Diagnostic Ability and Safety of Repeated Pancreatic Juice Cytology Using an Endoscopic Nasopancreatic Drainage Catheter for Pancreatic Ductal Adenocarcinoma: A Multicenter Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
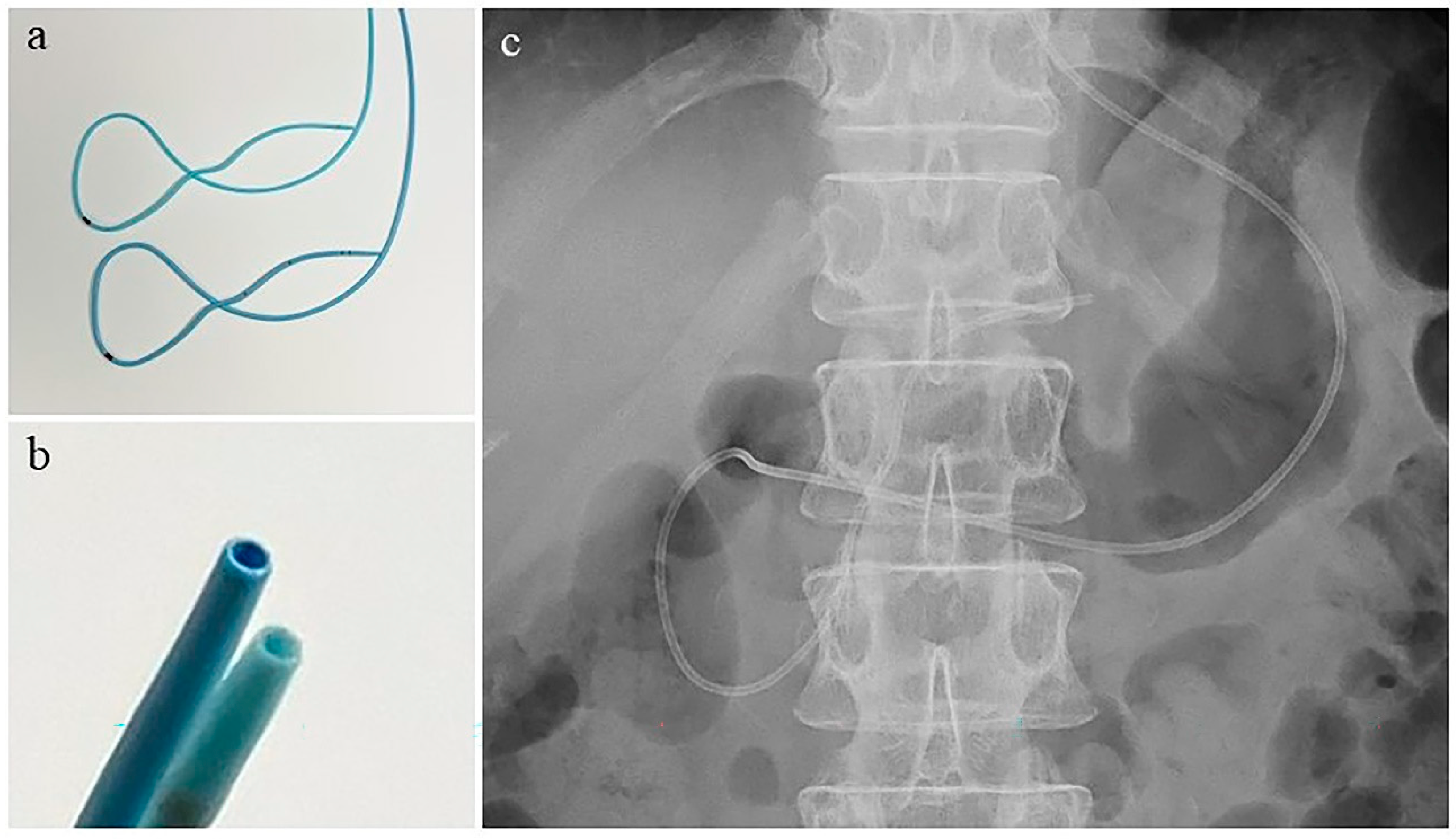
2.3. Endoscopic Retrograde Cholangiopancreatography and Nasopancreatic Drainage Catheter Placement
2.4. Protocol
2.5. Cytological Diagnosis
2.6. Endpoints and Definitions
2.7. Statistical Analyses
3. Results
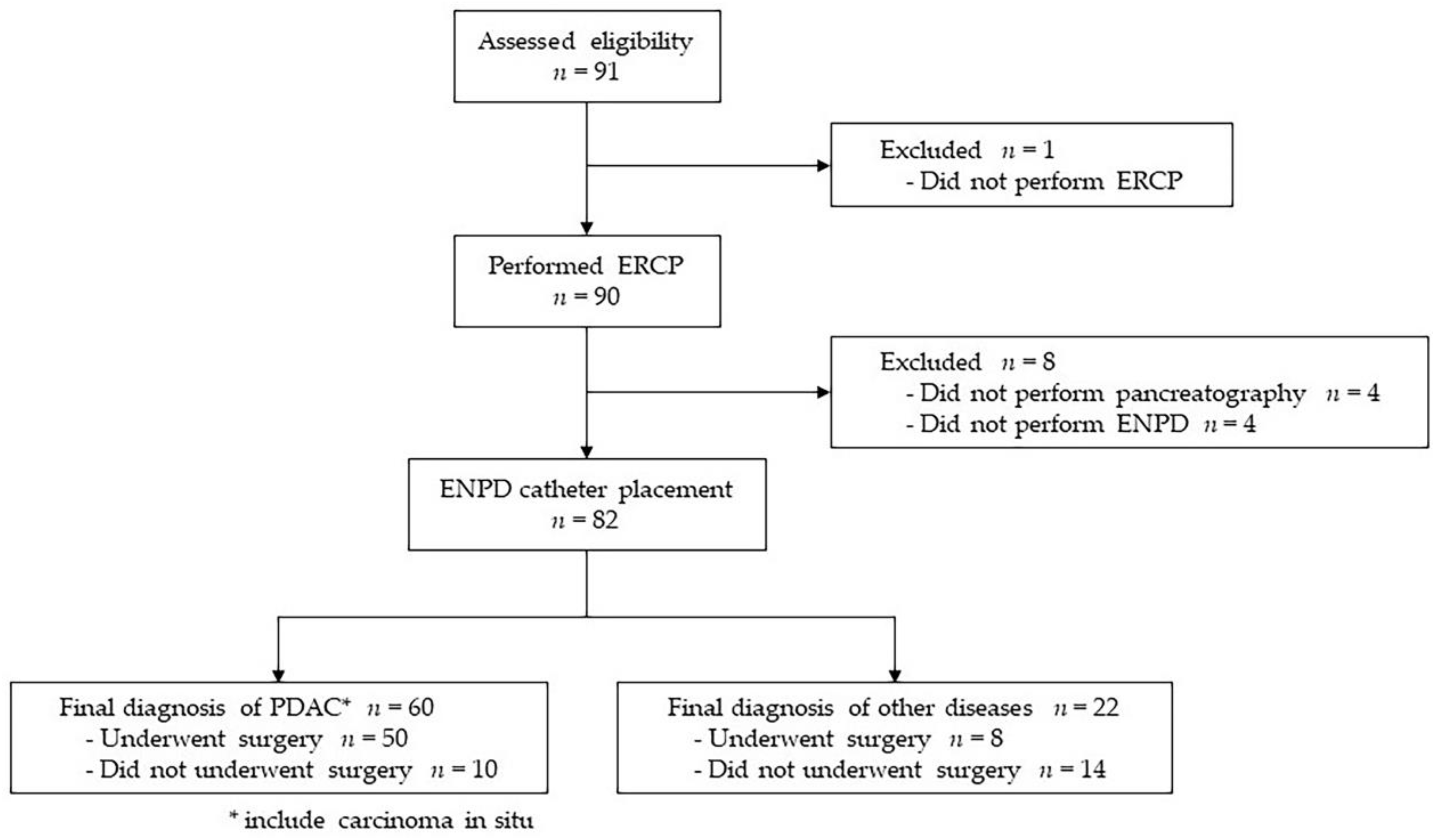
3.1. Study Flow Chart
3.2. Patient Characteristics
3.3. Diagnostic Ability of Pancreatic Juice Cytology via an ENPD Catheter
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murage, P.; Bachmann, M.O.; Crawford, S.M.; McPhail, S.; Jones, A. Geographical access to GPs and modes of cancer diagnosis in England: A cross-sectional study. Fam. Pract. 2019, 36, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Abraham, S.C.; Wilentz, R.E.; Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Boitnott, J.K.; Hruban, R.H. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: Are they all ‘chronic pancreatitis’? Am. J. Surg. Pathol. 2003, 27, 110–120. [Google Scholar] [CrossRef]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Ito, Y.; Mizuno, N.; Hanada, K.; Ozaka, M.; Morizane, C.; Takeyama, Y. Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2022 from the Japan Pancreas Society: A synopsis. Int. J. Clin. Oncol. 2023, 28, 493–511. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Uson Junior, P.L.S.; Dias e Silva, D.; de Castro, N.M.; da Silva Victor, E.; Rother, E.T.; Araújo, S.E.A.; Borad, M.J.; Moura, F. Does neoadjuvant treatment in resectable pancreatic cancer improve overall survival? A systematic review and meta-analysis of randomized controlled trials. ESMO Open 2023, 8, 100771. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Zhao, Y.; Dai, M.; Zhang, T. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology 2013, 13, 298–304. [Google Scholar] [CrossRef]
- Haba, S.; Yamao, K.; Bhatia, V.; Mizuno, N.; Hara, K.; Hijioka, S.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J. Gastroenterol. 2013, 48, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, M.; Ashida, R.; Kita, E.; Katanuma, A.; Itoi, T.; Mikata, R.; Nishikawa, K.; Matsubayashi, H.; Takayama, Y.; et al. Committee of Clinical Research, Japan Pancreas Society. Needle tract seeding after endoscopic ultrasound-guided tissue acquisition of pancreatic tumors: A nationwide survey in Japan. Dig. Endosc. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Satoh, T.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Muro, H.; Hanada, K. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (SPACE). Clin. J. Gastroenterol. 2017, 10, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: Establishing a new method for the early detection of pancreatic cancer in situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef]
- Mikata, R.; Ishihara, T.; Tada, M.; Tawada, K.; Saito, M.; Kurosawa, J.; Sugiyama, H.; Sakai, Y.; Tsuyuguchi, T.; Miyazaki, M.; et al. Clinical usefulness of repeated pancreatic juice cytology via endoscopic naso-pancreatic drainage tube in patients with pancreatic cancer. J. Gastroenterol. 2013, 48, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, J.; Serikawa, M.; Hanada, K.; Eguchi, N.; Sasaki, T.; Fujimoto, Y.; Sugiyama, S.; Yamaguchi, A.; Noma, B.; Kamigaki, M.; et al. Clinical analysis of early-stage pancreatic cancer and proposal for a new diagnostic algorithm: A multicenter observational study. Diagnostics 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Yokoe, M.; Takada, T.; Kataoka, K.; Yoshida, M.; Gabata, T.; Hirota, M.; Mayumi, T.; Kadoya, M.; Yamanouchi, E.; et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J. Hepatobiliary Pancreas Sci. 2010, 17, 37–44. [Google Scholar] [CrossRef]
- Endo, Y.; Morii, T.; Tamura, H.; Okuda, S. Cytodiagnosis of pancreatic malignant tumors by aspiration, under direct vision, using a duodenal fiberscope. Gastroenterology 1974, 67, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, A.R.; Smithies, A.; Wilkins, R.; Levi, A.J. Assessment of endoscopic retrograde cholangio-pancreatography (ERCP) and pure pancreatic juice cytology in patients with pancreatic disease. Gut 1976, 17, 14–21. [Google Scholar] [CrossRef]
- McGuire, D.E.; Venum, R.P.; Brown, R.D.; Etzkorn, K.P.; Glaws, W.R.; Abu-Hammour, A. Brush cytology for pancreatic carcinoma: An analysis of factors influencing results. Gastrointest. Endosc. 1996, 44, 300–304. [Google Scholar] [CrossRef]
- Uchida, N.; Kamada, H.; Tsutsui, K.; Ono, M.; Aritomo, Y.; Masaki, T.; Kushida, Y.; Haba, R.; Nakatsu, T.; Kuriyama, S. Utility of pancreatic duct brushing for diagnosis of pancreatic carcinoma. J. Gastroenterol. 2007, 42, 657–662. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Yokoyama, Y.; Ebata, T.; Igami, T.; Sugawara, G.; Kato, K.; Shimoyama, Y.; Nagino, M. Randomized controlled trial on timing and number of sampling for bile aspiration cytology. J. Hepatobiliary Pancreat. Sci. 2014, 21, 433–438. [Google Scholar] [CrossRef]
- Abdelghani, Y.A.; Arisaka, Y.; Masuda, D.; Takii, M.; Ashida, R.; Makhlouf, M.M.; Fouad, Y.M.; Tsuji, M.; Kurisu, Y.; Higuchi, K. Bile aspiration cytology in diagnosis of bile duct carcinoma: Factors associated with positive yields. J. Hepatobiliary Pancreat. Sci. 2012, 19, 370–378. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, R.; Ishii, Y.; Serikawa, M.; Tsuboi, T.; Tsushima, K.; Nakamura, S.; Hirano, T.; Ikemoto, J.; Kiyoshita, Y.; Saeki, S.; et al. Optimal indication of endoscopic retrograde pancreatography-based cytology in the preoperative pathological diagnosis of pancreatic ductal adenocancer. Pancreatology 2022, 22, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, O.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Horaguchi, J.; Obana, T.; Endo, T.; Nakahara, K.; Ishida, K.; et al. Clinicopathological study on the intraductal spread of small pancreatic cancer. J. Gastroenterol. 2007, 42, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Yokode, M.; Akita, M.; Fujikura, K.; Kim, M.J.; Morinaga, Y.; Yoshikawa, S.; Terada, T.; Matsukiyo, H.; Tajiri, T.; Abe-Suzuki, S.; et al. High-grade Pan IN presenting with localised stricture of the main pancreatic duct: A clinicopathological and molecular study of 10 cases suggests a clue for the early detection of pancreatic cancer. Histopathology 2018, 73, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Koshita, S.; Ogawa, T.; Kusunose, H.; Masu, K.; Sakai, T.; Yonamine, K.; Kawakami, Y.; Fujii, Y.; Miyamoto, K.; et al. Predictive Value of Localized Stenosis of the Main Pancreatic Duct for Early Detection of Pancreatic Cancer. Clin. Endosc. 2019, 52, 588–597. [Google Scholar] [CrossRef]
- Katanuma, A.; Maguchi, H.; Yane, K.; Hashigo, S.; Kin, T.; Kaneko, M.; Kato, S.; Kato, R.; Harada, R.; Osanai, M.; et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig. Dis. Sci. 2013, 58, 2093–2099. [Google Scholar] [CrossRef]
- Mouri, T.; Sasaki, T.; Serikawa, M.; Ishigaki, T.; Ishii, Y.; Shimizu, A.; Tsuboi, T.; Kurihara, K.; Tatsukawa, Y.; Miyaki, E.; et al. A comparison of 4-Fr with 5-Fr endoscopic nasopancreatic drainage catheters: A randomized, controlled trial. J. Gastroenterol. Hepatol. 2016, 31, 1783–1789. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shirai, Y.; Nakamura, N.; Sudo, K.; Nakamura, K.; Hironaka, S.; Hara, T.; Denda, T. Usefulness of brush cytology combined with pancreatic juice cytology in the diagnosis of pancreatic cancer: Significance of pancreatic juice cytology after brushing. Pancreas 2012, 41, 1225–1229. [Google Scholar] [CrossRef]
- Mie, T.; Sasaki, T.; Takeda, T.; Okamoto, T.; Mori, C.; Furukawa, T.; Yamada, Y.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; et al. Diagnostic Yield of Serial Pancreatic Juice Aspiration Cytologic Examination with Brush Cytology for Pancreatic Ductal Stenosis. Pancreas 2022, 51, 995–999. [Google Scholar] [CrossRef] [PubMed]
- International Academy of Cytology; International Agency for Research on Cancer; World Health Organization Joint Editorial Board. WHO Reporting System for Pancreaticobiliary Cytopathology, 1st ed.; Forthcoming; IAC-IARC-WHO Cytopathology Reporting System Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 2. [Google Scholar]
- Kimura, H.; Ohtsuka, T.; Matsunaga, T.; Watanabe, Y.; Tamura, K.; Ideno, N.; Aso, T.; Miyazaki, T.; Osoegawa, T.; Aishima, S.; et al. Predictors and Diagnostic Strategies for Early-Stage Pancreatic Ductal Adenocarcinoma: A Retrospective Study. Pancreas 2015, 44, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
| Values | |
|---|---|
| Age (years) | 72 (64–79) |
| Sex (male to female) | 50:32 |
| Indication for pancreatic juice cytology, n (%) | |
| Pancreatic mass | 60 (73.2%) |
| Pancreatic duct stenosis | 13 (15.9%) |
| Pancreatic duct dilatation | 6 (7.3%) |
| Pancreatic cyst | 3 (3.7%) |
| Diameter of the pancreatic mass on imaging, n (%) | |
| ≤10 mm | 9 (15.5%) |
| 11–20 mm | 27 (46.6%) |
| >20 mm | 22 (37.9%) |
| Location of the lesion | |
| Head | 38 (46.3%) |
| Body | 25 (30.5%) |
| Tail | 19 (23.2%) |
| Diameter of the ENPD catheter, n (%) | |
| 4-Fr | 62 (75.6%) |
| 5-Fr | 20 (24.4%) |
| Unexpected ENPD catheter displacement, n (%) | 3 (3.7%) |
| Due to complications | 2 (2.4%) |
| Self-removal | 1 (1.2%) |
| Number of PJC submissions via an ENPD catheter | |
| Surgical resection performed, n (%) | 58 (70.7%) |
| Final diagnosis, n (%) | |
| Pancreatic ductal adenocarcinoma | 58 (70.7%) |
| Carcinoma in situ | 2 (2.4%) |
| Intraductal papillary mucinous neoplasm | 2 (2.4%) |
| Chronic pancreatitis | 11 (13.4%) |
| Autoimmune pancreatitis | 1 (1.2%) |
| Pancreatic cyst | 5 (6.1%) |
| Others | 3 (3.7%) |
| Final Diagnosis by Histopathological Examination or Clinical Follow-Up | |||
|---|---|---|---|
| Cytological Diagnosis | PDAC, n (%) | Non-PDAC (Benign), n (%) | Total, n (%) |
| Positive | 28 (96.6%) | 1 (3.4%) | 29 (35.4%) |
| Atypical | 17 (73.9%) | 6 (26.1%) | 23 (28.0%) |
| Negative | 15 (50.0%) | 15 (50.0%) | 30 (36.6%) |
| Total, n (%) | 60 (73.2%) | 22 (26.8%) | 82 (100) |
| Values | |
|---|---|
| Sensitivity | 46.7% (28/60) |
| Specificity | 95.5% (21/22) |
| Positive predictive value | 96.6% (28/29) |
| Negative predictive value | 39.6% (21/53) |
| Accuracy | 59.8% (49/82) |
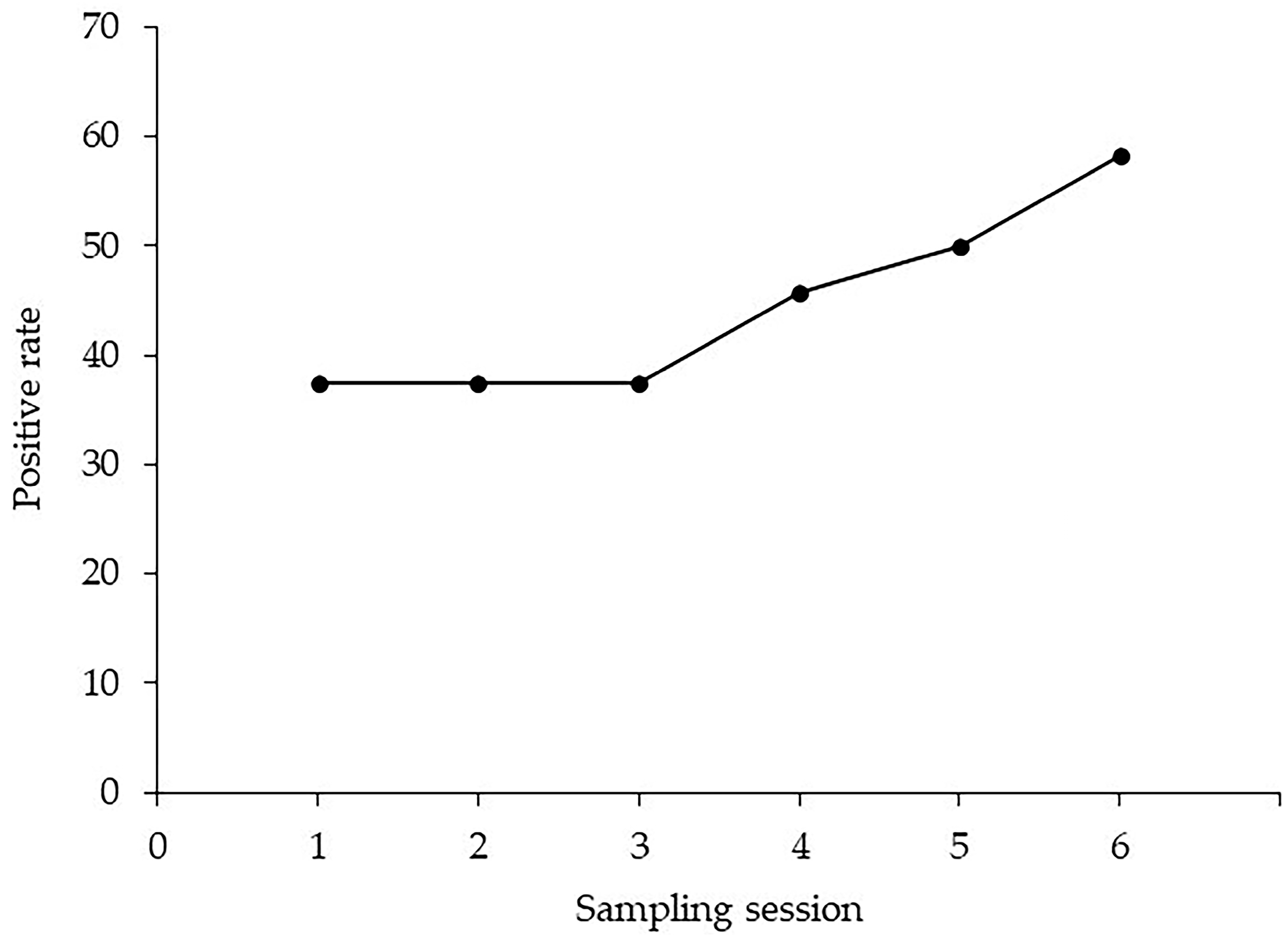
| Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 |
|---|---|---|---|---|---|
| 31.7% (19/60) | 25.9% (14/54) | 20.0% (10/50) | 35.0% (14/40) | 37.1% (13/35) | 45.8% (11/24) |
| Number of Patients | Number of Positivity | Sensitivity | p Value | |
|---|---|---|---|---|
| Tumor location | ||||
| Head | 29 | 17 | 58.6% | 0.045 |
| Body | 19 | 10 | 52.6% | |
| Tail | 12 | 2 | 16.7% | |
| Tumor size | 0.073 | |||
| ≤10 mm | 4 | 4 | 100% | |
| 11–20 mm | 28 | 14 | 50.0% | |
| >20 mm | 28 | 11 | 39.3% | |
| MPD stenosis | 0.032 | |||
| Present | 46 | 26 | 56.5% | |
| Absent | 14 | 3 | 21.4% | |
| Catheter diameter | 0.500 | |||
| 4-Fr | 50 | 23 | 46.0% | |
| 5-Fr | 10 | 6 | 60.0% | |
| Brush cytology | 0.511 | |||
| Performed | 19 | 8 | 42.1% | |
| Not performed | 41 | 21 | 51.2% |
| All Patients (n = 82) | PDAC (n = 60) | |
|---|---|---|
| Pancreatitis | 6 (7.3%) | 4 (6.7%) |
| Mild | 3 (3.7%) | 3 (5.0%) |
| Moderate | 2 (2.4%) | 0 |
| Severe | 1 (1.2%) | 1 (1.7%) |
| Hyperamylasemia | 44 (53.7%) | 36 (60.0%) |
| Cholangitis | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Ishii, Y.; Serikawa, M.; Hanada, K.; Eguchi, N.; Sasaki, T.; Fujimoto, Y.; Yamaguchi, A.; Sugiyama, S.; Noma, B.; et al. Diagnostic Ability and Safety of Repeated Pancreatic Juice Cytology Using an Endoscopic Nasopancreatic Drainage Catheter for Pancreatic Ductal Adenocarcinoma: A Multicenter Prospective Study. Diagnostics 2023, 13, 2696. https://doi.org/10.3390/diagnostics13162696
Nakamura S, Ishii Y, Serikawa M, Hanada K, Eguchi N, Sasaki T, Fujimoto Y, Yamaguchi A, Sugiyama S, Noma B, et al. Diagnostic Ability and Safety of Repeated Pancreatic Juice Cytology Using an Endoscopic Nasopancreatic Drainage Catheter for Pancreatic Ductal Adenocarcinoma: A Multicenter Prospective Study. Diagnostics. 2023; 13(16):2696. https://doi.org/10.3390/diagnostics13162696
Chicago/Turabian StyleNakamura, Shinya, Yasutaka Ishii, Masahiro Serikawa, Keiji Hanada, Noriaki Eguchi, Tamito Sasaki, Yoshifumi Fujimoto, Atsushi Yamaguchi, Shinichiro Sugiyama, Bunjiro Noma, and et al. 2023. "Diagnostic Ability and Safety of Repeated Pancreatic Juice Cytology Using an Endoscopic Nasopancreatic Drainage Catheter for Pancreatic Ductal Adenocarcinoma: A Multicenter Prospective Study" Diagnostics 13, no. 16: 2696. https://doi.org/10.3390/diagnostics13162696
APA StyleNakamura, S., Ishii, Y., Serikawa, M., Hanada, K., Eguchi, N., Sasaki, T., Fujimoto, Y., Yamaguchi, A., Sugiyama, S., Noma, B., Kamigaki, M., Minami, T., Okazaki, A., Yukutake, M., Mouri, T., Tatsukawa, Y., Ikemoto, J., Arihiro, K., & Oka, S. (2023). Diagnostic Ability and Safety of Repeated Pancreatic Juice Cytology Using an Endoscopic Nasopancreatic Drainage Catheter for Pancreatic Ductal Adenocarcinoma: A Multicenter Prospective Study. Diagnostics, 13(16), 2696. https://doi.org/10.3390/diagnostics13162696







