Prostate MRI and PSMA-PET in the Primary Diagnosis of Prostate Cancer
Abstract
:1. Introduction
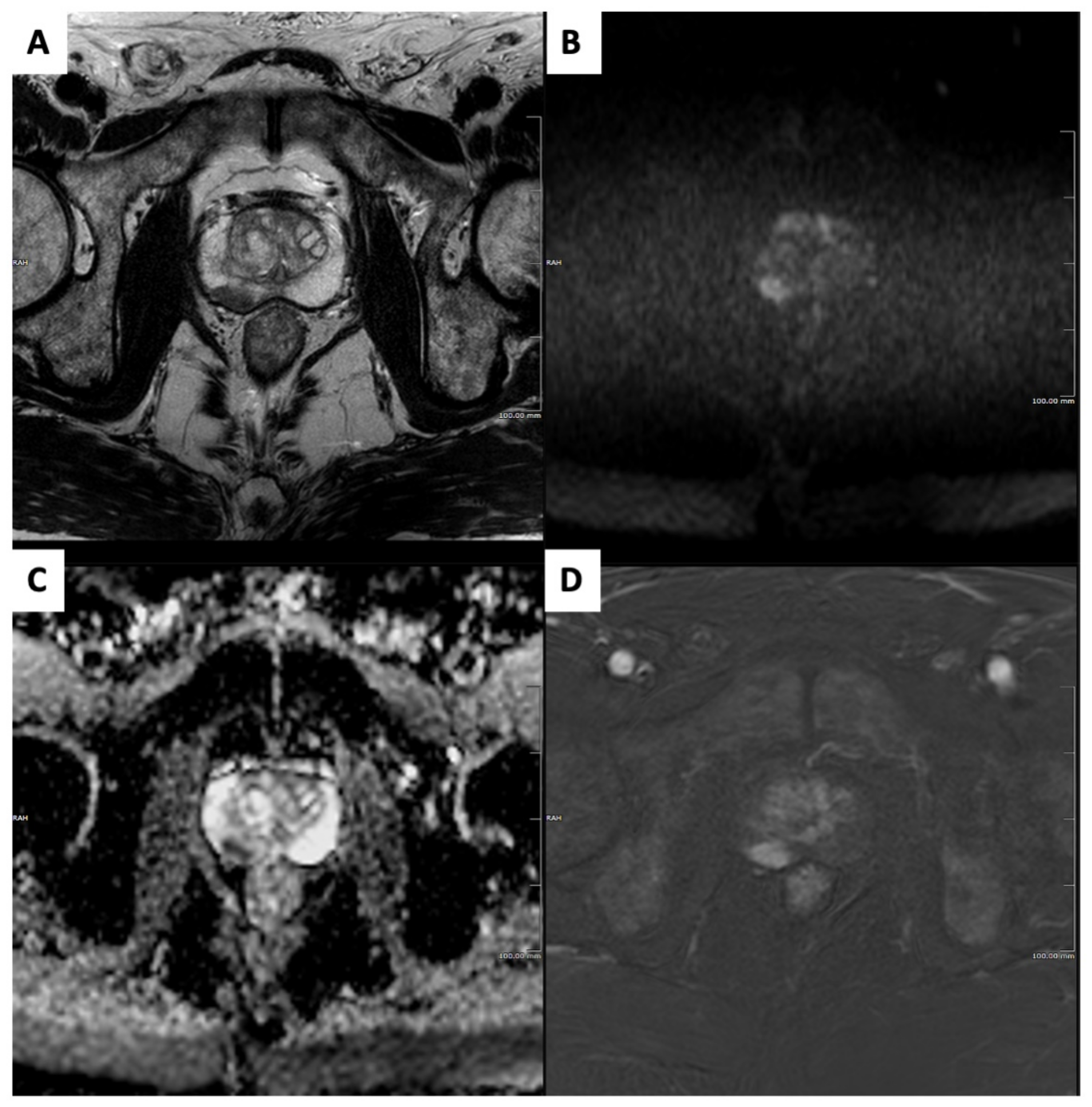
2. The Role of MRI
2.1. Diagnostic Accuracy
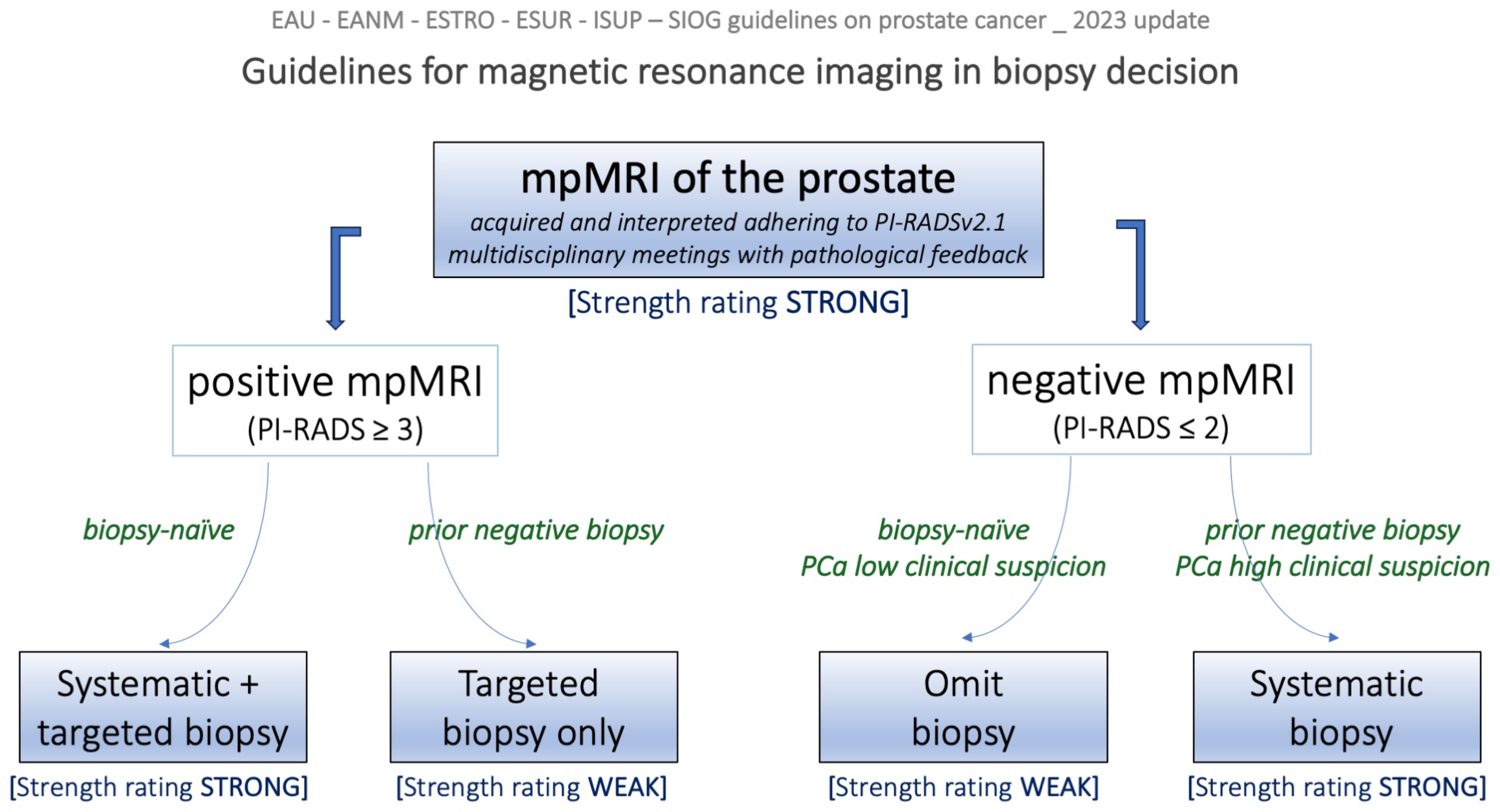
2.2. EAU Guidelines
2.3. Open Questions
3. Role of PSMA-PET
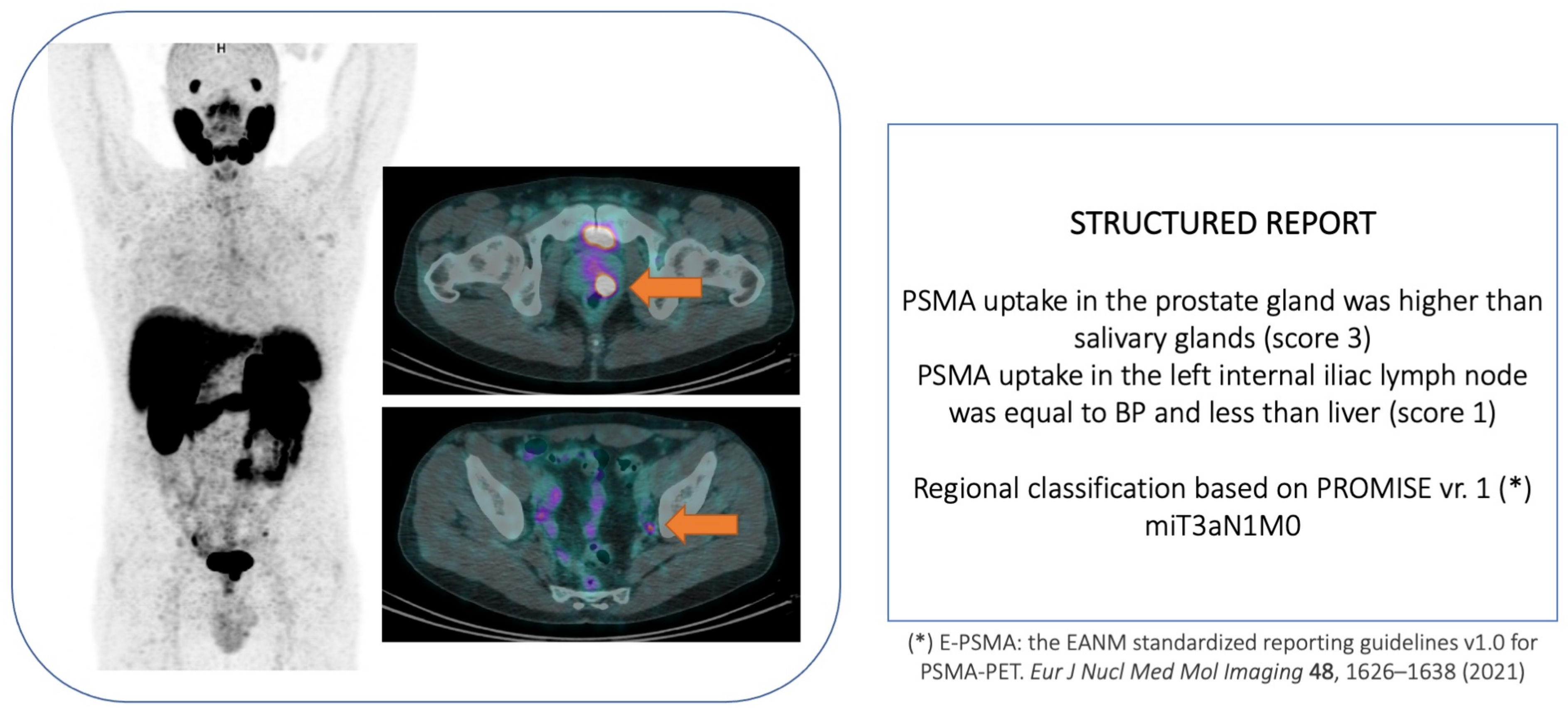
3.1. Primary Staging
3.2. Primary Diagnosis
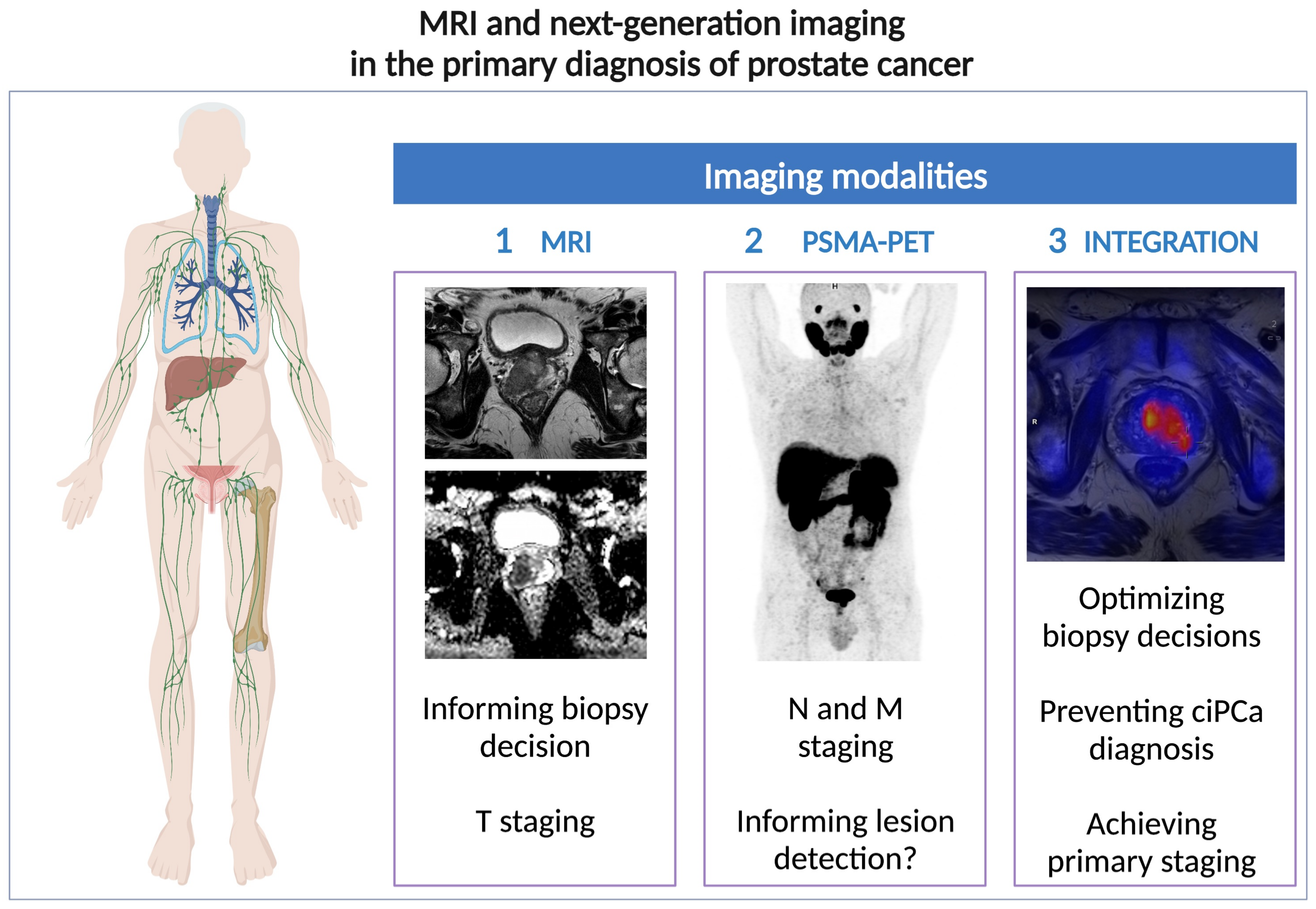
4. Pathways of Interaction between MRI and PSMA-PET
4.1. Pathway 1: “MRI vs. PET/CT”
4.2. Pathway 2: “PET/CT Following MRI”
4.3. Pathway 3: “MRI vs. PET/MRI”
4.4. Pathway 4: “PET/MRI Following MRI”
4.5. Pathway 5: “Prostate MRI within PET/MRI”
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Preisser, F.; Nazzani, S.; Tian, Z.; Bandini, M.; Gandaglia, G.; Fossati, N.; Montorsi, F.; Graefen, M.; Shariat, S.F.; et al. The Effect of Lymph Node Dissection in Metastatic Prostate Cancer Patients Treated with Radical Prostatectomy: A Contemporary Analysis of Survival and Early Postoperative Outcomes. Eur. Urol. Oncol. 2019, 5, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Russo, G.; Di Zazzo, E.; Pisapia, P.; Mirto, B.F.; Palmieri, A.; Pepe, F.; Bellevicine, C.; Russo, A.; La Civita, E.; et al. Liquid Biopsy in Prostate Cancer Management—Current Challenges and Future Perspectives. Cancers 2022, 14, 3272. [Google Scholar] [CrossRef]
- Coakley, F.V.; Oto, A.; Alexander, L.F.; Allen, B.C.; Davis, B.J.; Froemming, A.T.; Fulgham, P.F.; Hosseinzadeh, K.; Porter, C.; Sahni, V.A.; et al. ACR Appropriateness Criteria ® Prostate Cancer—Pretreatment Detection, Surveillance, and Staging. J. Am. Coll. Radiol. 2017, 14, S245–S257. [Google Scholar] [CrossRef]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 697–713. [Google Scholar] [CrossRef]
- Girometti, R.; Giannarini, G.; Greco, F.; Isola, M.; Cereser, L.; Como, G.; Sioletic, S.; Pizzolitto, S.; Crestani, A.; Ficarra, V.; et al. Interreader agreement of PI-RADS v. 2 in assessing prostate cancer with multiparametric MRI: A study using whole-mount histology as the standard of reference: Assessing Prostate Cancer With mpMRI. J. Magn. Reson. Imaging 2019, 49, 546–555. [Google Scholar] [CrossRef]
- De Rooij, M.; Israël, B.; Tummers, M.; Ahmed, H.U.; Barrett, T.; Giganti, F.; Hamm, B.; Løgager, V.; Padhani, A.; Panebianco, V.; et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: Quality requirements for image acquisition, interpretation and radiologists’ training. Eur. Radiol. 2020, 30, 5404–5416. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Wieler, H.J.; Baues, C.; Kuntz, N.J.; Richardsen, I.; Schreckenberger, M. The Impact of 68Ga-PSMA PET/CT and PET/MRI on the Management of Prostate Cancer. Urology 2019, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.B.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. JCO 2020, 38, 1963–1996. [Google Scholar] [CrossRef] [PubMed]
- Hagens, M.J.; Van Leeuwen, P.J. A Future Prebiopsy Imaging-guided Pathway to Safely Omit Systematic Biopsies and Prevent Diagnosis of Indolent Prostate Cancer. Eur. Urol. 2021, 80, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Morton, A.; Yaxley, J.; Ranasinghe, S.; Teloken, P.E.; Kyle, S.; Coughlin, G.; Esler, R.; Dunglison, N.; Gardiner, R.A.; et al. 68Ga-PSMA PET/CT better characterises localised prostate cancer after MRI and transperineal prostate biopsy: Is 68Ga-PSMA PET/CT guided biopsy the future? Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1843–1851. [Google Scholar] [CrossRef]
- Harland, N.; Stenzl, A. Micro-ultrasound: A way to bring imaging for prostate cancer back to urology. Prostate Int. 2021, 9, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Girometti, R.; Giannarini, G.; Peruzzi, V.; Amparore, D.; Pizzolitto, S.; Zuiani, C. MRI-informed prostate biopsy: What the radiologist should know on quality in biopsy planning and biopsy acquisition. Eur. J. Radiol. 2023, 164, 110852. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Lee, C.H.; Vellayappan, B.; Tan, C.H. Comparison of diagnostic performance and inter-reader agreement between PI-RADS v2.1 and PI-RADS v2: Systematic review and meta-analysis. BJR 2022, 95, 20210509. [Google Scholar] [CrossRef]
- Moldovan, P.C.; Van Den Broeck, T.; Sylvester, R.; Marconi, L.; Bellmunt, J.; Van Den Bergh, R.C.N.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Fossati, N.; et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 2017, 72, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Padhani, A.R. Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation. BJU Int. 2021, 127, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.-J.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.A.M.; Kanthabalan, A.; Arya, M.; Briggs, T.; Barratt, D.; Charman, S.C.; Freeman, A.; Gelister, J.; Hawkes, D.; Hu, Y.; et al. The PICTURE study: Diagnostic accuracy of multiparametric MRI in men requiring a repeat prostate biopsy. Br. J. Cancer 2017, 116, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Stabile, A.; Neves, J.B.; Giganti, F.; Valerio, M.; Shanmugabavan, Y.; Clement, K.D.; Sarkar, D.; Philippou, Y.; Thurtle, D.; et al. Magnetic Resonance Imaging-targeted Biopsy Versus Systematic Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2019, 76, 284–303. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Choi, S.H.; Kim, M.; Kim, J.K.; Jeong, I.G. Performance of Prostate Imaging Reporting and Data System Version 2.1 for Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Magn. Reson. Imaging 2021, 54, 103–112. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van der Bergh, R.C.N.; Briers, E.; Eberli, E.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer, 2023 update. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 14 June 2023).
- Wadera, A.; Alabousi, M.; Pozdnyakov, A.; Kashif Al-Ghita, M.; Jafri, A.; McInnes, M.D.; Schieda, N.; van der Pol, C.B.; Salameh, J.-P.; Samoilov, L.; et al. Impact of PI-RADS Category 3 lesions on the diagnostic accuracy of MRI for detecting prostate cancer and the prevalence of prostate cancer within each PI-RADS category: A systematic review and meta-analysis. BJR 2021, 94, 20191050. [Google Scholar] [CrossRef]
- Park, K.J.; Choi, S.H.; Lee, J.S.; Kim, J.K.; Kim, M. Interreader Agreement with Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 661–670. [Google Scholar] [CrossRef]
- Westphalen, A.C.; McCulloch, C.E.; Anaokar, J.M.; Arora, S.; Barashi, N.S.; Barentsz, J.O.; Bathala, T.K.; Bittencourt, L.K.; Booker, M.T.; Braxton, V.G.; et al. Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 2020, 296, 76–84. [Google Scholar] [CrossRef]
- Girometti, R.; Giannarini, G.; De Martino, M.; Caregnato, E.; Cereser, L.; Soligo, M.; Rozze, D.; Pizzolitto, S.; Isola, M.; Zuiani, C. Multivariable stratification of PI-RADS version 2.1 categories for the risk of false-positive target biopsy: Impact on prostate biopsy decisions. Eur. J. Radiol. 2023, 165, 110897. [Google Scholar] [CrossRef]
- Padhani, A.R.; Haider, M.A.; Rouviere, O. Balancing the benefits and harms of MRI-directed biopsy pathways. Eur. Radiol. 2022, 32, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Osses, D.; Roobol, M.; Schoots, I. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. IJMS 2019, 20, 1637. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.; Truong, M.; Bullen, J.A.; Ward, R.D.; Purysko, A.S.; Klein, E.A. Clinical utility of PSAD combined with PI-RADS category for the detection of clinically significant prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 846.e9–846.e16. [Google Scholar] [CrossRef] [PubMed]
- Nordström, T.; Akre, O.; Aly, M.; Grönberg, H.; Eklund, M. Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 57–63. [Google Scholar] [CrossRef]
- Girometti, R.; Giannarini, G.; Panebianco, V.; Maresca, S.; Cereser, L.; De Martino, M.; Pizzolitto, S.; Pecoraro, M.; Ficarra, V.; Zuiani, C.; et al. Comparison of different thresholds of PSA density for risk stratification of PI-RADSv2.1 categories on prostate MRI. BJR 2022, 95, 20210886. [Google Scholar] [CrossRef]
- Oerther, B.; Engel, H.; Bamberg, F.; Sigle, A.; Gratzke, C.; Benndorf, M. Cancer detection rates of the PI-RADSv2.1 assessment categories: Systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis. 2022, 25, 256–263. [Google Scholar] [CrossRef]
- Cuocolo, R.; Comelli, A.; Stefano, A.; Benfante, V.; Dahiya, N.; Stanzione, A.; Castaldo, A.; De Lucia, D.R.; Yezzi, A.; Imbriaco, M. Deep Learning Whole-Gland and Zonal Prostate Segmentation on a Public MRI Dataset. J. Magn. Reson. Imaging 2021, 54, 452–459. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Elkhoury, F.F.; Felker, E.R.; Kwan, L.; Sisk, A.E.; Delfin, M.; Natarajan, S.; Marks, L.S. Comparison of Targeted vs Systematic Prostate Biopsy in Men Who Are Biopsy Naive: The Prospective Assessment of Image Registration in the Diagnosis of Prostate Cancer (PAIREDCAP) Study. JAMA Surg. 2019, 154, 811. [Google Scholar] [CrossRef]
- Stabile, A.; Dell’Oglio, P.; Gandaglia, G.; Fossati, N.; Brembilla, G.; Cristel, G.; Dehò, F.; Scattoni, V.; Maga, T.; Losa, A.; et al. Not All Multiparametric Magnetic Resonance Imaging-targeted Biopsies Are Equal: The Impact of the Type of Approach and Operator Expertise on the Detection of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Venderink, W.; Van Der Leest, M.; Van Luijtelaar, A.; Van De Ven, W.J.M.; Fütterer, J.J.; Sedelaar, J.P.M.; Huisman, H.J. Retrospective comparison of direct in-bore magnetic resonance imaging (MRI)-guided biopsy and fusion-guided biopsy in patients with MRI lesions which are likely or highly likely to be clinically significant prostate cancer. World, J. Urol. 2017, 35, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Van Der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Boesen, L.; Nørgaard, N.; Løgager, V.; Balslev, I.; Bisbjerg, R.; Thestrup, K.-C.; Winther, M.D.; Jakobsen, H.; Thomsen, H.S. Assessment of the Diagnostic Accuracy of Biparametric Magnetic Resonance Imaging for Prostate Cancer in Biopsy-Naive Men: The Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Netw. Open 2018, 1, e180219. [Google Scholar] [CrossRef] [PubMed]
- Nordström, T.; Discacciati, A.; Bergman, M.; Clements, M.; Aly, M.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Jäderling, F.; Eklund, M.; et al. STHLM3 study group, Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): A prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021, 22, 1240–1249. [Google Scholar] [CrossRef]
- Ettala, O.; Jambor, I.; Perez, I.M.; Seppänen, M.; Kaipia, A.; Seikkula, H.; Syvänen, K.T.; Taimen, P.; Verho, J.; Steiner, A.; et al. Individualised non-contrast MRI-based risk estimation and shared decision-making in men with a suspicion of prostate cancer: Protocol for multicentre randomised controlled trial (multi-IMPROD V.2.0). BMJ Open 2022, 12, e053118. [Google Scholar] [CrossRef]
- Padhani, A.R.; Schoots, I.; Villeirs, G. Contrast Medium or No Contrast Medium for Prostate Cancer Diagnosis. That Is the Question. J. Magn. Reson. Imaging 2021, 53, 13–22. [Google Scholar] [CrossRef]
- Wegelin, O.; Exterkate, L.; van der Leest, M.; Kummer, J.A.; Vreuls, W.; de Bruin, P.C.; Bosch, J.L.H.R.; Barentsz, J.O.; Somford, D.M.; van Melick, H.H.E. The FUTURE trial: A multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur. Urol. 2019, 75, 582–590. [Google Scholar] [CrossRef]
- Exterkate, L.; Wegelin, O.; Barentsz, J.O.; van der Leest, M.; Kummer, J.A.; Vreuls, W.; de Bruin, P.C.; Bosch, J.L.H.R.; van Melick, H.H.E.; Somford, D.M. Is There Still a Need for Repeated Systematic Biopsies in Patients with Previous Negative Biopsies in the Era of Magnetic Resonance Imaging-targeted Biopsies of the Prostate? Eur. Urol. Oncol. 2020, 3, 216–223. [Google Scholar] [CrossRef]
- Prabhakar, S.; Schieda, N. Patient preparation for prostate MRI: A scoping review. Eur. J. Radiol. 2023, 162, 110758. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Hall, R.; Walter, F.M.; Hamilton, W.; Spencer, A.E. Systematic review and narrative synthesis of economic evaluations of prostate cancer diagnostic pathways incorporating prebiopsy magnetic rsonance imaging. Eur. Urol. Open Sci. 2023, 52, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Adock, L. Magnetic Resonance Imaging for Prostate Assessment: A Review of Clinical and Cost-Effectiveness [Internet]. In Canadian Agency for Drugs and Technologies in Health; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. [Google Scholar]
- Vickers, A. Effects of magnetic resonance imaging targeting on overdiagnosis and overtreatmen t of prostate cancer. Eur. Urol. 2021, 80, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, R.; Haffner, M.C.; Gulati, R. Divining harm-benefit tradeoffs of magnetic resonance imaging-targeted biopsy. Eur. Urol. 2021, 80, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Van Leenders, G.J.L.H.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristianse, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N. ISUP Grading Workshop Panel Members. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.F.; Mussi, T.C.; da Cunha, M.L.; Filippi, R.Z.; Baroni, R.H. Accuracy of 68Ga-PSMA PET/CT for lymph node and bone primary staging in prostate cancer. Urol. Oncol. 2022, 40, 104.e17–104.e21. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Piert, M. Performance of 68Ga-PSMA PET/CT for Prostate Cancer Management at Initial Staging and Time of Biochemical Recurrence. Curr. Urol. Rep. 2017, 18, 84. [Google Scholar] [CrossRef]
- Kuten, J.; Mabjeesh, N.J.; Lerman, H.; Levine, C.; Barnes, S.; Even-Sapir, E. Ga-PSMA PET/CT Staging of Newly Diagnosed Intermediate- and High-Risk Prostate Cancer. Isr. Med. Assoc. J. 2019, 21, 100–104. [Google Scholar]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Sonni, I.; Eiber, M.; Fendler, W.P.; Alano, R.M.; Vangala, S.S.; Kishan, A.U.; Nickols, N.; Rettig, M.B.; Reiter, R.E.; Czernin, J.; et al. Impact of 68Ga-PSMA-11 PET/CT on Staging and Management of Prostate Cancer Patients in Various Clinical Settings: A Prospective Single-Center Study. J. Nucl. Med. 2020, 61, 1153–1160. [Google Scholar] [CrossRef]
- Hope, T.A.; Eiber, M.; Armstrong, W.R.; Juarez, R.; Murthy, V.; Lawhn-Heath, C.; Behr, S.C.; Zhang, L.; Barbato, F.; Ceci, F.; et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021, 7, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, T.; Zhang, Z.; Zhang, N.; Du, P.; Yang, Y.; Liu, Y.; Yu, W.; Li, N.; Gorin, M.A.; et al. 68Ga-PSMA PET/CT Combined with PET/Ultrasound-Guided Prostate Biopsy Can Diagnose Clinically Significant Prostate Cancer in Men with Previous Negative Biopsy Results. J. Nucl. Med. 2020, 61, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Li, W.-C.; Xu, Z.; Jiang, N.; Zang, S.-M.; Xu, L.-W.; Huang, W.-B.; Wang, F.; Sun, H.-B. 68Ga-PSMA PET/CT targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Uprimny, C.; Kroiss, A.S.; Decristoforo, C.; Fritz, J.; von Guggenberg, E.; Kendler, D.; Scarpa, L.; di Santo, G.; Roig, L.G.; Maffey-Steffan, J.; et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Buteau, J.; Papa, N.; Moon, D.; Thompson, J.; Roberts, M.J.; Rasiah, K.; Pattison, D.A.; Yaxley, J.; Thomas, P.; et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur. Urol. 2021, 80, 682–689. [Google Scholar] [CrossRef]
- Amin, A.; Blazevski, A.; Thompson, J.; Scheltema, M.J.; Hofman, M.S.; Murphy, D.; Lawrentschuk, N.; Sathianathen, N.; Kapoor, J.; Woo, H.H.; et al. Protocol for the PRIMARY clinical trial, a prospective, multicentre, cross-sectional study of the additive diagnostic value of gallium-68 prostate-specific membrane antigen positron-emission tomography/computed tomography to multiparametric magnetic resonance imaging in the diagnostic setting for men being investigated for prostate cancer. BJU Int. 2020, 125, 515–524. [Google Scholar] [CrossRef]
- Seifert, R.; Emmett, L.; Rowe, S.P.; Herrmann, K.; Hadaschik, B.; Calais, J.; Giesel, F.L.; Reiter, R.; Maurer, T.; Heck, M.; et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur. Urol. 2023, 83, 405–412. [Google Scholar] [CrossRef]
- Wang, Y.; Galante, J.R.; Haroon, A.; Wan, S.; Afaq, A.; Payne, H.; Bomanji, J.; Adeleke, S.; Kasivisvanathan, V. The future of PSMA PET and WB MRI as next-generation imaging tools in prostate cancer. Nat. Rev. Urol. 2022, 19, 475–493. [Google Scholar] [CrossRef]
- Expert Panel on Urological Imaging; et al.; Akin, O.; Woo, S.; Oto, A.; Allen, B.C.; Avery, R.; Barker, S.J.; Gerena, M.; Halpern, D.J.; Gettle, L.M. ACR Appropriateness Criteria® Pretreatment Detection, Surveillance, and Staging of Prostate Cancer: 2022 Update. J. Am. Coll. Radiol. 2023, 20, S187–S210. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Tseng, N.-C.; Chen, Y.-K.; Huang, K.-H.; Lin, H.-Y.; Huang, Y.-Y.; Hwang, T.I.S.; Ou, Y.-C. The Detection Performance of 18F–Prostate-Specific Membrane Antigen-1007 PET/CT in Primary Prostate Cancer: A Systemic Review and Meta-analysis. Clin. Nucl. Med. 2022, 47, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Zattoni, F.; Cassarino, G.; Artioli, P.; Cecchin, D.; Dal Moro, F.; Zucchetta, P. PET/MRI in prostate cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Singh, H.; Kumar, R.; Mittal, B.R. Diagnostic Accuracy of 68Ga-PSMA PET/CT for Initial Detection in Patients With Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2021, 216, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Sari Motlagh, R.; Yanagisawa, T.; Kawada, T.; Laukhtina, E.; Rajwa, P.; Aydh, A.; König, F.; Pallauf, M.; Huebner, N.A.; Baltzer, P.A.; et al. Accuracy of SelectMDx compared to mpMRI in the diagnosis of prostate cancer: A systematic review and diagnostic meta-analysis. Prostate Cancer Prostatic Dis. 2022, 25, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Liu, X.; Yegang, C.; Yongjiao, Y.; Yawei, X.; Jiaqi, K.; Xianhao, W.; Yuxuan, S.; Rui, H.; Wei, Z.; et al. Accuracy of multiparametric magnetic resonance imaging for diagnosing prostate Cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1244. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.F.; Alameddine, M.; Zucker, I.; Tamariz, L.; Palacio, A.; Nemeth, Z.; Velasquez, M.C.; Savio, L.F.; Panizzutti, M.; Jue, J.S.; et al. Performance of Multiparametric MRI of the Prostate in Biopsy Naïve Men: A Meta-analysis of Prospective Studies. Urology 2020, 146, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Loy, L.M.; Lim, G.H.; Leow, J.J.; Lee, C.H.; Tan, T.W.; Tan, C.H. A systematic review and meta-analysis of magnetic resonance imaging and ultrasound guided fusion biopsy of prostate for cancer detection—Comparing transrectal with transperineal approaches. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 650–660. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 2: Likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr. 2007, 96, 487–491. [Google Scholar] [CrossRef]
- Graves, R.S. Users’ Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. J. Med. Libr. Assoc. 2002, 90, 483. [Google Scholar]
- Sonni, I.; Felker, E.R.; Lenis, A.T.; Sisk, A.E.; Bahri, S.; Allen-Auerbach, M.; Armstrong, W.R.; Suvannarerg, V.; Tubtawee, T.; Grogan, T.; et al. Head-to-Head Comparison of 68Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J. Nucl. Med. 2022, 63, 847–854. [Google Scholar] [CrossRef]
- Kalapara, A.A.; Nzenza, T.; Pan, H.Y.C.; Ballok, Z.; Ramdave, S.; O’Sullivan, R.; Ryan, A.; Cherk, M.; Hofman, M.S.; Konety, B.R.; et al. Detection and localisation of primary prostate cancer using 68gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. 2020, 126, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Greer, P.B.; Simpson, J.; Goodwin, J.; Fu, C.; Lau, P.; Siddique, S.; Heerschap, A.; Ramadan, S. Diagnosis of transition zone prostate cancer by multiparametric MRI: Added value of MR spectroscopic imaging with sLASER volume selection. J. Biomed. Sci. 2021, 28, 54. [Google Scholar] [CrossRef] [PubMed]
- Exterkate, L.; Hermsen, R.; Küsters-Vandevelde, H.V.N.; Prette, J.F.; Baas, D.J.H.; Somford, D.M.; van Basten, J.A. Head-to-Head Comparison of 18F-PSMA-1007 Positron Emission Tomography/Computed Tomography and Multiparametric Magnetic Resonance Imaging with Whole-mount Histopathology as Reference in Localisation and Staging of Primary Prostate Cancer. Eur. Urol. Oncol. 2023, S2588-9311(23)00082-2. [Google Scholar] [CrossRef]
- Li, M.; Huang, Z.; Yu, H.; Wang, Y.; Zhang, Y.; Song, B. Comparison of PET/MRI with multiparametric MRI in diagnosis of primary prostate cancer: A meta-analysis. Eur. J. Radiol. 2019, 113, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, M.J.; Chang, J.I.; Stricker, P.D.; Van Leeuwen, P.J.; Nguyen, Q.A.; Ho, B.; Delprado, W.; Lee, J.; Thompson, J.E.; Cusick, T.; et al. Diagnostic accuracy of 68Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: Impact of the addition of 68Ga-P. BJU Int. 2019, 124, 42–49. [Google Scholar] [CrossRef]
- Coşar, U.; Şen, İ.; Aydos, U.; Koparal, M.Y.; Uçar, M.; Tokgöz, N.; Gönül, İ.I.; Akdemir, Ü.Ö.; Atay, L.Ö.; Sözen, T.S. Diagnostic accuracy of 68 Ga-PSMA PET/MRI and multiparametric MRI in detecting index tumours in radical prostatectomy specimen. Int. J. Clin. Pract. 2021, 75, e14287. [Google Scholar] [CrossRef]
- Ferraro, D.A.; Laudicella, R.; Zeimpekis, K.; Mebert, I.; Müller, J.; Maurer, A.; Grünig, H.; Donati, O.; Sapienza, M.T.; Rueschoff, J.H.; et al. Hot needles can confirm accurate lesion sampling intraoperatively using [18F]PSMA-1007 PET/CT-guided biopsy in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1721–1730. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Yang, R.; Ma, X.; Tian, R. 68Ga-PSMA PET/MRI for the diagnosis of primary and biochemically recurrent prostate cancer: A meta-analysis. Eur. J. Radiol. 2020, 130, 109131. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Jena, A.; Taneja, R.; Taneja, S.; Singh, A.; Kumar, V.; Agarwal, A.; Subramanian, N. Improving Diagnosis of Primary Prostate Cancer With Combined 68Ga-Prostate-Specific Membrane Antigen-HBED-CC Simultaneous PET and Multiparametric MRI and Clinical Parameters. AJR Am. J. Roentgenol. 2018, 211, 1246–1253. [Google Scholar] [CrossRef]
- Taneja, S.; Jena, A.; Taneja, R.; Singh, A.; Ahuja, A. Effect of Combined 68Ga-PSMAHBED-CC Uptake Pattern and Multiparametric MRI Derived With Simultaneous PET/MRI in the Diagnosis of Primary Prostate Cancer: Initial Experience. AJR Am. J. Roentgenol. 2018, 210, 1338–1345. [Google Scholar] [CrossRef]
- Al-Bayati, M.; Grueneisen, J.; Lütje, S.; Sawicki, L.M.; Suntharalingam, S.; Tschirdewahn, S.; Forsting, M.; Rübben, H.; Herrmann, K.; Umutlu, L.; et al. Integrated 68Gallium Labelled Prostate-Specific Membrane Antigen-11 Positron Emission Tomography/Magnetic Resonance Imaging Enhances Discriminatory Power of Multi-Parametric Prostate Magnetic Resonance Imaging. Urol. Int. 2018, 100, 164–171. [Google Scholar] [CrossRef]
- Bodar, Y.J.L.; Zwezerijnen, B.G.J.C.; Van Der Voorn, P.J.; Jansen, B.H.E.; Smit, R.S.; Kol, S.Q.; Meijer, D.; De Bie, K.; Yaqub, M.; Windhorst, B.A.D.; et al. Prospective analysis of clinically significant prostate cancer detection with [18F]DCFPyL PET/MRI compared to multiparametric MRI: A comparison with the histopathology in the radical prostatectomy specimen, the ProStaPET study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1731–1742. [Google Scholar] [CrossRef]
- Doan, P.; Counter, W.; Papa, N.; Sheehan-Dare, G.; Ho, B.; Lee, J.; Liu, V.; Thompson, J.E.; Agrawal, S.; Roberts, M.J.; et al. Synchronous vs independent reading of prostate-specific membrane antigen positron emission tomography (PSMA-PET) and magnetic resonance imaging (MRI) to improve diagnosis of prostate cancer. BJU Int. 2023, 131, 588–595. [Google Scholar] [CrossRef] [PubMed]
| Number of Trial | Radiopharmaceutical Agent (Study Phase) | Primary End-Point | Ongoing |
|---|---|---|---|
| NCT04116086 | 68Ga-PSMA-11 (not available) | To evaluate the possible role of PSMA-PET-CT in the early detection of prostate cancer, reducing the rate of unnecessary prostate biopsies, and correct staging of the disease and corresponding management in cases of prostate cancer. | Unknown |
| NCT05820724 | 18F-DCFPyl (phase II) | To determine if PSMA-PET imaging plus MRI improves the detection of clinically significant prostate cancer as compared with MRI alone. | Not yet |
| NCT05815316 | 18F-PSMA-1007 (phase II) | To evaluate the role of fully hybrid PET/MRI with 18F-PSMA and MRI as a one-stop approach for the diagnosis of clinically significant prostate cancer. | Not yet |
| NCT05160597 | 68Ga-PSMA-11 (phase I) | To use 68Ga-PSMA-11 PET/CT in patients with negative prostate biopsies. | Yes |
| NCT05154162 (PRIMARY 2) | 68Ga-PSMA-11 (phase III) | To prove that the addition of PSMA-PET/CT is non-inferior to MRI for the detection of csPCa in men with PI-RADS 2–3 disease, while providing the advantages of reducing unnecessary biopsies and limiting to targeted-only TPPB. | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cereser, L.; Evangelista, L.; Giannarini, G.; Girometti, R. Prostate MRI and PSMA-PET in the Primary Diagnosis of Prostate Cancer. Diagnostics 2023, 13, 2697. https://doi.org/10.3390/diagnostics13162697
Cereser L, Evangelista L, Giannarini G, Girometti R. Prostate MRI and PSMA-PET in the Primary Diagnosis of Prostate Cancer. Diagnostics. 2023; 13(16):2697. https://doi.org/10.3390/diagnostics13162697
Chicago/Turabian StyleCereser, Lorenzo, Laura Evangelista, Gianluca Giannarini, and Rossano Girometti. 2023. "Prostate MRI and PSMA-PET in the Primary Diagnosis of Prostate Cancer" Diagnostics 13, no. 16: 2697. https://doi.org/10.3390/diagnostics13162697
APA StyleCereser, L., Evangelista, L., Giannarini, G., & Girometti, R. (2023). Prostate MRI and PSMA-PET in the Primary Diagnosis of Prostate Cancer. Diagnostics, 13(16), 2697. https://doi.org/10.3390/diagnostics13162697








