Abstract
We aimed to overview the most recent data on sternal metastases from a multidisciplinary approach (diagnosis strategies, outcome, and histological reports). This narrative review based on a PubMed search (between January 2020 and 22 July 2023) using key words such as “sternal”, “manubrium”, and “metastasis” within the title and/or abstract only included original papers that specifically addressed secondary sternal spreading of cancer in adults, for a total of 48 original articles (14 studies and 34 single case reports). A prior unpublished case in point is also introduced (percutaneous incisional biopsy was used to address a 10 cm sternal tumour upon first admission on an apparently healthy male). The studies (n = 14) may be classified into one of three groups: studies addressing the incidence of bone metastases (including sternum) amid different primary cancers, such as prostate cancer (N = 122 with bone metastases, 83% of them with chest wall metastases), head and neck cancers (N = 3620, 0.8% with bone metastases, and 10.34% of this subgroup with sternum involvement); and glioblastoma (N = 92 with bone metastases, 37% of them with non-vertebral metastases, including the sternum); assessment cohorts, including breast cancer (N = 410; accuracy and sensitivity of PET/CT vs. bone scintigraphy is superior with concern to sternum spreading) and bone metastases of unknown origin (N = 83, including a subgroup with sternum metastases; some features of PET/CT help the differentiation with multiple myeloma); and cohorts with various therapeutic approaches, such as palliative arterial embolization (N = 10), thymic neuroendocrine neoplasia (1/5 detected with sternum metastases), survival rates for sternum metastases vs. non-sternum chest wall involvement (N = 87), oligo-metastatic (sternal) breast cancer (3 studies, N = 16 for all of them), oligo-metastatic head and neck cancer (N = 81), conformal radiotherapy (N = 24,215, including an analysis on sternum spreading), and EBRT followed by MR-HIFU (N = 6). Core data coming from the isolated case reports (N = 34) showed a female to male ratio of 1.6; the females’ ages were between 34 and 80 (mean of 57.28) and the males’ ages varied between 33 and 79 (average of 58.78) years. The originating tumour profile revealed that the most frequent types were mammary (N = 8, all females) and thyroid (N = 9, both women and men), followed by bladder (N = 3), lung (N = 2), and kidney (N = 2). There was also one case for each of the following: adenoid cystic carcinoma of the jaw, malignant melanoma, caecum MiNEN, a brain and an extracranial meningioma, tongue carcinoma, cholangiocarcinoma, osteosarcoma, and hepatocellular carcinoma. To our knowledge, this is the most complex and the largest analysis of prior published data within the time frame of our methods. These data open up new perspectives of this intricate, dynamic, and challenging domain of sternum metastases. Awareness is a mandatory factor since the patients may have a complex multidisciplinary medical and/or surgical background or they are admitted for the first time with this condition; thus, the convolute puzzle will start from this newly detected sternal lump. Abbreviations: N = number of patients; n = number of studies; PET/CT = positron emission tomography/computed tomography; EVRT = external beam radiotherapy; MR-HIFU = magnetic resonance-guided high-intensity focused ultrasound; MiNEN = mixed neuroendocrine-non-neuroendocrine tumour.
Keywords:
sternal metastases; manubrium; cancer; biopsy; surgery; chest wall; thyroid cancer; thoracic surgery; breast cancer 1. Introduction
Chest wall tumours, including those at the sternum level, account for 5% of all thoracic tumours, and are regarded as challenging conditions that require early recognition and a multitude of surgical approaches for diagnosis through biopsy and resection in order to provide a good cancer-associated outcome [1,2,3,4,5,6]. So far, related statistical evidence is based on small sample studies; a recent expert consensus with regard to their resection and consecutive chest wall reconstruction was released in 2021 [7].
Generally, the prognosis depends on a pluri-factorial panel that takes into consideration the underlying histological report (which is generally found to be very heterogeneous in this particular instance), the tumour-related aggressive profile, and multidisciplinary management of the primary and secondary malignancy, such as surgery, chemotherapy, immunotherapy, radiotherapy, radioiodine treatment, somatostatin analogues, and PRRT (peptide receptor radionuclide therapy) [8,9,10].
When it comes to the sternal involvement originating from non-sternal malignancies (sternum metastases are reported in about 40% of all chest wall metastases), the current level of statistical evidence remains rather low (despite the fact that a few studies, particularly single-centre experiences, have been published) [11,12].
The most common types of published data are either reviews of reported cases concerning sternal metastases originating from a specific histological type of a primary tumour; follow-ups in subjects confirmed with this site of metastases in relationship to different surgical and non-surgical treatments; or the assessments of diagnosis tools, mostly of imaging techniques. Further case-identifying strategies, as well as the surgery candidates’ selection and treatment protocols are required in this difficult matter of metastatic sternum lesions.
We aim to overview the most recent data on sternal metastases from a multidisciplinary perspective, pinpointing various aspects such as the diagnosis strategies, the outcome with respect to different approaches, and the underlying histological reports.
2. Methods
This is a narrative review based on a PubMed search (between January 2020 and 22 July 2023) using the key words “sternal metastasis” (strategy 1), “sternum metastases” (strategy 2), and “manubrium metastasis” (strategy 3) within the title and/or abstract. This 3-year sample-based analysis only included original papers that specifically addressed secondary sternal spreading of cancer in adults (originating from any histological type of malignancy), regardless the level of statistical evidence (original studies, as well as case reports). We excluded papers concerning primary malignant tumours of the sternum, studies addressing chest wall tumours that did not provide specific data on the sternal involvement of a malignancy (metastasis), reviews and editorials, experimental studies, and non-English papers.
According to the three mentioned strategies of research, we identified 73, 53, and 4 articles, respectively. All the papers have been manually checked within the title and abstract for the key words, duplicates were excluded, and, finally, we selected and analysed 48 original articles (14 original studies and 34 single case reports) (Figure 1). A prior unpublished case in point is also introduced as the basis of the discussion in Section 4.
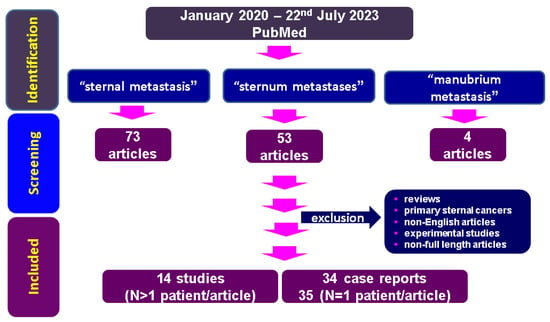
Figure 1.
Flowchart of research according to our methods concerning sternal metastases (between January 2020 and 22 July 2023).
The PubMed search identified a total of 627 articles; after restricting the search to the mentioned timeline according to the three types of key words combinations-based strategies, 130 papers were identified. Following the mentioned inclusion and exclusion criteria, a final number of 48 original papers are included in the final analysis.
3. Results: Sternal Metastases—A Multidisciplinary Perspective to Connect the Dots
3.1. First Recognition of Sternal Involvement: The Relationship with the Primary Tumour-Associated Histological Profile
Sternal metastases may be related to very frequent malignancies (such as mammary cancer or melanoma) or to unusual primary lesions (from an epidemiologic perspective); however, the rate of sternal involvement is not necessarily correlated with the frequency of the primary cancer among the general population. Generally, the spreading of cancer across the breastbone may be single or multiple (oligo- or multi-metastatic disease) [13,14].
3.1.1. Sternum Metastasis from Primary Malignancies with a High Prevalence in the General Population
Breast cancer is often described in relationship with secondary osseous sites; however, sternal involvement remains rather unusual, especially as a single lesion [15,16,17]. While metastatic breast cancer is a frequent condition, oligo-metastatic disease, particularly to the sternum, is unusual, with the first such report dating back to 1988 [18]. For example, Yao et al. [14] reported the case of a 49-year-old female diagnosed with an aggressive, triple-negative type of cancer that was complicated by pulmonary and sternal metastases that required second-line therapy with immune checkpoint inhibitors (anti-PD-1), in addition to a second regime of chemotherapy (sintilimab, paclitaxel, and carboplatin) following the failure of the initial treatment with paclitaxel and epirubicin [14]. Another interesting report introduced a 73-year-old woman who presented sternal metastases; 9 years had passed since the initial diagnosis, and the patient underwent therapy for the mammary cancer that required radiotherapy and re-starting hormonal treatment. This breastbone complication was followed 2 years later by severe spreading to the epicardium [15]. A similar late, distant metastasis was described in a 74-year-old woman who was found with superficial gastric and colonic metastases 23 years since having surgery for invasive lobular mammary cancer. Additional sternal and vertebral secondary lesions were confirmed on a positron emission tomography/computed tomography (PET/CT) scan [16].
Malignant melanoma, the most frequent dermatologic malignancy (as well as basal cell and squamous cell carcinoma), may spread to any body part, including bone. An exceptional scenario that involved melanoma-related sternum metastasis complicated with dissemination of the tumour thrombus at the level of the internal thoracic vein was reported by Ensle et al. [19] in 2023. This aspect was confirmed by an 18F-FDG (18-Fluor-2-desoxi-D-Glucose) PET/CT examination that functions as a very useful imaging tool [19].
A large retrospective study (between 2010 and 2019) on 16,209 subjects with head and neck primary cancers (N = 3620) identified 0.8% of them with bone metastases (N = 29; all the individuals were confirmed with a primary squamous carcinoma and a median period since the first sign of malignancy until bone involvement of 7.8 months was detected), the most frequent site being a vertebra (41%), followed by the pelvis (9%), sternum (10%, N = 3), and femur with a similar prevalence [20]. We identified a second original study on head and neck squamous cell carcinoma. This was a retrospective cohort on 81 patients with oligo-metastatic disease at different sites, including the sternum (admitted between 1998 and 2018); the 5-year survival rate was 40% (N = 32) after multimodal therapy. The results show that the site of metastasis did not influence the survival rate [21].
Alternatively, a bone metastatic score was calculated based on a prediction model in patients with newly diagnosed metastatic prostatic cancer following androgen deprivation therapy as the first -line treatment (N = 122 men; median age of 73; median follow-up duration of 11.5 months). The most frequent skeletal site of disease spreading was the pelvis at 92%, while 83% of them had chest involvement (sternum and ribs) with a hazard ratio (HR) of 2.093; 95% CI: 1.272–3.444, p = 0.004 [22].
3.1.2. Breastbone Spreading Originating from Unusual Primary Cancers
A few unusual scenarios have been reported within the time frame of our research, and awareness is necessary among specialists from different medical and surgical domains [23,24,25]. For example, we mention a 70-year-old female who was diagnosed with a primary intra-osseous adenoid cystic carcinoma at the level of the jaw with multiple bone (secondary) spreading, including the sternum. Sasaki et al. reviewed prior published data on this particular histological entity and identified only 51 similar cases [23]. Glioblastoma, a primary brain cancer with a very aggressive profile, rarely complicates with distant spreading. A meta-analysis of Strong et al. included PubMed-published studies of 92 cases with bone metastases (between 1952 and 2021). While the most frequent location was the spine (63%), 37% of these individuals had non-vertebral bone metastasis, including the sternum [24]. Cholangiocarcinoma, a condition that may spread to lymph nodes, the hepatic and pulmonary levels, and even the skeleton, was reported to involve, among other skeletal sites, the sternum of a 60-year-old woman. Singh et al. appreciated this case as the first one of single sternal involvement with respect to this rare malignancy originating from the biliary duct epithelium [25]. Another unusual case was an 80-year-old female with metastatic hepatocellular carcinoma requiring a radical sternum and ribs dissection and further chest wall reconstruction using titanium plates and an acellular dermal matrix [26] (Table 1).

Table 1.
Studies (N > 1 patient per study) focusing on bone metastases incidence (particularly, of the sternum); the table starts with the most recent publication time [20,22,24].
3.1.3. Thyroid Cancer and Sternal Involvement
Thyroid cancer represents the most frequent endocrine malignancy, with a rising incidence during the modern era. Skeletal metastasis of differentiated types, such as papillary and follicular, which represent 80–90% of all thyroid cancers, were reported in 2% to 13% of cases (other reports: 5–10%), follicular being more frequently affected than the papillary form. Skeleton spreading impacts the overall survival rate. The most common sites are the spine, pelvis, sternum, and ribs; usually, bone metastases of these cancers are considered radioiodine resistant, as opposed to other distant metastases, such as pulmonary. Depending on the location of the metastasis, the patients are suitable candidates for surgery and local radiotherapy [27,28,29]. Lately, oral multi-tyrosine kinase inhibitors, such as lenvatinib, have been used in multi-metastatic diseases (including bone) accompanying aggressive tumours [30]. Sternum metastases originating from rarer forms, such as anaplastic or poorly differentiated thyroid malignancies, are only exceptionally reported in association with sternum metastases [31].
One-stage diagnosis of a thyroid (malignant) nodule and associated sternal involvement was described according to the papers we found. For instance, in one case, a 59-year-old female was admitted for synchronous sternal metastases and originating malignancy, namely a papillary thyroid cancer (in combination with an insular carcinoma affecting 40% of the tumour mass). A complex one-stage procedure was performed combining a total thyroidectomy with lymph node resection in association with en bloc resection (a sternal mass of 7 cm) and chest wall rigid reconstruction (titanium bars covered with polymesh dual prosthesis). The thoracic surgery was followed by radioiodine therapy and the patient remained stable for 1 year after surgery while taking levothyroxine suppression medication [28]. Similarly, one case from 2022 involved the diagnosis of a papillary thyroid carcinoma associated with an anterior mediastinal metastatic mass including the sternum (from the notch to the third intercostal space) that required a total thyroid removal and en bloc resection of the sternum metastasis, followed by radioiodine therapy. Stability of the disease was achieved for the following 8 months [32].
Moreover, bone metastases may manifest before the actual recognition/diagnosis of the underlining thyroid condition. We reviewed a case of a 51-year-old female who had a 6-year history of left hip pain impairing her gait. This was caused by a loss of the diaphyseal femur; after 6 years, a check-up showed a large thyroid tumour with tracheal effects (a needle biopsy suggested follicular thyroid neoplasia) in addition to osteolytic lesions at the sternal level and one rib. A total thyroidectomy and neck dissection was performed. A papillary thyroid malignancy was post-operatively confirmed. The patient further underwent 131I radioiodine therapy (200 mCi), local radiotherapy, and monthly zoledronate therapy for bone involvement [33].
Of note, small-sized distant metastases from differentiated thyroid malignancies typically respond to standard 131I radioiodine therapy, while larger masses are more suitable for surgery; however, an individual decision should be made from a multidisciplinary perspective. This is why, despite a low level of statistical evidence, single lesions of the anterior chest wall might be selectively removed through thoracic surgery (sternal metastasectomy), followed by adjuvant radioiodine therapy [34,35]. Generally, mediastinal metastases from differentiated thyroid carcinomas embrace a more sever prognostic outlook than cervical (neck) recurrence or spreading. For example, the retrospective study of Moritani et al. [36] from 2022 identified 58 subjects diagnosed with papillary thyroid carcinoma who underwent upper mediastinal dissection by sternotomy (the subjects were admitted between 2006 and 2018). A 5-year cancer survival rate of 74.6%, and 10-year survival rate of 58.7% were achieved, which also confirms a more severe prognostic outcome in mediastinal vs. cervical recurrence, while mediastinal metastases larger than 3 cm or lower than the level of the paratracheal nodes were determined to be independent poor prognostic factors [36].
More aggressive histological subtypes are reported as well with regard to sternal metastases; however, the these are only reported in case reports. A sternal metastasectomy was performed on a senior confirmed with a follicular variant of a papillary thyroid cancer with no retrosternal extension, who also had a secondary sternum tumour (of 6 cm) [37]. Similarly, a 33-year-old male was diagnosed with the same histological subtype and, later, he presented with multiple bone metastases (including at the femur diaphysis, ribs, and sternum handle) 2 years after the initial thyroidectomy. In addition to the guided core-needle biopsy of the knee mass, 18F-FDG PET/CT assessed the entire bone spreading of the underlying cancer [38]. Hürthle (oncocytic) cell carcinoma, another subtype of differentiated thyroid malignancies with a more severe prognosis due to the fact that it is less radioiodine responsive (or even radioiodine refractory) [39,40], was reported to have distant metastases, including at the mediastinal lymph nodes and pulmonary and sternum levels (lytic lesions). Post-thyroidectomy spreading was identified via FDG PET/CT; the patient received sorafenib for 3 years and then switched to lenvatinib due to hepatic progression of the initial condition [41]. Another unique case was reported of a 79-year-old male who suffered from cutaneous metastasis 16 years after a total thyroidectomy was performed for a benign goitre. The spreading invaded the skin at the level of the upper anterior thoracic wall to the sternal periosteum; thus, a wide excision was performed, in addition to right neck dissection. A right pectoralis major island flap was used for skin reconstruction. Unexpectedly, the histological report showed a papillary thyroid carcinoma with Hürthle cells (which was not consistent with the initial post-thyroidectomy pathological report). The pathogenic traits behind this enigmatic malignant shift are still an open issue [42].
On the matter of the thyroglobulin-producing thyroid carcinoma with unusual sternal involvement, one case of a 59-year-old female was reported to have a negative radioiodine-based whole-body scan after a prior total thyroidectomy and I131 radioiodine therapy. Disease relapse in terms of sternal and lung metastases that were iodine-refractory were further detected. Upon exam, they the metastases were determined to be highly positive in somatostatin receptors; thus, PRRT was offered to the patient, and she achieved a thyroglobulin decrease, in addition to an improvement of the sternal pain and respiratory complaints [43]. Currently, PRRT (such as 68Ga/177Lu-DOTATATE) represents an important chapter in the management of iodine-refractory differentiated thyroid malignancies in addition to C cells derivate medullary thyroid cancer (as part of the larger frame, with respect to the neuroendocrine neoplasia); thus, we highlight the importance of determining the somatostatin receptors’ status through Octreoscan and/or immunohistochemistry (if feasible) in radioiodine negative differentiated thyroid carcinomas [44,45].
Recently, genetic analysis in papillary and follicular thyroid cancers (for example, KRAS, BRAF, RET, and P53 genes) has been considered as a useful tool for identifying an aggressive form, including with a higher risk of distant metastases and/or a radioiodine refractory profile [46]. In 2023, a new report of a recurrent papillary thyroid carcinoma (harboring both TERT promoter and BRAFV600E mutations) in a 71-year-old woman showed a large recurrent mass involving not only the neck area, but also several local and distant regions, such as the sternal end of the clavicle. [47]. Neoadjuvant treatment, namely, four cycles of anlotinib, a new multi-targeting tyrosine kinase inhibitor [48,49,50], allowed surgical resection by inducing partial tumour shrinkage; however, 6 months following the surgery, a recurrence was noted again [47].
Overall, a heterogeneous interplay between the thyroid and sternum is described. A collaborative team is required for decision-making, while individual management should be sustained by a multidisciplinary check-up.
3.2. Advances in Imaging Assessment
As expected, several studies addressed the importance of using PET/CT to assess the bone status, including the sternal metastases [41,51,52,53,54]. A study published in 2023 analysed the accuracy of investigating bone metastases by applying PET/CT vs. traditional bone scintigraphy (N = 410 women with mammary cancer who were followed between 2014 and 2020; 27% of them had associated distant metastases and 72% of these subjects had bone involvement) and identified a higher sensitivity for PET/CT (93.83% vs. 81.84%, p = 0.0442) in detecting bone metastases, including the upper and lower limbs, the spine (but not the cranium), as well as the sternum (N = 38 individuals; accuracy of 96% vs. 76% for PET/CT and1 bone scintigraphy, respectively; p = 0.0008; sensitivity of 94% vs. 52%, p = 0.0001) [51].
A report by Zhao et al. [52] showed that a PSMA (prostate-specific membrane antigen) PET/CT seems more useful for identifying bone metastases (including at the sternum level) than 18 F-FDG PET/CT (in the case of urothelial bladder carcinoma) [52]. A similar conclusion was drawn in a case of sternum metastatic osteosarcoma [55].
Another study on 18 F-FDG PET/CT analysed the bone (osteolytic) metastases in 63 individuals who were not previously diagnosed with a primary cancer; among the studied cohort, 20 patients were finally confirmed with multiple myeloma. Deng et al. [53] analysed the uptake profile for eight distinct skeletal sites (including the sternum). The lesions in multiple myeloma had short cross-section lengths and a more uniform distribution in these 20 subjects vs. the non-myeloma group (N = 43), suggesting the importance of PET/CT detailed features for an adequate interpretation and diagnosis [53].
Single-photon emission computed tomography/computed tomography (SPECT/CT) was found useful in different tumours that are either malignant or hormonally active, such as parathyroid tumours [56,57,58]. Kitajima et al. [56] applied three-dimensional (3D) quantitative bone SPECT/CT to assess the evolution of a patient diagnosed with pulmonary cancer who was treated with pembrolizumab. The initial uptake at the femoral level and left ribs was followed by local radiotherapy for the unilateral femoral head involvement; however, during follow-up, while the uptake was reduced in this site, there was an increased uptake at multiple skeletal sites, including novel sternum spreading [56].
Traditional CT scan and magnetic resonance imaging (MRI) might serve to pinpoint osteolytic lesions [59]. On the other hand, unexpected and undetected (according to a plain X-ray exam) sternal metastases impaired the outcome of a 66-year-old male previously diagnosed and treated for clear cell renal carcinoma; after 5 years of remission, the patient was admitted for aortic valve replacement due to severe stenosis of the bicuspid aortic valve and mitral valve plasty for mitral insufficiency. At the beginning of the cardiac surgery, massive bleeding occurred after the incision was made (at the level of the unsuspected sternal metastasis); in spite of a massive manubrium resection (of 15 cm), in addition to forced ligation of both mammary arteries (as a lifesaving procedure), the outcome was fatal. Approximatively half of the individuals diagnosed with this type of malignancy may present distant spreading, with bone being affected in one-third of them; however, a solitary metastasis, as seen here, is described only in 5% of cases, while in this particular type of kidney cancer, recurrence might occur after a well-established prolonged remission. This is why a check-up with a CT scan, even in asymptomatic subjects, within the remission period is useful before having major surgery [60] (Table 2).

Table 2.
Specific studies (N > 1 subject per study) to address the investigations required for sternum metastases diagnosis; the table starts with the most recent year of publication (2023) [51,53].
3.3. Management of Sternum Metastases
3.3.1. Surgery Notes
En bloc resection of chest wall tumours (with negative margins) represents the gold standard; reconstruction of the chest consecutive to the chest wall defects is mandatory to prevent cardiac, respiratory, and skeletal issues. Various reconstruction techniques with different materials, including 3D technologies for planning, designing, and 3D printing to achieve better surgical guides, have been applied; however, specific guidelines remain an open subject [26,61,62].
Titanium-based bar reconstruction represents a novel alternative, yet, with insufficient statistical evidence [63]. A retrospective analysis was published in 2022 representing the largest single-centre study on this type of malignant tumour with a long-term analysis (a maximum of 108 months). Out of the 87 patients (admitted between 2012 and 2018), 20 individuals had isolated metastases from distant cancers with various origins (such as breast, sarcomas, etc.). Complete resection was performed in 94% of the cases, while a partial sternum resection was performed in 15 subjects (29%). A 5-year survival rate of 57% was confirmed. Overall, the use of titanium bars and sternal plates correlated with a good long-term outcome; however, complications such as a post-surgery infection rate of 18% (mostly, soon after the operation, within the first 12 months, a ratio that seems rather low compared to prior studies) and persistent chest pain (defined as chronic pain for more than 3 months requiring daily medication) affected 24% of the cohort. When compared with the overall survival rate between the subjects with primitive chest wall cancers and secondary malignancies, a long rank p-value was not statistically significant (p = 0.574) [64].
Generally, neuroendocrine neoplasia represents a difficult dynamic domain; the particular subgroup in the thymic location is a very rare (representing less than 0.4% of all neuroendocrine neoplasia and 5% of all thymic tumours) and aggressive type (that may be typical or atypical carcinoid, large cell neuroendocrine, or small cell carcinomas) [65]. A series (N = 5, male to female ratio of 3 to 2; average age of 53.6 years) published by Huang et al. [66] included a 45-year-old asymptomatic man (N = 1/5) with increased neuron-specific enolase levels in the blood, who was confirmed with stage IV large cell neuroendocrine carcinoma (a mediastinal tumour of 6 cm the largest diameter) with sternum spreading, as well as spreading to other sites, such as the lymph nodes and lungs. The subject underwent tumour resection in addition to the removal of the sternal metastases, upper cava vein, and partial right atrium (with post-operatory remnants at this level) via a median sternotomy and cardio-pulmonary bypass. A post-surgery 4-month survival was associated with rapid progression with distant metastases while being treated with chemotherapy [66]. Limited data have been published so far in order to address the specific field of thymic neuroendocrine tumours; however, a surgical approach is associated with the most significant improvement, including resection of the lymph nodes and distant metastases (if feasible). Additional radiotherapy, chemotherapy, treatment with somatostatin analogues, and PRRT was recommended based on an individual decision [67].
Of note, neuroendocrine neoplasia, particularly the cases displaying carcinoid syndrome originating from gastrointestinal sites, usually correlates with the presence of liver metastases (the liver being involved in 5-hydroxytriptamine metabolism) and, in this case, the patients are candidates for somatostatin analogues, such as octreotide or lanreotide. In spite of this, surgery remains the first choice, if feasible (even debulking procedures might help). In one case of a 59-year-old female with carcinoid syndrome related to a caecum primary tumour, the patient was treated for 6 months with chemotherapy and interferon, followed by Octreoscan-based confirmation of liver and bone metastases (including at the level of the right sternum–clavicle joint); thus, a switch to octreotide LAR was made, allowing for stabilisation of the disease for 2 years when the disease progression required PRRT (177Lu-DOTATATE) [68].
Minimally invasive procedures may also include, for example, percutaneous osteoplasty, as similarly seen in percutaneous vertebroplasty (injection of bone cement). This seems to be a promising alternative to painful sternal metastases, as well, which are no longer responsive to standard therapy, but this aspect is still under debate [69].
3.3.2. Non-Surgical Management of the Sternal Metastases
As an alternative to radiation therapy, or in cases with a poor response to standard management, palliative arterial embolization has been applied. The case series of Papalexis et al. [70] included 10 subjects (male to female ratio of 1, average age of 58.1 years, aged between 37 and 70) diagnosed with sternum metastases from different primary tumours (the patients were admitted between 2007 and 2022). They received palliative arterial Lipiodol embolization (four of them underwent a second procedure). Approximately 90% of them had an occlusion of pathological-associated vessels proven by angiography to correlated with the tumour size reduction, in addition to a clinical improvement, as reflected by the pain scores (according to a median duration of 9.5 months) and a reduction of analgesic medication use [70].
Proton beam therapy was offered to a patient who had prior radiation therapy following a mastectomy for a primary mammary malignancy, according to a report from 2022. The evolution of the malignancy was complicated with a solitary sternum metastasis 6 months after radiotherapy, while the patient was treated with tamoxifen. After experiencing an early complication from this procedure (proton beam therapy-associated dermatitis), a 3-year complete remission was confirmed (in the absence of surgery or chemotherapy, which the subject refused) [71]. Oligo-metastatic mammary cancer with single (isolated) sternum lesions was also the topic of a small study (N = 4) that introduced multimodal treatment (with curative intent) followed by proton pencil beam scanning to the sternum. The disease-free median of the post-diagnosis follow-up was of 28 months [72].
A single-centre experience reported a study on 10 patients with breast cancer who received stereotactic ablative body (3D-conformal) radiotherapy for oligo-metastatic disease at the sternum level. After a median period of time of 32 months, 9/10 subjects had in-field control; among the 7/10 patient with sternal pain, the results after 3 months showed that 3/7 individuals had a decreased pain score and 2/7 subjects had pain remission, suggesting overall a potential analgesic effect. Generally, this type of radiotherapy at the sternum is considered to be more complicated due to the close skin exposure of a larger area [73]. Another large retrospective, multi-centre study on conformal radiotherapy for bone metastases included 24,215 patients (between 2009 and 2016). Sternum spreading was shown, among other sites, to be most likely treated with advanced radiotherapy (OR of 5.2, p < 0.001) [74].
Bone pain relief was associated with external beam radiotherapy (EBRT) with good pain control in 60% of cases. In 2021, a first study to address this issue by using magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) was released; this was a singlearm study on six patients with a median age of 67 (a median lesion of 5.6 cm), who were offered MR-HIFU within 4 days after EBRT. Five of the six subjects registered a good pain response at 7 days, and at 4 weeks, a 60% stabilization rate was registered [75]. Opposingly, a hyper-progression of a sternal metastasis (among other sites) was registered under radiotherapy and immunotherapy, as reported in a case with high-grade urothelial bladder carcinoma [76].
An alternative to surgery for sternal metastases is represented by systemic chemotherapy to target synchronous primary and secondary tumours [77]. For instance, Iijima et al. [78] reported an unusual squamous cell carcinoma on the left side of a 42-year-old woman’s tongue. The histological report was confirmed after lingual ulcer biopsy, while PET/CT confirmed the metastases at the sternum level. She was offered EXTREME regimen (cisplatin and 5-fluorouracil, in association with cetuximab as a loading dose) to be administered six times, then she continued with weekly administration of cetuximab (for 3 years) and achieved sustained remission for 5 years. The authors suggested that the p16 positive status (as revealed by immunohistochemistry exam) was prone to be associated with a better outcome with these drugs [78]. Lately, oral multityrosine kinase inhibitors, such as lenvatinib, have been used in multi-metastatic disease (including bone) accompanying aggressive forms [30] (Table 3).

Table 3.
Studies and case series (N > 1 individual per study) aiming to analyse different aspects of management with regard to sternum metastases; the table starts with the most recent year of publication [18,21,64,66,70,72,73,74,75].
4. Discussion
4.1. A Matter of Differential Diagnosis: Sternal Metastases
Differentiating sternum metastases from other entities represent a challenge in some instances. As mentioned, we only included secondary malignancies of the sternum, not primary tumours of breastbone origin, nor their local recurrence [79,80]. It should be noted that chondrosarcoma is regarded as the most frequent primary type (representing 15% of all chest wall tumours), but the issue of primary cancers with sternum origins is very complicated and included unusual tumours, such as multifocal osteosarcomas [81,82,83,84]. Moreover, sternum metastases are distinct from the skin over the sternal area at the metastatic site, although clinical differentiation is not always feasible on the initial admission [85].
Another important differential diagnosis is tuberculosis with bone involvement, which is considered to be a “great mimicker” of a malignancy; however, the sternum is among the rarest sites described under these circumstances, as opposed to, for instance, the spine or hip [86,87]. For example, Engin et al. [87] introduced a case of extra-pulmonary tuberculosis with multiple locations, including the sternum, that initially presented as a suspected malignancy at 18F-FDG PET/CT examination. This highlights that awareness is mandatory for differential diagnosis, especially in countries where tuberculosis has an elevated incidence in the general population [87]. Another infectious condition with metastases-like appearance, including at the sternum level, was reported to be alveolar echinococcosis [88].
Interestingly, Yin et al. [89] introduced the case of 42-year-old male confirmed with dermatomyositis after previously experiencing retrosternal pain, rash, and muscle asthenia. After successful corticotherapy, CT showed osteolytic sternum metastases due to a newly detected squamous cell carcinoma of the lungs, as well as tuberculosis, a diagnosis that could not be established at the first presentation for the autoimmune condition [89].
Another distinction should be made in females with mammary cancer that have routine sternum assessments during periodic MRI (or other similar imagery techniques) for disease surveillance [90,91]. Through these assessments, a metastasis at the sternal level may be easily detected, while a sternal incidentaloma underlying, for instance, a hemangioma needs to be differentiated [91].
4.2. Insight of a Manubrium Metastasis at First Admission
4.2.1. A Case in Point
This was the case of a 61-year-old male who was a heavy smoker and was initially referred to a tertiary centre of endocrinology for a suspected retrosternal goitre. He was clinically stable and had no relevant personal or family medical history. On admission, the clinical exam showed a sternal lump of almost 10 cm with no connection to the thyroid gland. The patient described its progressive increase during the previous few months. He delayed the presentation amid the COVID-19 pandemic (Figure 2).
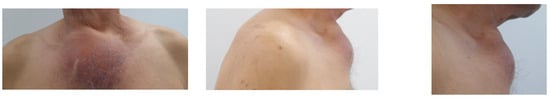
Figure 2.
A 61-year-old male admitted for a sternal lump that developed within a few months (different planes).
Blood exams showed mild anaemia in addition to normal thyroid function and negative thyroid antibodies. A thyroid ultrasound revealed a multinodular goitre with a hypoechoic pattern with several nodules of less than 0.6 cm; the largest nodule of 1.1 by 0.6 cm was detected at the upper part of the right thyroid lobe, with no tracheal deviation and no connection to the sternal mass (Figure 3).
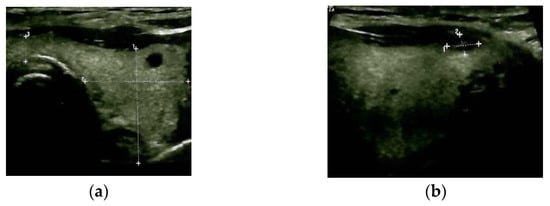
Figure 3.
Thyroid ultrasound: multinodular goitre with hypoechoic pattern (a): transverse plane; (b) (right) sagittal plane.
CT with intravenous contrast was performed and showed a sternal tumour of 10 by 11.6 cm at the largest diameter, with no retrosternal extension of the goitre (Figure 4).
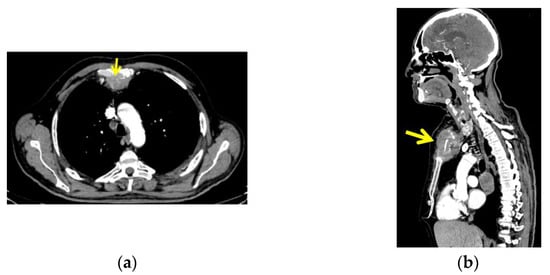
Figure 4.
CT with intravenous contrast: sternal tumour of 10 by 11.6 cm; (a) transversal plane; (b) right: sagittal plane.
The CT scan also revealed two tumours at the level of the dorsal upper pulmonary right lobe, measuring 4 by 3 cm and 3.8 by 3 cm, respectively. Multiple mediastinal lymph nodes and a similar lesion at the level of right lateral cerebellum measuring 3.5 by 2.3 cm, and another at the left temporal lobe measuring 1.9 by 2 cm were also detected (metastases).
A multidisciplinary decision was made to pursue a biopsy of the sternal tumour in order to provide a histological report; thus, the patient was transferred to the department of thoracic surgery. A percutaneous incisional biopsy of the sternal tumour was performed. After sterile prepping and dressing of the tumour zone, local anesthesia was administered with 10 cc of lidocaine 1%. A small elliptical incision was made, and a tissue sample of 2 by 2 cm was excised. Local hemostasis was achieved with both electrocautery and hemostatic powder. Two 2.0 nylon interrupted stitches were placed. The wound was packed, and the patient was discharged early with no complications. Unfortunately, he died suddenly in his sleep a few days later while experiencing no other clinical complications. The histological report showed lung adenocarcinoma with a cribriform and glandular pattern (Figure 5).

Figure 5.
Histological report: sternal metastasis originating from pulmonary adenocarcinoma with a cribriform and glandular pattern; hematoxylin—eosin.
Immunohistochemistry analysis revealed a positive CK7 and TTF1 reaction within the tumour cells and a Ki67 proliferation marker of 55% (Figure 6).
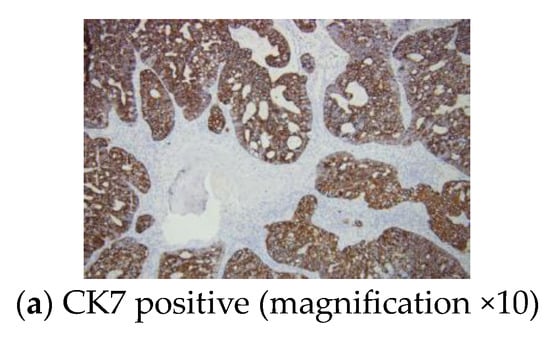
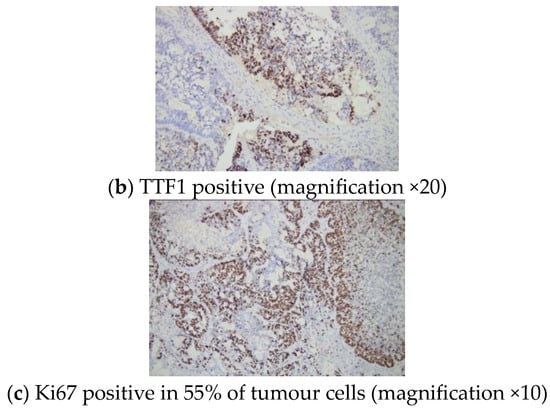
Figure 6.
Immunohistochemistry report: sternal metastasis originating from a pulmonary adenocarcinoma with a cribriform and glandular pattern.
4.2.2. Biopsy of Sternal Metastases
According to the data we reviewed and this case, a major player in investigating a newly detected sternal mass is represented by biopsy. Generally, there are several types of biopsies: incisional, excisional (surgical), endoscopic, bone marrow biopsy, fine needle aspiration biopsy, core needle biopsy, punch biopsy, and shave biopsy. The incisional type implies partial tumour removal, while an excisional biopsy removes the entire mass. Fine and core needle biopsies use a smaller or larger needle to collect cells/fluids (fine) or tissue samples (core), with or without imaging guidance. In this case, we performed an incisional biopsy, a surgical procedure in which only a small part of the tumour mass is removed for subsequent pathology examination. It is applied in cases of large, deep-seated tumours, or when a complete surgical excision is not possible because of the medical and surgical risks involved. It can be performed under local or general anesthesia. Percutaneous incisional biopsy of a sternal tumour under local anesthesia is a safe and efficient procedure and can be used in cases when general anesthesia has relative or absolute contraindications. Typically, a biopsy of a sternal mass can be performed by core needle, incisional, or excisional biopsy, depending on the situation. For larger tumours invading adjacent structures, a core needle or an incisional biopsy are preferred. The advantage of the percutaneous core needle biopsy is represented by a reduced risk of hemorrhage and reduced pain and peri-procedural morbidity. Percutaneous incisional biopsy, on the other hand, has the advantage of providing a larger tissue sample for analysis. Excisional biopsy implies the removal of the whole tumour, within oncological limits, and can be performed in tumours up to 5 cm (which was not feasible in this case in point) [23,30,37,62].
In this mentioned case of pulmonary carcinoma with sternal, brain, and lymph node metastases, the patient experienced no local side effects following the biopsy and most probably, the unexpected outcome was related to the brain metastases. As we mentioned, we identified several papers to address biopsy options for sternal masses (within our mentioned methods) [18,23,30,37,62].
Of a similar note, we highlight the case of a 42-year-old male who was admitted for a large mass overlying the manubrium (of 10 by 12 cm). Fine needle aspiration cytology was provided, which was suggestive for a serous cyst; however, after a wide excision was performed, the post-operative histological report confirmed an extra-gonadal seminoma (germ cell tumour). Chemotherapy was administered [92]. This was one of the rarest reports in men diagnosed with seminomas and extra-gonadal involvement [93,94]; however, in the case published by Rathod et al. [92], the spreading mimicked mediastinal involvement, which was not confirmed. Due to the location of the lesion (over the sternum) at the level of soft tissue, fine needle aspiration was a convenient choice, yet it lacked cytological and histological concordance [92].
Another challenging circumstance was described by Hayashi et al. [95]. This was a case of a woman in her 70s who was first admitted for left mammary cancer and axillary lymph node metastases (confirmed via ultrasound-guided biopsy and assessed through 18F-FDG-PET/CT). After neoadjuvant chemotherapy, both sites were negative on a subsequent 18F-FDG-PET/CT; however, a novel lesion showed a positive sternum accumulation. A sternal (bone) biopsy was performed and found no cancer. The authors suggested that chemotherapy in association with G-CSF (granulocyte colony-stimulating factor) might be responsible for the false positive PET/CT lesions [95]. Direct access via a biopsy avoids unnecessary medication if metastasis is suspected in patients with prior/concomitant non-sternal malignancies [95,96].
Moreover, a CT-guided trans-sternal 125I seeds implantation at the level of the mediastinum for primary or secondary tumours at this level represents an additional therapeutic application. A study of 20 patients with 22 different types of lesions (between 2017 and 2019) showed a satisfaction rate concerning the iodine distribution of 90%; a local control rate of 53% was found 1 year after the surgical procedure. In regard to side effects, there was one case of mild pneumothorax and another of hemoptysis [97].
An open biopsy was also used for sternal swelling that developed more than 2 years after the final treatment for a meningioma in a 75-year-old male. The histological report confirmed aetastasis from the initial brain tumour and resection was performed; unfortunately, the disease rapidly progressed to the thoracic cavity and then the abdominal cavity [98]. The same procedure, an open bone (sternum) biopsy was performed on 68-year-old male diagnosed with bladder cancer and osteoblastic lesions with discordant evolution under chemotherapy when compared to the primary site. In cases with multiple skeletal involvements, the decision of performing a biopsy is multidisciplinary, to a certain extent, and it primarily takes into account imaging assessments, such as CT and whole-body bone scintigram, as seen here [99].
Another application of the sternum biopsy was described in a newly admitted adult presenting a 5 cm sternal mass associated with chest pain for 2 months (without a prior history of malignancy). The post-biopsy pathological analysis indicated a renal cell carcinoma as the originating tumour that was complicated by this isolated metastasis. Further targeted therapy was offered to this 76-year-old male [100].
A benign lung and sternum metastasizing leiomyoma was confirmed though biopsy at both levels a decade after a hysterectomy for the originating benign smooth muscle uterine tumour was performed. This most unusual tumour (only a few cases have been previously published), while not being a sarcoma, is slowly growing and hormonally responsive since it displays oestrogen receptors. In this case, therapy with tamoxifen was administrated [101].
A needle biopsy of the sternum was performed for adequate diagnosis following thoracoscopic resection of an exceptional tumour, a primary pulmonary (extracranial) malignant meningioma. The woman presented with persistent tight pain and a further PET/CT was performed and confirmed multiple bone metastases, including the ribs, vertebras, and sternum. Due to the rarity of the initial cancer (only a few cases have been published before this report from 2020), histological confirmation was mandatory; thus, a sternal approach was chosen. These multiple skeletal lesions were then addressed with monthly denosumab and local radiotherapy for femur lesions [102].
As mentioned, the case of an elderly women reported by Pradeep et al. [37] in 2020 involved a diagnosis on first admission of thyroid cancer complicated with manubrium and lung metastases, as highlighted by FDG-PET/CT. For thyroid evaluation, ultrasound-guided fine needle aspiration cytology was performed, while for the sternal mass, the same procedure was applied to confirm the metastases within the thyroid origin. These investigations led to a multidisciplinary decision to perform a total thyroidectomy, unilateral selective neck dissection, and sternal (upper part) metastasectomy (with Dacron mesh, cement, and pectoralis major muscle reconstruction), followed by radioiodine ablative therapy [37] (Table 4).

Table 4.
Case reports/series addressing sternal or trans-sternal biopsy or fine needle aspiration for diagnosis or therapeutic purposes in non-metastatic lesions of the sternum; the table starts with the most recent publication [92,95,97].
4.3. A 43-Month Sample-Based Study: A Heterogeneous Picture
Overall, we identified 14 studies (of more than 1 subject/article) [18,20,21,22,24,51,53,64,66,70,72,73,74,75] and 34 single case reports [14,15,16,17,19,23,25,26,38,41,42,43,47,52,55,56,59,60,62,68,71,76,77,78,89,98,99,100,101,102] within our methods with respect to sternal metastases (n = 48 papers) (Table 5).

Table 5.
Single case reports of sternal metastases according to our methods; the articles are cited starting with the most recent time of publication [14,15,16,17,19,23,25,26,30,32,33,37,38,41,42,43,47,52,55,56,59,60,62,68,71,76,77,78,89,98,99,100,101,102].
The case we mentioned brought up the importance of addressing a sternal biopsy into the larger frame of multi-lined, complex management. The detection of sternal metastases might start with the progression of a palpable lump (self-detected by one patient) or with clinical complaints, such as respiratory troubles or chest pain, or it may be evaluated during an imaging assessment for a prior known condition that may or may not be related (incidentaloma) to the sternal mass. Further on, once identified, a tumour of the breastbone should undergo a complex workup and, in selected cases, the decision of performing a sternum biopsy might represent the next logical step of the therapeutic approach (Figure 7).
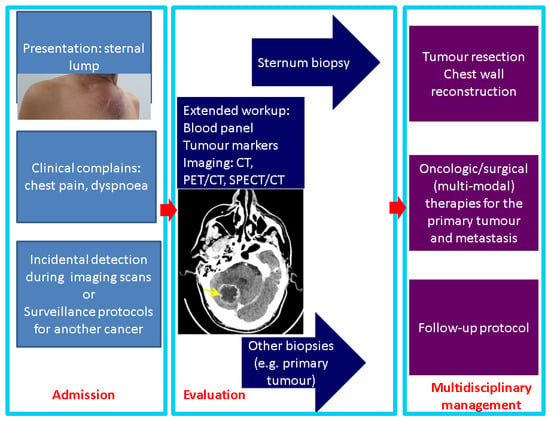
Figure 7.
Panel of management in sternal metastases: from presentation to therapy (the photo of the sternal lump represents the case previously described in a 61-year-old male who was admitted for the first time for this breastbone mass, which was finally confirmed as metastatic lung cancer; the image in the second box represents a CT scan with brain metastasis in the same patient).
Another aspect to pinpoint is the fact that apparently, in this case, a delay of the presentation was related to the recent COVID-19 pandemic. Numerous aspects of medical and surgical practices have been impacted amid this 3-year time frame, and new conditions are being reported or more severe presentations are confirmed due to the deficiency in medical health systems during the dynamic regulations related to the pandemic [103,104]. The impact of prompt, adequate recognition with regard to sternum metastases grossly depends on the underlining primary tumour, the co-morbidities, and the multimodal therapy [105,106]. A multidisciplinary decision is mandatory; yet, in many cases, as mentioned, this decision is personalized rather than being a matter of a specific guideline. Finally, the resection of the mentioned chest wall metastatic tumour and chest wall reconstruction implies another major milestone in decision making and it should be integrated in the overall management in secondary malignant lesions of the breastbone, as seen in primitive sternum cancers [107] (Figure 8).
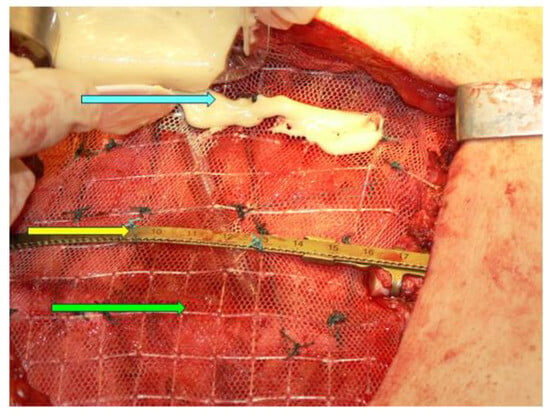
Figure 8.
Sternal reconstruction after resection of the medial third of the sternum on an adult patient; Stratos titanium bar (yellow arrow), polypropylene mesh (green arrow), kryptonite bone cement (blue arrow).
This sample-related analysis is based on a single database research (PubMed); however, this represents the largest study on published data we could identify. A narrative review allows a more flexible approach since the study design, the studied population, and the assessments/therapies were inhomogeneous. The 14 studies we mentioned may be classified into one of three groups: studies that addressed the incidence of bone metastases (including the sternum) amid different primary cancers, such as prostate cancer (N = 122 with bone metastases, 83% of them with chest wall metastases), head and neck cancers (N = 3620, 0.8% with bone metastases, and 10.34% of this subgroup with sternum involvement), and glioblastoma (N = 92 with bone metastases, 37% of them with non-vertebral metastases, including the sternum); assessments-based cohorts, namely, one on breast cancer (N = 410; accuracy and sensitivity of PET/CT vs. bone scintigraphy is superior with concern to sternum spreading) and another on bone metastases of unknown origin (N = 83, including a subgroup with sternum metastases; some features of PET/CT help with differentiation from multiple myeloma); and the third category is represented by cohorts with various therapeutic approaches, such as palliative arterial embolization (N = 10), thymic neuroendocrine neoplasia-associated management (1/5 subject detected with sternum metastases), survival rates for subjects with sternum metastases vs. non-sternum chest wall involvement (N = 87), oligo-metastatic (sternal) breast cancer (N = 4; in two other studies, N = 10 and N = 2, respectively), oligo-metastatic head and neck cancer (N = 81), applications of conformal radiotherapy (N = 24,215, including an analysis on sternum spreading), and EBRT followed by MR-HIFU (N = 6) [18,20,21,22,24,51,53,64,66,70,72,73,74,75].
The core data coming from the 34 single case reports show the following: female to male ratio of 1:6; the females’ age was between 34 and 80, with a mean value of 57.28 years; and the males’ age varied between 33 and 79 (2 patients were ≤41 years), an average of 58.78 years. The originating tumour profile revealed that the most frequent types were mammary cancer (N = 8, all females) and thyroid cancer (N = 9, both women and men), followed by bladder carcinoma (N = 3), lung carcinoma (N = 2), and malignancy of renal origin (N = 2). One case was identified for each of the following sites: adenoid cystic carcinoma of the jaw, malignant melanoma, caecum MiNEN, brain and an extracranial meningioma, tongue carcinoma, cholangiocarcinoma, osteosarcoma, and hepatocellular carcinoma [14,15,16,17,19,23,25,26,30,32,33,37,38,41,42,43,47,52,55,56,59,60,62,68,71,76,77,78,89,98,99,100,101,102] (Figure 9).
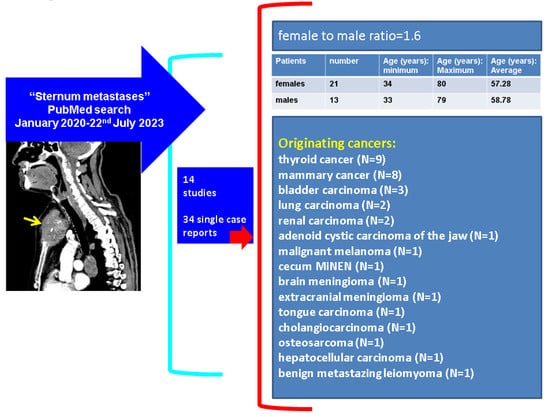
Figure 9.
Overview of published case reports (N = 34) according to our methods [14,15,16,17,19,23,25,26,30,32,33,37,38,41,42,43,47,52,55,56,59,60,62,68,71,76,77,78,89,98,99,100,101,102]. Abbreviations: N = number of patients; MiNEN = mixed neuroendocrine–non-neuroendocrine tumour.
The limitations of the current work are related to a single database search, a 3-year retrospective review, and the analysis according to a narrative review; however, as mentioned, the complexity of the topic and its multidisciplinary approach allowed a wide and insightful standpoint in the matter of breastbone-associated secondary malignancy.
5. Conclusions
To our knowledge, this is the most complex and the largest analysis of prior published data within the time frame of our methods. These data open up new perspectives of this intricate, dynamic, and challenging domain of sternum metastases. Awareness is a mandatory factor, since the patients may have a complex multidisciplinary medical and/or surgical background or they are admitted for the first time with this condition; thus, the convolute puzzle will start from this newly detected sternal lump.
Author Contributions
Conceptualization, M.C., D.T., F.V., A.-P.C., A.C. and C.N.; methodology, M.C., D.T., F.V., A.-P.C., A.C. and C.N.; software, M.C., F.V., A.-P.C., A.C. and C.N.; validation, M.C., A.-P.C., A.C. and C.N.; formal analysis, M.C., D.T., F.V., A.C. and C.N.; investigation, M.C. and C.N.; resources, M.C., D.T., F.V., A.-P.C., A.C. and C.N.; data curation, M.C., F.V., A.-P.C., A.C. and C.N.; writing—original draft preparation, M.C. and A.-P.C.; writing—review and editing, M.C., D.T., F.V., A.-P.C., A.C. and C.N.; visualization, M.C., D.T., F.V., A.-P.C., A.C. and C.N.; supervision, M.C., A.-P.C., A.C. and C.N.; project administration, M.C. and C.N.; funding acquisition, M.C., D.T., F.V., A.-P.C., A.C. and C.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Dr. Carol Davila Central Military Emergency University Hospital, Bucharest, Romania (protocol code 607/06/28/2023).
Informed Consent Statement
Informed consent was obtained from the patient at the time of admission; the presented data were retrospectively collected.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge both the endocrine and thoracic surgical teams.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CT | Computed tomography |
| CI | Confidence interval |
| 3D | three-dimensional |
| EBRT | external beam radiotherapy |
| 18F-FDG | 18-Fluor-2-desoxi-D-Glucose |
| HR | Hazard ratio |
| G-CSF | Granulocyte colony-stimulating factor |
| MRI | Magnetic resonance imaging |
| MR-HIFU | Magnetic resonance-guided high-intensity focused ultrasound |
| PET/CT | Positron emission tomography/computed tomography |
| PRRT | Peptide receptor radionuclide therapy |
| PSMA | Prostate-specific membrane antigen |
| SPECT/CT | Single-photon emission computed tomography/computed tomography |
References
- Asanuma, K.; Tsujii, M.; Hagi, T.; Nakamura, T.; Kita, K.; Shimamoto, A.; Kataoka, T.; Takao, M.; Sudo, A. Full-thickness chest wall resection for malignant chest wall tumors and postoperative problems. Front. Oncol. 2023, 13, 1104536. [Google Scholar] [CrossRef] [PubMed]
- Aramini, B.; Masciale, V.; Radaelli, L.F.Z.; Sgarzani, R.; Dominici, M.; Stella, F. The sternum reconstruction: Present and future perspectives. Front. Oncol. 2022, 12, 975603. [Google Scholar] [CrossRef] [PubMed]
- Foroulis, C.N.; Kleontas, A.D.; Tagarakis, G.; Nana, C.; Alexiou, I.; Grosomanidis, V.; Tossios, P.; Papadaki, E.; Kioumis, I.; Baka, S.; et al. Massive chest wall resection and reconstruction for malignant disease. OncoTargets Ther. 2016, 9, 2349–2358. [Google Scholar] [CrossRef][Green Version]
- Gonfiotti, A.; Salvicchi, A.; Voltolini, L. Chest-Wall Tumors and Surgical Techniques: State-of-the-Art and Our Institutional Experience. J. Clin. Med. 2022, 11, 5516. [Google Scholar] [CrossRef] [PubMed]
- Bukharov, A.; Derzhavin, V.; Yadrina, A.; Erin, D.; Elkhov, D.; Aliev, M.D. Surgical treatment of patients with chest wall metastases. Khirurgiia 2022, 8, 25–30. [Google Scholar] [CrossRef]
- Wang, L.; Yan, X.; Zhao, J.; Chen, C.; Chen, C.; Chen, J.; Chen, K.-N.; Cao, T.; Chen, M.-W.; Duan, H.; et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl. Lung Cancer Res. 2021, 10, 4057–4083. [Google Scholar] [CrossRef] [PubMed]
- Duranti, L.; Tavecchio, L. New perspectives in prosthetic reconstruction in chest wall resection. Updates Surg. 2023, 75, 1093–1102. [Google Scholar] [CrossRef]
- Kader, S.; Watkins, A.; Servais, E.L. The oncologic efficacy of extended thoracic resections. J. Surg. Oncol. 2023, 127, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Gritsiuta, A.I.; Bracken, A.; Abbas, A.E.; Petrov, R.V. Complex anterior chest wall reconstruction after extensive oncologic resections: A narrative review. Shanghai Chest 2021, 5, 41. [Google Scholar] [CrossRef]
- Salo, J.T.K.; Tukiainen, E.J. Oncologic Resection and Reconstruction of the Chest Wall: A 19-Year Experience in a Single Center. Plast. Reconstr. Surg. 2018, 142, 536–547. [Google Scholar] [CrossRef]
- Čerškutė, M.; Kinčius, M.; Januškevičius, T.; Cicėnas, S.; Ulys, A. Sternal resection of a solitary renal cell carcinoma metastasis: A case report and a literature review. Acta Med. Litu. 2019, 25, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, B.; Morandi, U.; De Santis, G.; Catani, F.; Stefani, A.; Pinelli, M.; Baccarani, A.; Starnoni, M.; Artioli, F.; Aramini, B. Can surgery relieve pain and act as first-line treatment for a large metastasis of the sternum? Int. J. Surg. Case Rep. 2018, 63, 125–128. [Google Scholar] [CrossRef]
- Oshima, K.; Kikumori, K.; Yanagawa, T.; Egawa, C.; Takatsuka, Y.; Shinke, G.; Katsuyama, S.; Kawai, K.; Hiraki, M.; Katsura, Y.; et al. A Case of Late Recurrence of Breast Cancer with Chest Wall Recurrence 43 Years after Surgery. Gan Kagaku Ryoho Cancer Chemother. 2021, 48, 1846–1848. [Google Scholar]
- Yao, G.; Huang, J.; Zhang, Q.; Hu, D.; Yuan, F.; Han, G. Excellent response of refractory triple-negative breast cancer to sintilimab plus chemotherapy: A case report. Immunotherapy 2023, 15, 221–228. [Google Scholar] [CrossRef]
- Rebegea, L.; Ilie, A.M.; Paslaru, A.M.; Firescu, D.; Serban, C.; Voinescu, C.; Sapira, V.; Stoleriu, G.; Lungu, M.; Zgura, A.; et al. Management of Epicardial Metastasis in Breast Cancer. Chirurgia 2021, 116, 627–633. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tashima, T.; Nagata, K.; Sakuramoto, S.; Osaki, A.; Ryozawa, S. Colorectal and gastric metastases from lobular breast cancer that resembled superficial neoplastic lesions. Clin. J. Gastroenterol. 2021, 14, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Guo, Y.; Jiang, X.; Li, K.; Fu, W.; Cao, Y. Concomitant fulvestrant with reirradiation for unresectable locoregional recurrent estrogen receptor positive (ER+) breast cancer: A case report and narrative review. Medicine 2020, 99, e21344. [Google Scholar] [CrossRef]
- Mohamed, S.; Mazhar, K.; Osman, A.; Patel, A.; Srinivasan, L.; Ghosh, S. Excision of metastatic breast cancer from sternum and reconstruction in two patients with solitary metastatic spread. J. Surg. Case Rep. 2020, 2020, rjaa272. [Google Scholar] [CrossRef] [PubMed]
- Ensle, F.; Schaab, J.A.; Dimitriou, F.; Huellner, M.W.; Maurer, A. Internal Thoracic Vein Tumor Thrombus From Sternal Melanoma Metastasis on 18F-FDG PET/CT. Clin. Nucl. Med. 2023, 48, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Gupta, S.; Dubey, V.; Verma, S.; Ali, T.; Palod, S.; Lodhi, A. Incidence of bone metastasis in head and neck malignancy: A retrospective study. J. Cancer Res. Ther. 2022, 18, S210–S214. [Google Scholar] [CrossRef]
- Vincent, A.G.; Wang, W.; Shokri, T.; Ducic, Y. Treatment of Oligometastatic Disease in Squamous Cell Carcinoma of the Head and Neck. Laryngoscope 2020, 131, E1476–E1480. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ding, L.; Zheng, Y.; Wu, C.; Wang, K.; Xia, W.; Ge, P. Development and validation of a novel risk model in newly diagnosed de novo bone metastatic prostate cancer (M1b): A retrospective study. PeerJ 2023, 11, e14615. [Google Scholar] [CrossRef]
- Sasaki, E.; Yamagata, K.; Hagiwara, T.; Takasaki, R.; Fukuzawa, S.; Uchida, F.; Ishibashi-Kanno, N.; Bukawa, H. A Case of Primary Intraosseous Adenoid Cystic Carcinoma of the Mandible. Case Rep. Dent. 2023, 2023, 2422086. [Google Scholar] [CrossRef]
- Strong, M.J.; Koduri, S.; Allison, J.A.; Pesavento, C.M.; Ogunsola, S.; Ogunsola, O.; Yee, T.J.; Khalsa, S.S.S.; Saadeh, Y.S.; Joseph, J.R.; et al. Bone metastasis from glioblastoma: A systematic review. J. Neuro-Oncol. 2022, 158, 379–392. [Google Scholar] [CrossRef]
- Ramahi, A.; Singh, B.; Chan, K.H.; Kaur, P.; Guron, G.; Shaaban, H. Diffuse bone metastasis from cholangiocarcinoma involving the sternum: A case report and review of literature. Int. J. Crit. Illn. Inj. Sci. 2021, 11, 43–46. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Lee, J.; Jeong, J.Y. Radical resection and reconstruction of the sternum for metastasis of hepatocellular carcinoma. J. Cardiothorac. Surg. 2020, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Son, H.Y.; An, S.-Y.; Kim, E.Y.; Ahn, S.B.; Lee, B.C. Selective embolization for hypervascular metastasis from differentiated thyroid cancer: A case series. J. Med. Case Rep. 2014, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- El Haj, N.I.; Hafidi, S.; Karam, R.; Boubia, S.; Karkouri, M.; Ridai, M. Sternal metastasis as first manifestation of a papillary thyroid carcinoma: A case report. Int. J. Surg. Case Rep. 2021, 80, 105663. [Google Scholar] [CrossRef]
- Osorio, M.; Moubayed, S.P.; Su, H.; Urken, M.L. Systematic review of site distribution of bone metastases in differentiated thyroid cancer. Head Neck 2017, 39, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, S.; Pinillos, G.M.; Chaves-Conde, M. Lenvatininb as Treatment for Naïve Patients with Aggressive Thyroid Cancer Bone Metastases and Bad Performance Status. Case Rep. Oncol. Med. 2020, 2020, 8679149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Youn, I. Metastasis of Poorly Differentiated Thyroid Carcinoma to the Sternum: A Case Report. J. Korean Soc. Radiol. 2020, 81, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Paspala, A.; Papakonstantinou, D.; Pikoulis, E.; Tomos, P.; Nastos, C. Synchronous Sternal Metastasectomy and Total Thyroidectomy for Differentiated Thyroid Cancer: A Rare Case Report. Cureus 2022, 14, e31294. [Google Scholar] [CrossRef] [PubMed]
- Suwardjo, S.; Avanti, W.S.; Dwianingsih, E.K.; Harahap, W.A.; Anwar, S.L. Complete diaphysis resorption of the femur: A case report in a metastatic papillary thyroid cancer. Ann. Med. Surg. 2020, 60, 614–618. [Google Scholar] [CrossRef]
- Lan, H.-J.; Wu, Z.-Q.; Gong, D.-G.; Zheng, W.-Y.; Jin, Y. Partial resection and reconstruction of the sternum for treatment of metachronous sternal metastasis of thyroid carcinoma: A case report. Medicine 2017, 96, e8786. [Google Scholar] [CrossRef]
- Syazni, M.A.; Gendeh, H.S.; Kosai, N.R.; Ramzisham, A.R.; Gendeh, B.S.; Basiron, N.H.; Imran, F.-H. Follicular thyroid cancer with sternal metastasis-challenges and outcomes. Med. J. Malays. 2017, 72, 80–82. [Google Scholar]
- Moritani, S.; Takenobu, M.; Yasunaga, M.; Kawamoto, K.; Fujii, T.; Ishida, Y.; Kitano, H. Surgical indications for upper mediastinal dissection by sternotomy in patients with papillary thyroid carcinoma. Endocr. J. 2022, 69, 1245–1251. [Google Scholar] [CrossRef]
- Pradeep, S.; Hedne, N.; Vidhyadharan, S.; Rajiv, S. Sternal metastatectomy in a case of papillary thyroid carcinoma. BMJ Case Rep. 2020, 13, e235967. [Google Scholar] [CrossRef]
- Candanedo-Gonzalez, F.; Rodriguez-Orihuela, D.; Arista-Nasr, J. Macrofollicular variant of papillary thyroid carcinoma with metastasis to femur. Thyroid. Res. 2020, 13, 10. [Google Scholar] [CrossRef]
- Syed, A.; Vanka, S.A.; Escudero, I.; Ismail, R.; Krayem, H. Oncocytic Cell Carcinoma of the Thyroid: A Case Report and an Overview of the Diagnosis, Treatment Modalities, and Prognosis. Cureus 2022, 14, e30298. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.J.; Jiang, X.S. Top Ten Oncocytic Head and Neck Lesions to Contemplate. Head Neck Pathol. 2023, 17, 53–65. [Google Scholar] [CrossRef]
- Loharkar, S.; Basu, S. Hurthle Cell Thyroid Carcinoma with Liver and Paraaortic Abdominal Nodal Metastasis: Progression on Sorafenib Therapy after Initial Disease Stabilization. World J. Nucl. Med. 2022, 22, 70–74. [Google Scholar] [CrossRef]
- Klonaris, D.; Kefalogianni, T.; Karakostas, E.; Mastorakis, G.; Lagoudianakis, G. Cutaneous thyroid carcinoma sixteen years after benign total thyroidectomy: A unique case. Hippokratia 2020, 24, 88–90. [Google Scholar]
- Ataei-Nakhaei, S.; Aryana, K.; Mostafavi, S.M.; Kosari, H.M.; Esmatinia, M.; Aghaee, A. Peptide receptor radionuclide therapy (PRRT) in radioiodine-refractory thyroid cancer: A case report of significant response to lu177 DOTA-TATE treatment. Arch. Endocrinol. Metab. 2022, 66, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gubbi, S.; Koch, C.A.; Klubo-Gwiezdzinska, J. Peptide Receptor Radionuclide Therapy in Thyroid Cancer. Front. Endocrinol. 2022, 13, 896287. [Google Scholar] [CrossRef]
- Dadgar, H.; Jafari, E.; Ahmadzadehfar, H.; Rekabpour, S.J.; Ravanbod, M.R.; Kalantarhormozi, M.; Nabipour, I.; Assadi, M. Feasibility and therapeutic potential of the 68Ga/177Lu-DOTATATE theranostic pair in patients with metastatic medullary thyroid carcinoma. Ann. Endocrinol. 2023, 84, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fung, R.; Fasen, M.; Warda, F.; Natter, P.; Nedrud, S.; Fernandes, R.; Alkhasawneh, A.; Gandhi, G.Y. Clavicular Metastasis as an Initial Presentation of Papillary Thyroid Cancer. Case Rep. Endocrinol. 2021, 2021, 6662071. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, P.; Shi, R. Anlotinib as a molecular targeted therapy for tumors (Review). Oncol. Lett. 2020, 20, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Cai, S.-J.; Liang, B.-Y.; Yan, S.-Y.; Wang, B.; Li, M.-Y.; Zhao, W.-X. Efficacy of anlotinib combined with radioiodine to treat scalp metastasis of papillary thyroid cancer: A case report and review of literature. World J. Clin. Cases 2023, 11, 2839–2847. [Google Scholar] [CrossRef]
- Su, Y.-J.; Cheng, S.-H.; Qian, J.; Zhang, M.; Liu, W.; Zhan, X.-X.; Wang, Z.-Q.; Liu, H.-D.; Zhong, X.-W.; Cheng, R.-C. Neoadjuvant therapy with anlotinib in a locally advanced and pulmonary metastasis PTC patient harboring TERT promoter and BRAFV600E mutations: A case report. Arq. Bras. Endocrinol. Metabol. 2023, 67, e000659. [Google Scholar] [CrossRef]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Abreu, M.H.; Santos, M.S.; Duarte, H.; Alpoim, T.; Próspero, I.; Sousa, S.; Abreu, P.H. Bone Metastases Detection in Patients with Breast Cancer: Does Bone Scintigraphy Add Information to PET/CT? Oncologist 2023, 28, e600–e605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.M.; Dong, A.; Zuo, C. Prostate-Specific Membrane Antigen–Avid Bone Metastases From Urothelial Carcinoma of the Bladder. Clin. Nucl. Med. 2022, 47, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Han, D.; Zhang, X.; Lv, Z.; Li, D. Differential performances in lesions and radio-tracer of 18F-FDG PET/CT between multiple myeloma and unknown osteolytic metastasis. Curr. Med. Imaging 2023, 19, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Hung, V.; Everaert, H.; Gorobets, O.; Van Parijs, H.; Verfaillie, G.; Vanhoeij, M.; Storme, G.; Fontaine, C.; Lamote, J.; Perrin, J.; et al. Breast cancer preoperative 18FDG-PET, overall survival prognostic separation compared with the lymph node ratio. Breast Cancer 2021, 28, 956–968. [Google Scholar] [CrossRef]
- Can, C.; Gündoğan, C.; Kömek, H. Is 68Ga–Prostate-Specific Membrane Antigen PET/CT Superior than 18F-FDG PET/CT for Evaluation of Metastatic Osteosarcoma? Clin. Nucl. Med. 2021, 46, e233–e235. [Google Scholar] [CrossRef]
- Kitajima, K.; Tsuchitani, T.; Takahashi, Y.; Minami, T.; Yokoi, T.; Nakamura, A.; Hashimoto, M.; Kuribayashi, K.; Kijima, T.; Hasegawa, S.; et al. Usefulness of Quantitative Bone Single-Photon Emission Computed Tomography/Computed Tomography for Evaluating the Treatment Response of Bone Metastasis in a Lung Cancer Patient. Case Rep. Oncol. 2021, 14, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.; Boicean, L.C.; Popa, F.L. The role of combined techniques of scintigraphy and SPECT/CT in the diagnosis of primary hyperparathyroidism: A case report. Medicine 2019, 98, e14154. [Google Scholar] [CrossRef]
- Carsote, M.; Paduraru, D.N.; Nica, A.E.; Valea, A. Parathyroidectomy: Is vitamin D a player for a good outcome? J. Med. Life 2016, 9, 348–352. [Google Scholar]
- Chen, X.; Lei, L.; Tian, C.; Ning, P. Invasive pleomorphic lobular carcinoma of the breast with multiple metastases: A case report. Int. J. Surg. Case Rep. 2021, 80, 105581. [Google Scholar] [CrossRef]
- Dergel, M.; Balik, M.; Pacovsky, J.; Vobornik, M.; Mandak, J.; Laco, J. Solitary metastasis of clear cell renal cell carcinoma in sternum diagnosed unexpectedly during cardiac surgery—A rare but potentially fatal trap. Urol. Case Rep. 2021, 38, 101730. [Google Scholar] [CrossRef]
- Young, J.S.; McAllister, M.; Marshall, M.B. Three-dimensional technologies in chest wall resection and reconstruction. J. Surg. Oncol. 2023, 127, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.A.S.; Lau, R.W.H.; Yu, P.S.Y.; Siu, I.C.H.; Chan, J.W.Y.; Ng, C.S.H. Use of custom made 3-dimensional printed surgical guide for manubrio-sternal resection of solitary breast cancer metastasis: Case report. AME Case Rep. 2020, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, I.; Dovgalski, L.; Evans, P.L. 3D Printing Technology for Chest Wall Reconstruction With a Sternum-Ribs-Cartilage Titanium Implant: From Ideation to Creation. Innovations 2023, 18, 67–72. [Google Scholar] [CrossRef]
- Clermidy, H.; Fadel, G.; De Lemos, A.; Pradere, P.; Mitilian, D.; Girault, A.; Menager, J.-B.; Fabre, D.; Mussot, S.; Leymarie, N.; et al. Long-term outcomes after chest wall resection and repair with titanium bars and sternal plates. Front. Surg. 2022, 9, 950177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-H.; Gao, J.; Zhang, Y.; Wang, H.; Tan, L.-J.; Ding, J.-Y. Modified Subxiphoid Thoracoscopic Thymectomy for Locally Invasive Thymoma. Ann. Thorac. Surg. 2020, 112, 1095–1100. [Google Scholar] [CrossRef]
- Huang, C.; Sun, Y.-G.; Wu, Q.-J.; Ma, C.; Jiao, P.; Wang, Y.-Z.; Huang, W.; Tian, W.-X.; Yu, H.-B.; Li, D.-H.; et al. Surgical treatment of intermediate to high grade thymic neuroendocrine neoplasms: Case series of five patients and literature review. Transl. Cancer Res. 2022, 11, 3535–3547. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Cvasciuc, T.I.; Simpson, D.; de Jong, M.C.; Parameswaran, R. Continuing challenges of primary neuroendocrine tumours of the thymus: A concise review. Eur. J. Surg. Oncol. 2022, 48, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Polymeris, A.; Kogia, C.; Kazakou, P.; Psachna, S.; Lilis, D.; Drakou, M.; Michalakis, K.; Ioannidis, D. A rare case of poorly differentiated mixed neuroendocrine-nonneuroendocrine tumor of the caecum with long term survival: A case report. Endocr. Regul. 2022, 56, 249–253. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, K.-H. Percutaneous osteoplasty for painful bony lesions: A technical survey. Korean J. Pain 2021, 34, 375–393. [Google Scholar] [CrossRef]
- Papalexis, N.; Peta, G.; Vara, G.; Spinnato, P.; Errani, C.; Martella, C.; Miceli, M.; Facchini, G. Palliative Arterial Embolization for Metastases of the Sternum. Cardiovasc. Interv. Radiol. 2023, 46, 794–798. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Suzuki, M.; Yamaguchi, H.; Seto, I.; Machida, M.; Takagawa, Y.; Jingu, K.; Kikuchi, Y.; Murakami, M. Successful treatment with proton beam therapy for a solitary sternal metastasis of breast cancer: A case report. J. Med. Case Rep. 2022, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Depauw, N.; Zieminski, S.; Jimenez, R. Proton Radiotherapy for Patients With Oligometastatic Breast Cancer Involving the Sternum. Int. J. Part. Ther. 2021, 8, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, M.P.; Kelly, D.; Tan, J.; Siva, S.; Kron, T.; David, S. Single-fraction stereotactic ablative body radiotherapy for sternal metastases in oligometastatic breast cancer: Technique and single institution experience. J. Med. Imaging Radiat. Oncol. 2020, 64, 580–585. [Google Scholar] [CrossRef]
- Chan, M.; Olson, R.; Lefresne, S.; McKenzie, M. Advanced Radiation Therapy Technology Use in the Treatment of Bone Metastases in a Public, Salary-Funded, Non-Incentivized Health Care System. JCO Oncol. Pract. 2021, 17, e178–e185. [Google Scholar] [CrossRef] [PubMed]
- Bartels, M.M.; Verpalen, I.M.; Ferrer, C.J.; Slotman, D.J.; Phernambucq, E.C.; Verhoeff, J.J.; Eppinga, W.S.; Braat, M.N.; van den Hoed, R.D.; van't Veer-Ten Kate, M.; et al. Combining radiotherapy and focused ultrasound for pain palliation of cancer induced bone pain; a stage I/IIa study according to the IDEAL framework. Clin. Transl. Radiat. Oncol. 2021, 27, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, I.M.; Kouloulias, V.; I Koukourakis, M. Radio-Immunotherapy: A Case Report of ‘Abscopal Hyper-Progression’? Cureus 2020, 12, e10117. [Google Scholar] [CrossRef]
- Yue, L.; Wentao, L.; Xin, Z.; Jingjing, H.; Xiaoyan, Z.; Na, F.; Tonghui, M.; Dalin, L. Human epidermal growth factor receptor 2-positive metastatic breast cancer with novel epidermal growth factor receptor -ZNF880 fusion and epidermal growth factor receptor E114K mutations effectively treated with pyrotinib: A case report. Medicine 2020, 99, e23406. [Google Scholar] [CrossRef]
- Iijima, H.; Ebisumoto, K.; Sakai, A.; Maki, D.; Teramura, T.; Yamauchi, M.; Ogura, G.; Nakamura, N.; Okami, K. A p16 Positive M1 Oral Tongue Cancer Completely Responsive to the EXTREME Regimen: A Case Report. Tokai J. Exp. Clin. Med. 2021, 46, 97–100. [Google Scholar] [PubMed]
- Jalil, R.A.; Abdallah, F.A. Multiple local recurrences of primary sternal chondrosarcoma: Tumor manipulation or self-seeding. J. Cardiothorac. Surg. 2023, 18, 114. [Google Scholar] [CrossRef]
- Laitinen, M.K.; Kask, G.; Laurila, K.; Tukiainen, E.J.; Rönty, M.; Haapamäki, V.; Salo, J.T. Chondrosarcoma of the Chest Wall: A Single Institution Review of 50 Cases. Ann. Plast. Surg. 2023, 90, 151–155. [Google Scholar] [CrossRef]
- Lo, E.Y.W.; Lo, C.; Hing, A.; Langbart, M.; French, B. Case report: Management of infiltrative basosquamous carcinoma of the sternum. Int. J. Surg. Case Rep. 2023, 106, 108107. [Google Scholar] [CrossRef]
- Abdulfatah, E.; Rottmann, D.; Morag, Y.; Pantanowitz, L.; Udager, A.M.; Hao, W.; Lucas, D.R. Conventional chondrosarcoma of the rib cage and sternum: Clinicopathological and molecular analysis of 27 patients treated at a single institution. Hum. Pathol. 2023, 136, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Patil, A.; James, T.; Kumar, N.; Premalata, C.S. Multifocal Osteosarcoma: Multiple Primaries or Metastases? A Report of Rare Case and Review of Literature. J. Orthop. Case Rep. 2020, 10, 97–100. [Google Scholar] [PubMed]
- Lenze, U.; Angelini, A.; Pohlig, F.; Knebel, C.; Trovarelli, G.; Berizzi, A.; Mavrogenis, A.F.; Theisen, J.; VON Eisenhart-Rothe, R.; Ruggieri, P. Chondrosarcoma of the Chest Wall: A Review of 53 Cases from Two Institutions. Anticancer. Res. 2020, 40, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, R.; Singh, P. Coughing up: “Adenosquamous carcinoma lung with unusual initial presentation as an ulceroproliferative growth”-case report and review of literature. J. Cancer Res. Ther. 2020, 16, 922–925. [Google Scholar] [CrossRef]
- Jauhary, T.; Hayati, F. Unusual sites of tuberculosis mimicking skeletal metastases: A case report. Radiol. Case Rep. 2022, 17, 1931–1937. [Google Scholar] [CrossRef]
- Engin, G.; Yirgin, K.; Kandemir, H. Extrapulmonary Tuberculosis Mimics Diffuse Metastatic Disease: A Case Report. Indian J. Radiol. Imaging 2021, 31, 1039–1042. [Google Scholar] [CrossRef]
- Karaman, A.; Araz, Ö.; Koru, A. Metastases of Alveolar Echinococcosis to the Skin, Pleura, Ribs, and the Xiphoid Process: A Case Report. Arch. Bronconeumol. 2021, 57, 228. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chi, J.; Bai, Y. A case report of dermatomyositis with the missed diagnosis of non-small cell lung cancer and concurrence of pulmonary tuberculosis. Open Med. 2022, 17, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Price, J. The sternum: An important review area when reporting pre-surgical staging breast MRI studies. J. Med. Imaging Radiat. Oncol. 2022, 66, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, K.; Ikeda, T.; Araki, K.; Hamada, M. A Case of Advanced Late Recurrence of Hormone Receptor Positive Breast Cancer Successfully Treated with Abemaciclib and Anastrozole. Gan Kagaku Ryoho Cancer Chemother. 2021, 48, 1259–1263. [Google Scholar]
- Rathod, S.; Wagh, D.; Niveditha, S. A case of soft-tissue tumor over the sternum: A rare case report. J. Cancer Res. Ther. 2022, 18, S481–S485. [Google Scholar] [CrossRef]
- Silva, J.S.; Torres, C.; Teixeira, J.A.; Garcia, P.; Calvinho, P. Manubrium resection and reconstruction for mediastinal tumor: A case report. Rev. Port. Cir. Cardio-Torac. Vasc. 2018, 25, 87–89. [Google Scholar]
- Aoki, T.; Ozeki, Y.; Watanabe, M.; Tanaka, S.; Isaki, H.; Terahata, S. Development of primary leiomyosarcoma of the sternum postirradiation: Report of a case. Surg. Today 1998, 28, 1326–1328. [Google Scholar] [CrossRef]
- Hayashi, K.; Fujioka, T.; Hara, M.; Kumaki, Y.; Oda, G.; Yamaga, E.; Mori, M.; Ohnishi, I.; Kubota, K.; Nakagawa, T. Focal Uptake in the Sternum on 18F-FDG-PET/CT Caused by G-CSF Therapy after Chemotherapy Mimicking Bone Metastasis of Breast Cancer. Diagnostics 2022, 12, 2308. [Google Scholar] [CrossRef]
- Fardanesh, R.; Beavers, K.; Jochelson, M.S.; Ulaner, G.A. Value of subspecialist second opinion reads of 18F-FDG PET-CT examinations for patients with breast cancer. Nucl. Med. Commun. 2023, 44, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, F.; Zhou, Z.; Cui, Y.; Du, K.; Li, S.; Liu, T.; Chen, Y.; Li, Y.; Gao, F. CT-guided trans-sternal 125I seeds implantation for masses in the anterior or middle mediastinum. J. Contemp. Brachyther. 2022, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Matsuo, T.; Hirai, S.; Katayama, T.; Nishisaka, T.; Tominaga, A.; Shinbo, K.; Mochizuki, Y.; Nishida, K.; Nakamura, M.; et al. Sternal Metastasis of Brain Meningioma—A Case Report. Anticancer. Res. 2021, 41, 4039–4043. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Tanaka, T.; Shindo, T.; Hashimoto, K.; Fukuta, F.; Kobayashi, K.; Sugawara, T.; Hasegawa, T.; Masumori, N. Two cases of osteoblastic bone metastasis from muscle-invasive bladder cancer with discrepancy in response to chemotherapy: Problems and limitations of bone biopsy. Int. Cancer Conf. J. 2020, 9, 235–239. [Google Scholar] [CrossRef]
- Irzi, M.; Mhanna, T.; Aynaou, M.; Ouraghi, A.; Alaoui, M.W.; Barki, A. Renal cell carcinoma revealed by sternal tumefaction: A rare case report and literature review. Urol. Case Rep. 2020, 31, 101137. [Google Scholar] [CrossRef]
- Padhi, P.; Topalovski, M. Benign Metastasizing Leiomyoma of the Uterus with Pulmonary and Bone Metastases. Case Rep. Obstet. Gynecol. 2021, 2021, 5536675. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Sato, S.; Koyanagi, H.; Kinowaki, Y. Malignant primary pulmonary meningioma with bone metastasis. Oxf. Med. Case Rep. 2020, 2020, omaa005. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.-E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients-Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, S.; Hioki, M.; Hisayoshi, T.; Yamashita, K.; Yamashita, Y.; Kawamura, J.; Hirata, T.; Yamagishi, S.; Koizumi, K.; Shimizu, K. Resection of Sternal Tumors and Reconstruction of the Thorax: A Review of 15 Patients. Surg. Today 2006, 36, 225–229. [Google Scholar] [CrossRef]
- Bains, L.; Bhatia, S.; Kaushik, R.; Jain, S.K.; Singh, C.B.; Mandal, S.; Kaur, D. Pre-sternal thyroid swellings: A case of rare aberrant site recurrence and review of literature. Thyroid. Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Aprile, V.; Korasidis, S.; Crisci, R.; Ambrogi, M.C. Chest wall reconstruction with a novel titanium mesh after partial sternectomy for chondrosarcoma. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 149–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).