for test | a Single-Shot Detector (SSD) model No augmentation, no preprocessing | For the two test sets, the mean accuracy, precision, recall, and F1 score were 0.86, 0.85, 0.84, and 0.84. | TMJ OA |
| [41] | 1189 OPG, 2378 joints, 800 are normal, 779 are indeterminate, and 799 are OA (based on the CBCT) | Inception ResNet V2 Data augmentation was done by image rotation, image shift, brightness, and contrast, no preprocessing. 700 epochs, learnin rate of 1.0 × 10, ADAM optimizer | Accuracy: 0.78, sensitivity: 0.73, and specificity: 0.82 (When the indeterminate OA was removed). | TMJ OA |
| [42] | A collective sum of 858 panoramic images of the TMJ were included, comprising 395 images classified as normal and 463 images categorized as TMJ-OA. These images were obtained from a cohort of 518 individuals. Ratio of train, validation, and test was (6:2:2). | Resnet152 and EfficientNetB7 learning rate of , 1000 epochs, ADAM optimizer, and a weight decay of . Augmentation techniques: Horizontal flip, color jitter, random affine, random rotation. ROI preprocessing. | The classification accuracies of ResNet152 and EfficientNetB7 were 0.87 and 0.88, respectively. | TMJ OA |
| [43] | 17 TMJOA patient, 17 control subjects 259 condyles were used for training, 34 for test. | a NN, No augmentation. | The accuracy of the NN’s staging of TMJOA in comparison to the repeated clinicians’ consensus was found to be 73.5% and 91.2%, respectively. | TMJ OA |
| [44] | 293 CBCTs in total (259 training set and 34 testing set). In training, 105 control subjects and 154 patients of TMJ OA. | The shape variation analyzer (implemented as a deep neural network) utilized a NN architecture consisting of 4 hidden layers with neuron counts of (4096, 2048, 1024, 512), along with a dropout layer with a dropout probability of 0.5. The network also incorporated a softmax layer with 7 output units. The learning rate was configured as , and the training process spanned 100 epochs, employing a batch size of 32. Augmentation was applied, no preprocessing | They achieved an exact classification accuracy of 47%. However, if they allowed for an error of , the accuracy increased to 91%. | TMJ OA |
| [45] | 92 subjects, 46 TMJ OA patients, and 46 healthy controls 5 fold cross valiation | TMJOAI (a diagnostic tool) has three parts: The feature preparation, selection and model evaluation. | The optimal performance was attained by averaging the predictions of the XGBoost and LightGBM models. Furthermore, the incorporation of an additional 32 markers from the mandibular fossa of the joint resulted in an enhancement of the prediction performance of the AUC, increasing it from 0.83 to 0.88. | TMJ OA |
| [46] | Panoromic images, 1292 patients For condyle detection (800 train, 167 test) For condyle discrimination (2066 train, 518 test) For condyle detection (1154 train, 778 test) | R-CNN for learning, VGG16 for condyle discrimination Duplicating and rotating. No preprocessing | The R-CNN achieved an average precision of 99.4% (right side) and 100% (left side) for condyle detection at intersection over union (IoU) > 0.5. As for the TMJ OA classification algorithm using a CNN, its sensitivity, specificity, and accuracy were 0.54, 0.94, and 0.84, respectively. | TMJ OA |
| [47] | CBCT The training dataset included 259 condyles, with 105 condyles sourced from control subjects and 154 from TMJ OA patients. For testing purposes, 34 condyles (17 right and 17 left) from a total of 17 patients were used. | A classifier based on a deep neural network for 3D condylar morphology (referred to as SVA), along with a versatile web-based system designed for data storage, computation, and integration (referred to as DSCI). 1 hidden layer, 2001 iterations, 50 epochs | The SVA classifier achieved a close agreement of 91% with the clinician consensus. | TMJ OA |
| [48] | A total of 2000 sagittal sections were extracted from CBCT DICOM images of 290 patients. These sections consisted of 500 images each for the categories of healthy, erosion, osteophyte, and flattening. | YOLOv5, 500 epoch No augmentation, no preprocessing | The model’s sensitivity, precision, and F1 scores for TMJ OA classification were 1, 0.7678, and 0.8686, respectively, with an accuracy value of 0.7678. The classification model’s prediction values were 88% for healthy joints, 70% for flattened joints, 95% for joints with erosion, and 86% for joints with osteophytes. As for the YOLOv5 model for TMJ segmentation, its sensitivity, precision, and F1 score are 1, 0.9953, and 0.9976, respectively, while the AUC value is 0.9723. | TMJ OA |
| [10] | Vibration and sEMG signals, a measurement system was developed, and sample outcomes from the rehabilitation process of a 27-year-old female patient with temporomandibular joint articular dysfunction are showcased. 15 patients(ten women and five men) for test. | k-NN k = 1 | The k-NN algorithm produced results ranging from 62.5% to 82.1%. In the opening with protruding exercise, effectiveness of 71.1% and 75.0% was achieved using vibration and EMG, respectively, while the combined classification achieved 79.1% effectiveness. After data fusion, the recognition efficiency for fast opening increased from 73.6% to 85.3%. However, the results for the remaining exercises, slow opening, and slow protruding, were not as promising. | TMD |
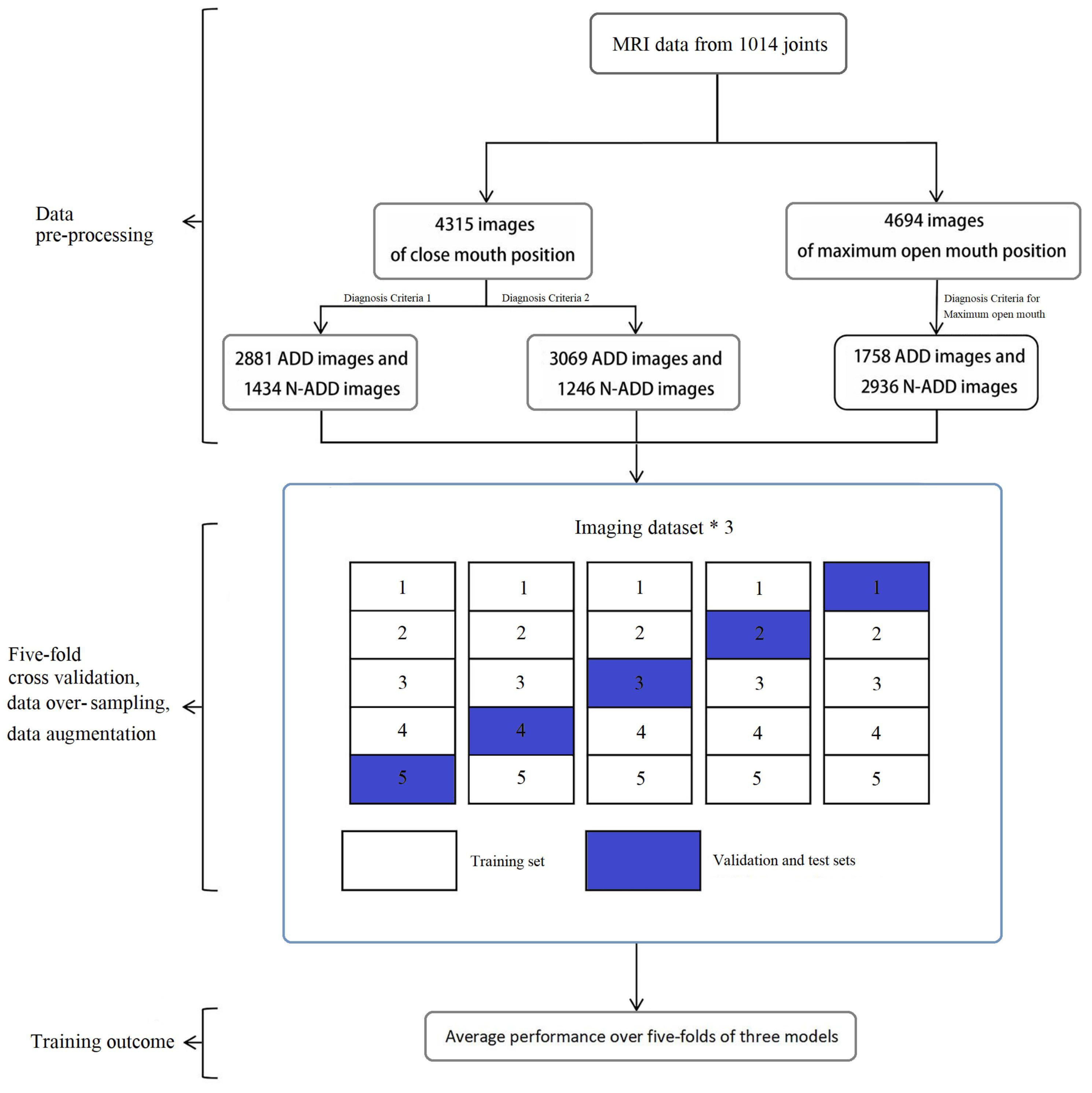
| [49] | 507 patients (426 female, 81 male) 9009 MRI %80 train, %10 validation, %10 test 5-fold cross validation | ResNet34, learning rate 0.001, 20 rounds of training Horizontal flipping, 10% zooming, 10% shifting | Theopen mouth position model exhibited accuracy and AUC of 0.970 (±0.007) and 0.990 (±0.005), respectively. The closed mouth position models, diagnostic Criteria 1 demonstrated higher accuracy and AUC with values of 0.863 (±0.008) and 0.922 (±0.009), respectively, which were significantly superior to those of diagnostic Criteria 2 (0.839 ± 0.013, p = 0.009; AUC 0.885 ± 0.018, p = 0.003). | TMD |
| [50] | MRI, 214 TMJs from 107 patients %80 train, %20 test | Logistic regression, random forest, decision tree, k-NN, XGBoost, SVM | The k-NN and RF classifiers were identified as the most effective machine learning models for predicting TMJ pathologies. | TMD |
| [51] | IT, 78 patients (37 control and 41 TMD) %70 training, %30 test cross validation used | k-NN, SVM and MLP k = 5 (for k-NN), The 2. layer has 8 neurons, the 3. and 4. layers have 6 neurons, the 5. has 4 (for MLP) Hopkins’s statistic, Shapiro–Wilk, ANOVA and Tukey tests were used, the significance level was 5% (p < 0.05). | Statistically significant differences were observed in the accuracy, precision, and sensitivity values between the semantic and radiomic-semantic associations compared with the radiomic features (p = 0.008, p = 0.016, and p = 0.013, respectively). | TMD |
| [52] | MRI, 299 joints from 289 patients (168 non perforated, 131 perforated) %80 train, %20 validation | Random forest, MLP No augmentation, no preprocessing | The MLP demonstrated the highest performance with an AUC of 0.940, followed by random forest with an AUC of 0.918, and disc shape alone with an AUC of 0.791. | TMD |
| [53] | 4744 participants’ 37 independent variables of TMDs (demographic factors, working conditions, socioeconomic status, and health-related determinants). %75 train, %25 test | Decision trees, logistic regression, random forest, naïve Bayes, SVM and an ANN The decision tree employed GINI, the random forest consisted of 1000 trees, radial basis function was utilized as the kernel for the SVM, and the ANN featured 2 hidden layers (10-10) with quasi-Newton (lbfgs) as the weight optimization method. | It has been observed that the factors selected by the Random Forest algorithm are similar to those chosen by the doctor. | TMD |
| [54] | Sound data 48 different individuals (21 healthy and 27 TMJ patients). 76 data (48 healthy, 28 TMD) For ANN, 54 training, 11 validations, 11 test For DL, the data was divided by 3 and the number was tripled. | Signal processing, ANN, DL For ANN, 10 hidden layers, sigmoid activation function For DL, each intermediate layer consists of a Convolution2DLayer, a ReLU-Layer, and a Maxpooling2DLayer. After the intermediate layers, there is a fully connected layer, a softmax layer, and a classification output layer. | The average success rate of the frequency-based feature extraction method using ANN classification is 78.6%. For the statistical-based feature extraction method, the average success rate with ANN classification is 89%. Additionally, the average success rate of the deep learning-based method is 94.5%. | TMD |
| [55] | The dataset comprises information from 2300 patients with TMJ syndrome, presenting symptoms such as locking of the jaw or limited movement, painful clicking, popping, or grating sounds, alterations in the fitting of upper and lower teeth, radiating pain in the face, jaw or neck, aching pain in and around the ear, jaw muscle stiffness, and underlying factors like genetic predisposition, diabetes, parathyroid issues, and vitamin D deficiency. | ANN 17 epoch, 20 neurons in hidden layer (1 hidden laye) | 98.9% of the correlation coefficient in terms of regression curve for the TMJ disorder prediction | TMD |
| [56] | 200 patient (patients between 20 and 50 years) Demographic information includes variables such as age, gender, and education level. Parafunctional aspects encompass bruxism and clenching behaviors, as well as habits such as nail biting and gum chewing. Occlusal factors involve dental relationships, lateral occlusion scheme, horizontal disparities between centric occlusion and maximal intercuspation (MI), as well as discrepancies between MI and the mandibular resting position. | The analysis of TMD was conducted using Chi-square tests and independent sample t-test at a significance level of = 0.05. Additionally, binomial logistic regression analysis was carried out, taking into account potential confounding variables. | The prevalence of TMD was found to be 58.9%. Among the parafunctional and occlusal factors examined, only bruxism demonstrated a statistically significant difference between the TMD and non-TMD groups ( 0.05). However, other parafunctional and occlusal factors did not exhibit significant influence on the occurrence of TMD. | TMD |
| [58] | MRI 295 cases, and 590 right and left sides of TMJs (54 male; 241 female) 10-fold cross-validation | The accuracy of the Bayesian Belief Network (BBN) was evaluated by comparing it with 11 different algorithms, (including “necessary path Rebane–Pearl poly tree, condition, Chow–Liu tree, path condition, greedy search-and-score with Bayesian information criterion, tree augmented naive Bayes model, minimum description length, maximum log likelihood, Akaike information criterion, K2, and C4.5), a multiple regression analysis and an ANN” using resubstitution validation and 10-fold cross-validation. | The BBN path condition algorithm using resubstitution validation and 10-fold cross validation was 0.99% accurate. | TMD |
| [59] | Survey (9922 invited participants aged 18 years or older) The main dataset utilized in the study consisted of 530 individuals (information of clinical oral examination, severe headaches). From the main dataset, they created dataset 1 (n = 345) and dataset 2 (n = 464) | Bayesian logistic regression models | The presence of migraine at follow-up was not associated with either of the baseline TMD-related pain variables (posterior effect estimates: −0.12, 95% credible interval [CI] −0.49– 0.24, and 0.11, 95% CI −0.38–0.59, for mTMD and jTMD, respectively). However, mTMD at baseline was associated with the presence of tension-type headache (TTH) at follow-up (posterior effect estimate 0.36, 95% CI 0.02–0.69), whereas jTMD at baseline was not significantly associated with TTH at follow-up (posterior effect estimate −0.32, 95% CI −0.94–0.25). | TMD |
| [60] | MRI images of 2520 TMJs (861 men, 399 women; average age 37.33 ± 18.83 years). 2051 for train, 468 for test, %20 of train for validation | VGG16 (fine-tuning, from scratch and freeze were evaluated separately) 3 different augmentations (randomly flip in vertical and affine transform image. Contrast changes: randomly changed contrast values and applied histogram equalization. Brightness changes: randomly changed brightness of image). Learning rate of 1 × 10 for the fine-tuning and from-scratch (their epochs were 15 and 30 respectively), the freeze model utilized 5 × 10 with 150 epochs. All 3 had the ADAM optimizer. | The fine-tuning model achieved a prediction performance with an AUC of 0.8775 and an accuracy of 0.83%. The AU C values for the from-scratch and freeze models were 0.8269 and 0.5858, respectively. | TMD |
| [61] | MRI, clinical examinations for pain and mouth opening limitation. 96 TMJS (48 patients, 39 female and 9 male) | Multiple logistic regression | The data analysis revealed statistically significant correlations between pain and the degree of disc displacement on MR imaging ( 0.05). The likelihood of experiencing pain in moderate to significant cases was 9.69 times higher compared to normal cases. | TMD |
| [62] | sEMG signals from the TMJ muscles during jaw movements 42 volunteers participated | Self-Organizing Map (SOM) combined with cross-correlation analysis | Intra cross-correlation coefficient (CC) provided information about muscle activity similarity within and between patients, indicating TMJ function stability. SOM analysis helps interpret muscle activation and assess TMJ health, while cross-correlation identifies similarities in sEMG data between patients, potentially aiding clinical TMD diagnosis and treatment assessment | TMD |
| [64] | 451 cases (320 females and 131 males) from patient notes between 8 and 93 years old, average age is 43.4 years. 141 variables (3 continuous, 138 dichotomous: age, pain severity and max mouth opening in millimeters) 5-fold cross validation | High Frequency Value (HFV) multilabel, random forest multilabel, random forest, Logit, SVM, and k-NN HFV method used THF = 0:67, random forest had 100 trees, SVM used a slack variable cost C = 1 and Logit used a SAG solver. | The accuracy varies between 75.60% and 96.8%, recall varies between 46.27% and 98.21%, precision varies between 52.40% and 97.38% and the F-1 score ranges from 0.56 to 0.96. | TMD |
| [63] | Medical records of 290 patients (61 male and 229 female; average age, 31.2 ± 15.8) with genuine TMD and 29 patients (14 male and 15 female; average age, 39.5 ± 23.2) with TMD-mimicking conditions. 10-fold cross validation | natural language processing and recursive partitioning. | The model exhibited a predictive performance of 96.6%, achieving a sensitivity of 69.0% and a specificity of 99.3% in anticipating TMD-mimicking conditions. | TMD |
| [67] | MRI of 52 patients with TMJ Disc displacement (TMJDD) and 32 healthy controls. 300 images (105 normal, and 195 patient) 5-fold cross validation 100 MRI used for U-Net | U-Net for joint cavity detection and InceptionResNetV2, InceptionV3, DenseNet169, and VGG16 for classification. | Among the models evaluated, InceptionV3 and DenseNet169 demonstrated the best performance. In the case of InceptionV3, the recall, precision, accuracy, and F1 score were 1, 0.81, 0.85, and 0.9, respectively. Similarly, for DenseNet169, the corresponding values were 0.92, 0.86, 0.85, and 0.89. | TMD |
| [65] | Mouth opening and closing videos of 91 patients (17 males and 74 females; mean age, 38.42 years old) The graph paths were: straight, sideways-skewed, and limited-straight line graphs | The automated model encompasses the following components: (I) the creation of an automated system for detecting landmarks on both the upper and lower lips within the video footage; and (II) the generation of a visual representation of the tracing graph and the subsequent automatic computation of both the graph’s height (indicating mouth opening length) and width (representing sideways values). | Subjects with a normal disc position exhibited mainly straight line graphs in 85.72% of cases. The presence of sideways skewed or limited -straight line graphs was identified in 85.0% of cases within the anterior disc displacement with reduction group and in 89.47% of cases within the anterior disc displacement without reduction group. This observation exhibited a statistically significant correlation of (2 = 38.113, 0.001) between the two groups. | TMD |
| [66] | Clinical cases | MLP consisted of an input layer with 18 neurons, followed by 5 hidden layers, and concluded with 1 output layer. Backpropagation training | The accuracy of the MLP outperformed that of the general dental clinicians, with a statistically significant difference (p = 0.0072). | TMD |
| [68] | 2576 MR images of 200 patients (4 classes) %80 for train, %20 for test and %20 of training for validation Contrast, flip and rotation augmentation methods were applied. | Performance of a CNN, fine-tuned Xception, ResNet-101, MobileNetV2, InceptionV3, DenseNet-121 and ConvNeXt, and transformers were evaluated. 100 epochs, binary cross-entropy loss function, ADAM optimizer and 1 × 10 learning rate. | It was determined that MobileNetV2 exhibited the best performance for the “closed mouth disc position” group, while Xception performed the best for the “open mouth disc position” group. In terms of “joint cavity effusion,” ResNet-101 showed superior results, and for the “mandibular condyle degeneration” group, MobileNetV2 demonstrated the highest performance. | TMD |
| [57] | Gender, economic class, age and marital status. 1000 patients (aged 15 to 70, 57% were over 30 years of age and 83% were women. 53% were not married) were divided into 3 class which were Class A (high social class), Classes B/C (middle class) and Classes D/E (very poor social class). | Pearson’s chi-square test for proportions, fisher’s exact test, nonparametric mann-whitney test and binary logistic regression analysis | No participants belonged to Class A, 72% belonged to Classes B/C and 28% belonged to Classes D/E | TMD |
| [69] | TMJ sound data 11 orthodontic patients (22 joints) aged between 9 and 13 years old with lateral cross-bite, as well as 21 orthodontic patients (42 joints) within the age range of 9 to 13 years old with Class II Division I type malocclusions. | Discrete evolutionary transform, MLP 16 input neurons, (corresponding to each joint moment), a hidden layer, and an output layer containing 4 neurons (TMJ signal class). Back-propagation learning, 5000 iterations. | The classification of TMJ sounds based on recorded signals agreed to some extent with physician examination methods (palpation and auscultation). The accelerometer recordings provided additional detail in crepitation signals not detected by physical examination. In patients with lateral cross-bite, 6 out of 11 showed a decrease in TMJ vibrations after treatment, while 2 patients had increased vibrations and 5 showed no change. In Class II Division I patients, 6 out of 21 showed a decrease in vibrations, 17 had increased vibrations, and 5 showed no change after treatment. More patients demonstrated increased TMJ vibrations in the Class II Division I group, and decreased vibrations in the lateral cross-bite group. | Sound |
| [70] | Soundscoming from the TMJs during opening/closing motion | Nearest linear combination (NLC), Nearest neighbor (NN), and Nearest constrained linear combination (NCLC) were used on either only time-shift, or both time-shift and scale-invariant representations of the signal Reduced Interference distribution (RID)’s | The automated analysis techniques yielded classification outcomes comparable to the previous manual sound classification. Excluding scale invariance significantly enhanced the performance of the classifier. Scale invariance was found to interfere with the frequency content of the signal | Sound |
| [71] | Set of simulated data was generated and tested by the algorithm. Then TMD data were used. 14 subjects (4 with TMD, 10 healthy) Each subject was captured performing four cycles of chewing motion using color video camera. 400 video frames were obtained per subject. | Singular spectrum analysis | It was interpreted that the SSA technique can be successfully used as a detection method, or at least for extraction of the main signal. | Sound |
| [72] | 26 patients (21 female, 5 males. Aged between 21 and 57.) with 44 TMJs (25 left, 9 right). Patients were used Anterior Repositioning Splint (ARS) for 6 weeks 3 classes (disc displacement (19), disc dislocation with reduction (4), acute disc dislocation without reduction (3)) | Evolutionary spectral analysis Parameters (sound type, amplitude, duration and energy) were evaluated | The results showed that 6-week ARS usage reduced amplitude and energy parameters of TMJ sounds | Sound |
| [73] | 35 type 1 (0 to 600 Hz), 31 type 2 (600 to 1200 Hz) and 38 type 3 (above 1200 Hz) TMJ clicks | The Nearest Neighbor (NN), Nearest Linear Combination (NLC) and Nearest Constrained Linear Combination (NCLC) | Linear combinations improved classification of the Reduced Interference Distributions (RID)’s of TMJ sound. | Sound |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozsari, S.; Güzel, M.S.; Yılmaz, D.; Kamburoğlu, K. A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases. Diagnostics 2023, 13, 2700. https://doi.org/10.3390/diagnostics13162700
Ozsari S, Güzel MS, Yılmaz D, Kamburoğlu K. A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases. Diagnostics. 2023; 13(16):2700. https://doi.org/10.3390/diagnostics13162700
Chicago/Turabian StyleOzsari, Sifa, Mehmet Serdar Güzel, Dilek Yılmaz, and Kıvanç Kamburoğlu. 2023. "A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases" Diagnostics 13, no. 16: 2700. https://doi.org/10.3390/diagnostics13162700
APA StyleOzsari, S., Güzel, M. S., Yılmaz, D., & Kamburoğlu, K. (2023). A Comprehensive Review of Artificial Intelligence Based Algorithms Regarding Temporomandibular Joint Related Diseases. Diagnostics, 13(16), 2700. https://doi.org/10.3390/diagnostics13162700







