Study on the Mechanism of the Pink Tooth Phenomenon Using Bovine Teeth: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Creation of the Experimental Model
2.2. Color Evaluation Method
2.3. Intra-Examiner and Inter-Examiner Reliability in Color Evaluation
2.4. Comparison of Color Tone Due to External Environmental Factors
2.5. Relationship with Carboxyhemoglobin and Hemoglobin Degradation Products
2.6. Relationship with Red Pigment-Producing Bacteria
3. Results
3.1. Establishing the Experimental Model
3.2. Color Evaluation Method and Evaluation of Intra- and Inter-Examiner Reliability
3.3. Color Change Due to External Environmental Factors
3.4. Relationship with Carboxyhemoglobin and Hemoglobin Degradation Products
3.5. Examination of the Relationship with Red Pigment-Producing Bacteria
3.6. Limitations in Research
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, B. The Anatomy, Physiology, and Diseases of the Teeth; Higley: London, UK, 1829; pp. 12–13. [Google Scholar]
- Borrmant, H.; Du Chesne, A.; Brinkmann, B. Medico-legal aspects of postmortem pink teeth. lnt. J. Leg. Med. 1994, 106, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Chissokushi toku ni keibuappaku niyoru chissokushi no shiga narabini shigashijisoshiki ni oyobosu eikyou no jikkenntekikennkyuu. Kokubyo Gakkai Zasshi 1941, 15, 248–270. (In Japanese) [Google Scholar]
- Soriano, E.P.; Carvalho, M.V.; Santos, F.B.; Mendoza, C.C.; Socorroe, M.D.; Campello, R.I. The post-mortem pink teeth phenomenon: A case report. Med. Oral. Patol. Oral. Cri. Bucal. 2009, 14, 337–339. [Google Scholar]
- Ortmann, C.; Chesne, A.D. A partially mummified corpse with pink teeth and pink nails. Int. J. Legal. Med. 1997, 111, 35–37. [Google Scholar] [CrossRef]
- Franco, A.; Mendes, S.D.S.C.; Picoli, F.F.; Rodrigues, L.G.; Silva, R.F. Forensic thanatology and the pink tooth phenomenon: From the lack of relation with the cause of death to a potential evidence of cadaveric decomposition in dental autopsies—Case series. Forensic Sci. Int. 2018, 291, 8–12. [Google Scholar] [CrossRef]
- Brites, A.N.; Rezende Machado, A.L.; Franco, A.; da Silva, R.H. Revisiting autopsies of death by mechanical asphyxia in the search for post-mortem pink teeth. J. Forensic Odontostomatol. 2020, 38, 34–38. [Google Scholar]
- Mitttal, P.; Karagwal, P.; Gupta, D. Pink teeth phenomenon and asphyxia: A reassessment and update. J. Forensic Res. 2021, 12, 1–10. [Google Scholar]
- Stavrianos, C.; Vasiliadis, L.; Papadopoulos, C.; Pantelidou, O.; Tolidis, K.; Dagkalis, P. The post-mortem pink teeth phenomenon. Res. J. Biol. Sci. Res. 2011, 6, 124–127. [Google Scholar]
- Campobasso, C.P.; Di Vella, G.; De Donno, A.; Santoro, V.; Favia, G.; Introna, F. Pink teeth in a series of bodies recovered from a single shipwreck. Am. J. Forensic Med. Pathol. 2006, 27, 313–316. [Google Scholar] [CrossRef]
- Takuur, R.; Ahmed, M.; Bajaj, N. Pink tooth: A speculative forensic phenomenon. Indian J. Forensic Med. Toxicol. 2013, 7, 254–287. [Google Scholar]
- Minegishi, S.; Saitoh, H.; Utsuno, H.; Ohta, J.; Namiki, S.; Toya, M.; Sumi, N.; Sakurada, K. Association of cadaveric factors with the degree and region of discoloration on pink teeth: An approach to serial cases. Appl. Sci. 2022, 12, 4242. [Google Scholar] [CrossRef]
- Sakurada, K. Effects on oral tissues of asphyxiation caused by cervical compression: The pink teeth phenomenon in Kato’s studies (1941). Leg. Med. 2023, 64, 102284. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Chissokushi toku ni keibuappaku niyoru chissokushi no shiga narabini shigashijisoshiki ni oyobosu eikyou no jikkenntekikennkyu (sono 2). Kokubyo Gakkai Zasshi 1941, 15, 327–334. (In Japanese) [Google Scholar]
- Kato, K. Kakusyu shintaitekijyoukyou niokeru kousatsu no shiga narabini shigashijisoshiki ni oyobosu eikyou no jikkenntekikennkyu. Kokubyo Gakkai Zasshi 1941, 15, 347–360. (In Japanese) [Google Scholar]
- Kato, K. Keibuappaku niyoru chissokushi no shiga narabini shigashijisoshiki ni oyobosu henka no hasseikijyo ni kansuru jikkenntekikennkyu. Kokubyo Gakkai Zasshi 1941, 15, 391–411. (In Japanese) [Google Scholar]
- Ikeda, N.; Harada, A.; Takahashi, H.; Suzuki, T. Experimental formation of pink teeth and their analysis. Jpn. J. Legal. Med. 1988, 42, 179–183. (In Japanese) [Google Scholar]
- Stern, A.W.; Clark, A.M.; Byrd, J.H.; Leser, K.M.; Russo, H. The pink teeth phenomenon in dogs and a cat. Forensic Sci. 2022, 2, 650–656. [Google Scholar] [CrossRef]
- Lopes, M.B.; Sinhoreti, M.A.C.; Gonini Júnior, A.; Consani, S.; McCabe, J.F. Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz. Dent. J. 2009, 20, 279–283. [Google Scholar] [CrossRef]
- Beeley, J.A.; Harvey, W. Pink teeth appearing as a post-mortem phenomenon. J. Forensic Sci. Soc. 1973, 13, 297–305. [Google Scholar] [CrossRef]
- Kato, Y. Shiga no pinkshi gensyou ni kannsuru houiakutekikennkyuu. Nihonnhoui Gaku Zasshi 1986, 40, 299–307. (In Japanese) [Google Scholar]
- Takano, M.; Sato, K.; Fujishiro, M.; Shinnmenn, N.; Umezawa, H.; Xiaopeng, L.; Kato, Y.; Tsutsumi, K.; Ito, H.; Komuro, T.; et al. Shiga no pinkushi tyakusyokugennsyou ni kannsuru kyapirari wo zougesaikannmoderu tosita zikkenntekikennkyuu. Syouwaikaishi 2009, 69, 387–394. (In Japanese) [Google Scholar]
- Sakuma, A.; Saitoh, H.; Ishii, N.; Iwase, H. The effects of racemization rate for age estimation of pink teeth. J. Forensic Sci. 2015, 60, 450–452. [Google Scholar] [CrossRef]
- Camps, F.E. Medical and scientific investigations in the Christie case. Postgrad. Med. J. 1954, 30, 382–383. [Google Scholar]
- Whittaker, D.K.; Thomas, V.C.; Thomas, R.I. Post-mortem pigmentation of teeth. Br. Dent. J. 1976, 140, 100–102. [Google Scholar] [CrossRef]
- Hubbard, R.; Rimington, C. The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens). Biochem. J. 1950, 46, 220–225. [Google Scholar] [CrossRef]
- Williams, R.P.; Green, J.A.; Rappoport, D.A. Studies on pigmentation of Serratia marcescens I. J. Bacteriol. 1956, 71, 115–120. [Google Scholar] [CrossRef]
- Lewis, S.M.; Corpe, W.A. Prodigiosin-producting bacteria from marine sources. Appl. Microbiol. 1964, 12, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kathumata, N.; Yata, A. Pinkushi no seiinn ni kannsuru hougakuteki kekyuu. Nihon Houiaku Zasshi 1984, 38, 527. [Google Scholar]
- Dumser, T.K.; Turkay, M. Postmortem changes of human bodies on the Bathyal Sea Floor-Two cases of aircraft accidents above the open sea. J. Forensic Sci. 2008, 53, 1049–1052. [Google Scholar] [CrossRef]
- Gowda, B.K.C.; Sivapathasundharam, B.; Chatterji, A.; Chatterji, B.L. Histological appearance of postmortem pink teeth: Report of two cases. J. Forensic Dent. Sci. 2015, 7, 168–170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittal, P.; Mittal, M.; Sharma, G. Pink teeth and the dead: A review with reports of two cases. J. India Acad. Forensic Med. 2016, 38, 487–489. [Google Scholar] [CrossRef]
- Wallman, J.F. Body farms. Forensic Sci. Med. Pathol. 2017, 13, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; de Oliveira, M.N.; Gomes-Lima, L.K.; Pereira-de-Oliveira, V.H.F.; Franco, R.P.A.V.; Blumenberg, C.; Silva, R.F.; da Silva, R.H.A.; Makeeva, I.; Santos-Filho, P.C.F.; et al. Case-specific characteristics of pink teeth in dental autopsies—A systematic review. J. Forensic Leg. Med. 2019, 68, 101869. [Google Scholar] [CrossRef]
- Hayakawa, T. Konpozittorezinn no misyorikennmazougesitu heno settyaku nikannsuru kennkyuu. Sikazairyou Kiki 1993, 12, 445–465. (In Japanese) [Google Scholar]
- Mizunuma, T. Zougeshitu no kagakusyuusyoku ya settyakusei no kannkei- 4-META/MMA-TBB kei rezinn no maesyorizougeshithu heno settyaku. Sikazairyou Kiki 1983, 2, 446–450. (In Japanese) [Google Scholar]
- Watanabe, K. Sumea- sou ga zougeshitu heno settyaku ni ataerueikyou sessakuzyoukenn to settyakutuyosa no kannkei. Sikazairyou Kiki 1994, 13, 2101–2118. (In Japanese) [Google Scholar]
- Matsuda, H.; Kubota, S.; Sato, H. Comparison of hair color using image analysis. Houkagakugizyutu 2008, 13, 151–166. (In Japanese) [Google Scholar] [CrossRef]
- Usumoto, Y.; Hikiji, W.; Sameshima, N.; Kubo, K.; Tsuji, A.; Ikeda, N. Estimation of postmortem interval based on the spectrophotometric analysis of postmortem lividity. Leg. Med. 2010, 12, 19–22. [Google Scholar] [CrossRef]
- Nath, A.; Patowary, A.J.; Ropmay, A.D.; Slong, D.; Pratim, K.P.; Rymbai, B.K.; Marbaniang, R.B.Y. A cross-sectional study of time science death from image analysis of corneal opacity. Cureus 2021, 13, 14975. [Google Scholar]
- Bohnert, M.; Weinmann, W.; Pollak, S. Spectrophotometric evaluation of postmortem lividity. Forensic Sci. Int. 1999, 99, 149–158. [Google Scholar] [CrossRef]
- Masuda, Y. Kyouzaiyou jisyoukotsu huunyu hyouhonn no sakusei. Rinnsyou Jika 1978, 5, 102–103. (In Japanese) [Google Scholar]
- Takatori, K.; Nagao, T. NEW Essennsyaru Houigaku, 6th ed.; Ishiyakusyuppannkabushikigaisya: Bunnkyoku, Japan, 2019; p. 267. [Google Scholar]
- Ishikawa, K.; Fujibayashi, S.; Kuni, Y.; Omichi, M. A sensitive method for estimating carboxy-hemoglobin and its applications. Sanngyouigaku 1972, 14, 123–129. (In Japanese) [Google Scholar]
- Commins, B.T.; Lawther, P.J. A sensitive method for the determination of carboxyhaemoglobin in a finger prick sample of blood. Br. J. Ind. Med. 1965, 22, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Oshida, T.; Yoshikawa, Y.; Kobayashi, Y.; Sakata, R.; Tanaka, K. Butaketueki no youketuteido to kyuukoudo hemusikiso tono kannkei. Nihonn Youton Gakkaishi 1988, 25, 113–118. (In Japanese) [Google Scholar] [CrossRef]
- Suzuki, Y.; Sakagisi, R. Birirubin no ziazo-kappurinngu-hannou de seisei suru tyukanntai no saiketugou ni motoduku birirubinn no seisei. Rinnsyoukagaku 1993, 22, 104–110. [Google Scholar]
- Hayashi, C.; Arisue, K.; Tanaka, F.; Kobayashi, Y. A new instrument for bilirubin measurement. Jpn. J. Clin. Chem. 1972, 1, 471–474. (In Japanese) [Google Scholar]
- Iwaya, A.; Nakagawa, S.; Iwakura, N.; Taneike, I.; Kurihara, M.; Kuwano, T.; Gondaira, F.; Endo, M.; Hatakeyama, K.; Yamamoto, T. Rapid and quantitative detection of blood Serratia marcescens by a real-time PCR assay: Its clinical application and evaluation in a mouse infection model. FEMS Microbiol. Lett. 2005, 248, 163–170. [Google Scholar] [CrossRef][Green Version]
- Silva-Junior, W.P.; Martins, A.S.; Xavier, P.C.N.; Appel, K.L.A.; Oliveira Junior, S.A.; Palhares, D.B. Etiological profile of early neonatal bacterial sepsis by multiplex qPCR. J. Infect. Dev. Ctries. 2016, 10, 1318–1324. [Google Scholar] [CrossRef]
- Zheng, J.; Huo, D.; Wen, H.; Shang, Q.; Sun, W.; Xu, Z. Corneal-Smart Phone: A novel method to intelligently estimate postortem interval. J. Forensic Sci. 2021, 66, 356–364. [Google Scholar] [CrossRef]
- Shigezane, J. Postmortem formation of carbon monoxide by bacteria. Jpn. J. Leg. Med. 1986, 40, 111–118. (In Japanese) [Google Scholar]
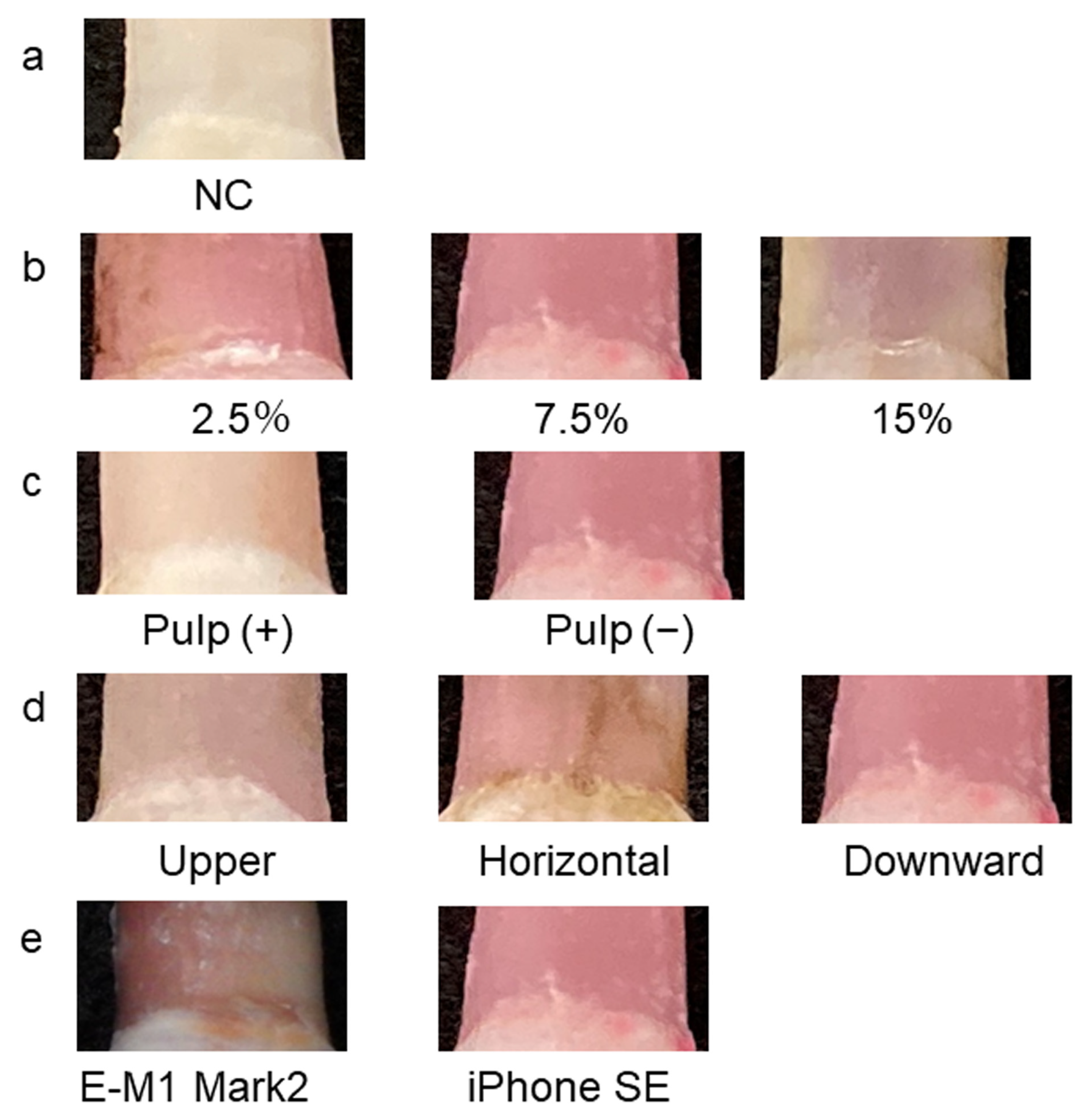
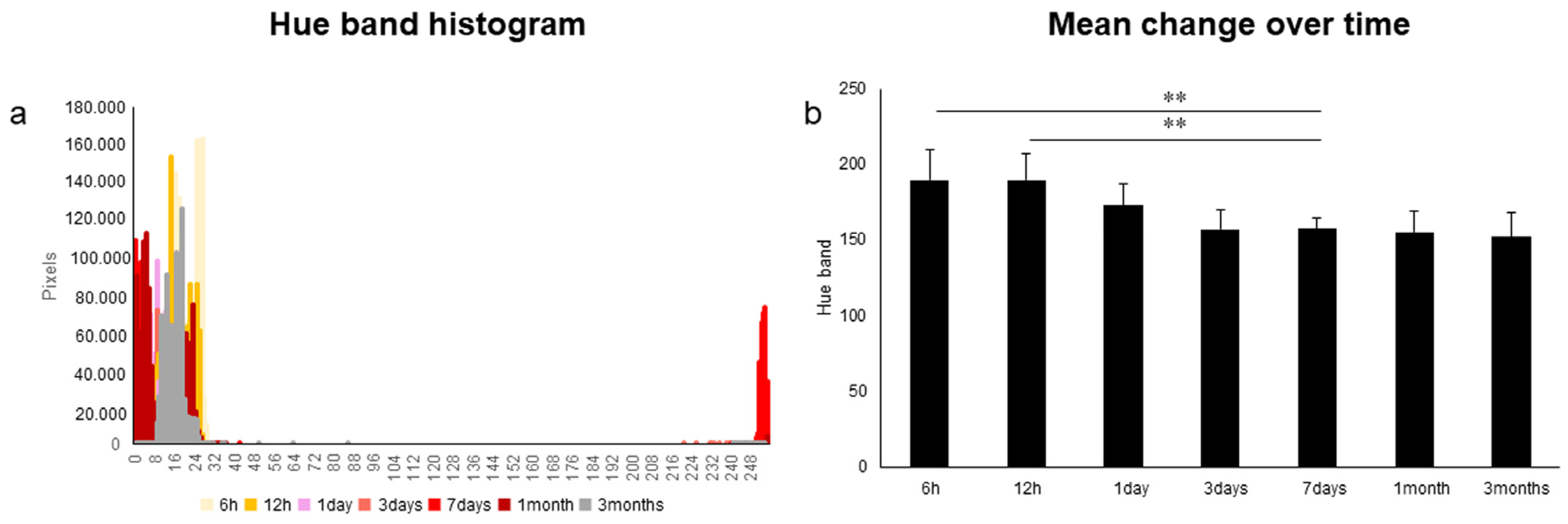
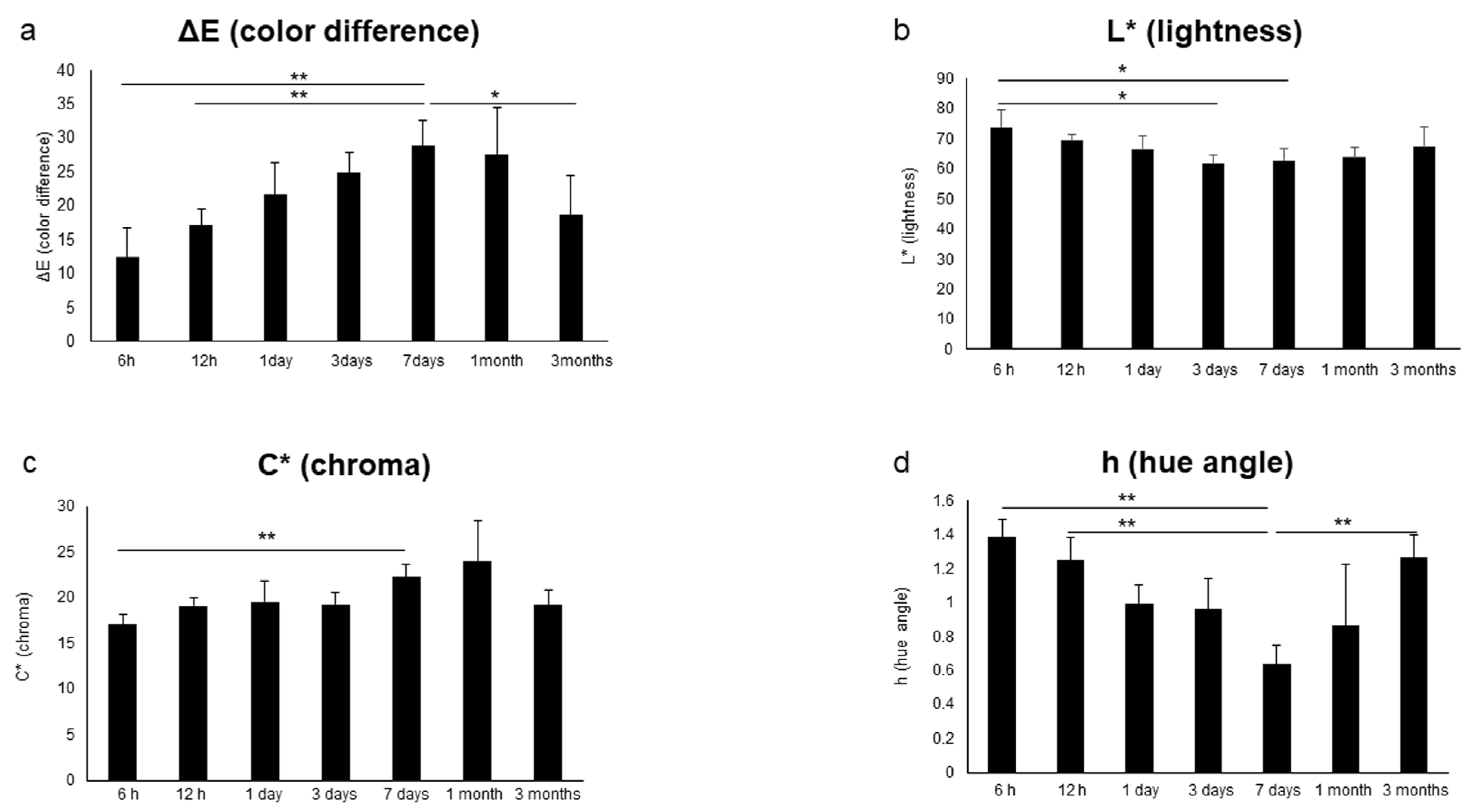
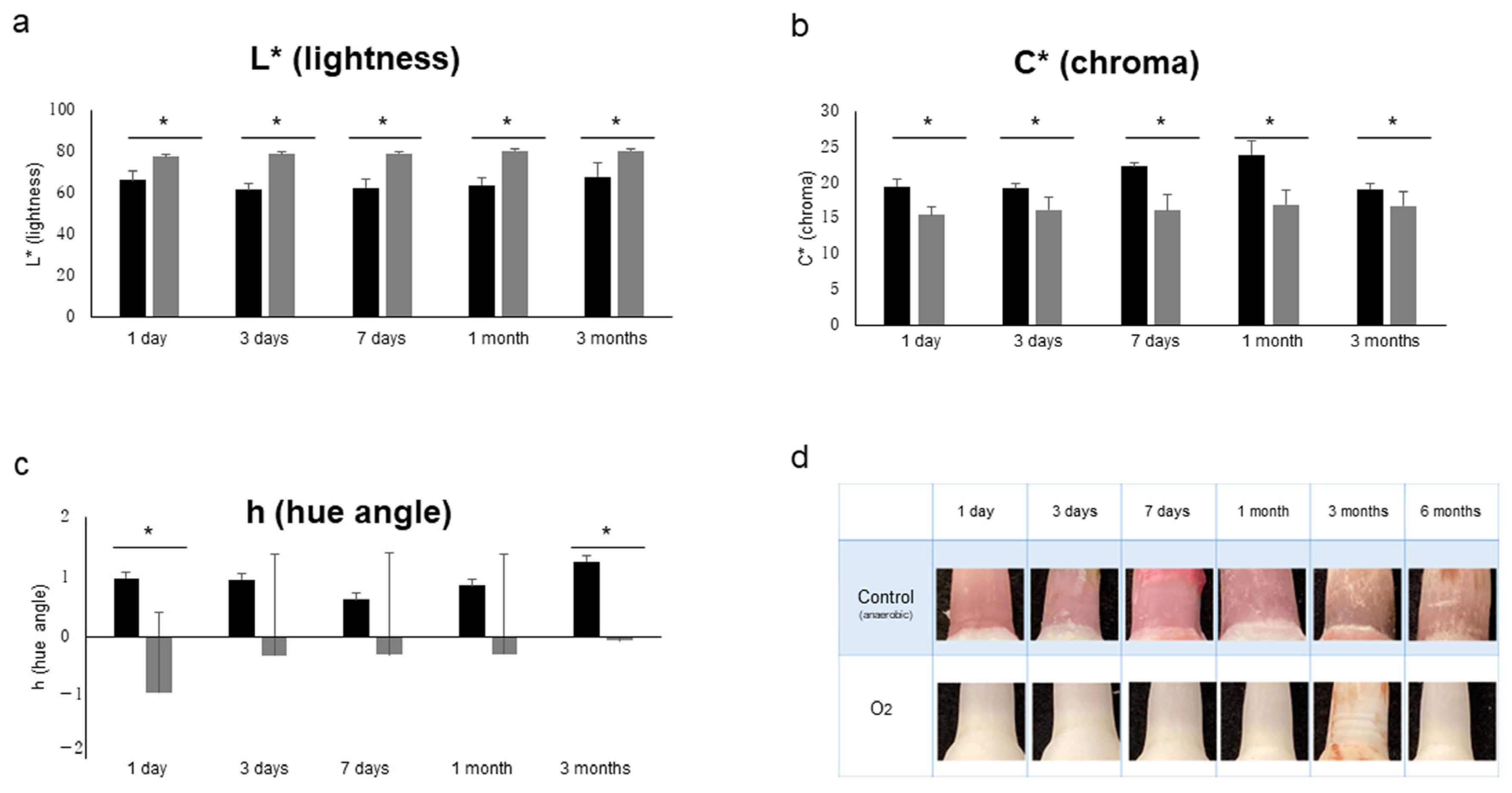




| Inter-Rater | R | p-Value | G | p-Value | B |
| NC | 0.997989 | <0.01 | 0.997125 | <0.01 | 0.997276 |
| 7 d | 0.996241 | 0.997392 | 0.996532 | ||
| 3 m | 0.994347 | 0.991968 | 0.993904 | ||
| Inter-Device | R | p-Value | G | p-Value | B |
| NC | 0.993376 | <0.01 | 0.990016 | <0.01 | 0.991847 |
| 7 d | 0.993974 | 0.995711 | 0.995423 | ||
| 3 m | 0.963627 | 0.939182 | 0.97046 |
| L*/Mean | C*/Mean | h/Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | 4 °C | p-Value | Cont. | 4 °C | p-Value | Cont. | 4 °C | p-Value | |
| 1 d | 66.36 | 69.68 | ns | 19.52 | 23.00 | <0.05 | 0.99 | 0.98 | ns |
| 3 d | 61.67 | 65.73 | <0.05 | 19.25 | 22.98 | <0.05 | 0.96 | 0.82 | <0.05 |
| 7 d | 62.67 | 62.44 | ns | 22.48 | 20.85 | ns | 0.64 | 0.89 | ns |
| 1 m | 63.87 | 61.95 | ns | 23.99 | 23.98 | ns | 0.86 | 0.82 | ns |
| 3 m | 67.23 | 61.22 | ns | 18.90 | 19.80 | ns | 1.269 | 1.18 | ns |
| Cont. | Humidity | p-Value | Cont. | Humidity | p-Value | Cont. | Humidity | p-Value | |
| 1 d | 66.36 | 73.26 | <0.05 | 19.52 | 18.05 | ns | 0.99 | 1.45 | <0.05 |
| 3 d | 61.67 | 68.40 | <0.05 | 19.25 | 21.18 | ns | 0.96 | 0.84 | <0.05 |
| 7 d | 62.67 | 65.84 | ns | 22.48 | 24.44 | ns | 0.64 | 0.72 | ns |
| 1 m | 63.87 | 68.33 | <0.05 | 23.99 | 21.70 | ns | 0.86 | 1.26 | ns |
| 3 m | 67.23 | 69.39 | ns | 18.90 | 24.43 | <0.05 | 1.269 | 1.35 | ns |
| Cont. | Soft tissue | p-Value | Cont. | Soft tissue | p-Value | Cont. | Soft tissue | p-Value | |
| 1 d | 66.36 | 67.70 | <0.05 | 19.52 | 16.22 | <0.05 | 0.99 | 0.94 | ns |
| 3 d | 61.67 | 63.97 | <0.05 | 19.25 | 19.71 | ns | 0.96 | 0.66 | <0.05 |
| 7 d | 62.67 | 61.40 | ns | 22.48 | 25.02 | ns | 0.64 | 0.63 | ns |
| 1 m | 63.87 | 60.43 | ns | 23.99 | 23.86 | ns | 0.86 | 0.82 | Ns |
| 3 m | 67.23 | 64.20 | ns | 18.90 | 19.75 | ns | 1.269 | 0.99 | Ns |
| Measurement Item | Qty (CP)/Mean | SD | p-Value |
|---|---|---|---|
| 6 h | 6.225 × 10−6 | 9.086 × 10−6 | Ns |
| 12 h | 4.723 × 10−6 | 7.577 × 10−6 | Ns |
| 1 d | 3.723 × 10−6 | 1.387 × 10−6 | Ns |
| 3 d | 1.545 × 10−5 | 7.058 × 10−5 | Ns |
| 7 d | 1.49 × 10−5 | 1.98 × 10−5 | Ns |
| 1 m | 0.12361 | 0.0969252 | <0.01 |
| Measurement Item | Ratio | CV | p-Value |
|---|---|---|---|
| 6 h | 0.023 | 3.95 × 10−2 | Ns |
| 12 h | 0.025 | 3.03 × 10−2 | Ns |
| 1 d | 0.00062 | 2.24 × 10−1 | Ns |
| 3 d | 0.00060 | 1.18 | Ns |
| 7 d | 5.50 | 3.40 × 10−4 | Ns |
| 1 m | 1.15 | 8.26 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumi, N.; Minegishi, S.; Ohta, J.; Utsuno, H.; Sakurada, K. Study on the Mechanism of the Pink Tooth Phenomenon Using Bovine Teeth: A Pilot Study. Diagnostics 2023, 13, 2699. https://doi.org/10.3390/diagnostics13162699
Sumi N, Minegishi S, Ohta J, Utsuno H, Sakurada K. Study on the Mechanism of the Pink Tooth Phenomenon Using Bovine Teeth: A Pilot Study. Diagnostics. 2023; 13(16):2699. https://doi.org/10.3390/diagnostics13162699
Chicago/Turabian StyleSumi, Nozomi, Saki Minegishi, Jun Ohta, Hajime Utsuno, and Koichi Sakurada. 2023. "Study on the Mechanism of the Pink Tooth Phenomenon Using Bovine Teeth: A Pilot Study" Diagnostics 13, no. 16: 2699. https://doi.org/10.3390/diagnostics13162699
APA StyleSumi, N., Minegishi, S., Ohta, J., Utsuno, H., & Sakurada, K. (2023). Study on the Mechanism of the Pink Tooth Phenomenon Using Bovine Teeth: A Pilot Study. Diagnostics, 13(16), 2699. https://doi.org/10.3390/diagnostics13162699






