Vein Wall Invasion Is a More Reliable Predictor of Oncological Outcomes than Vein-Related Margins after Pancreaticoduodenectomy for Early Stages of Pancreatic Ductal Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
Data Analysis
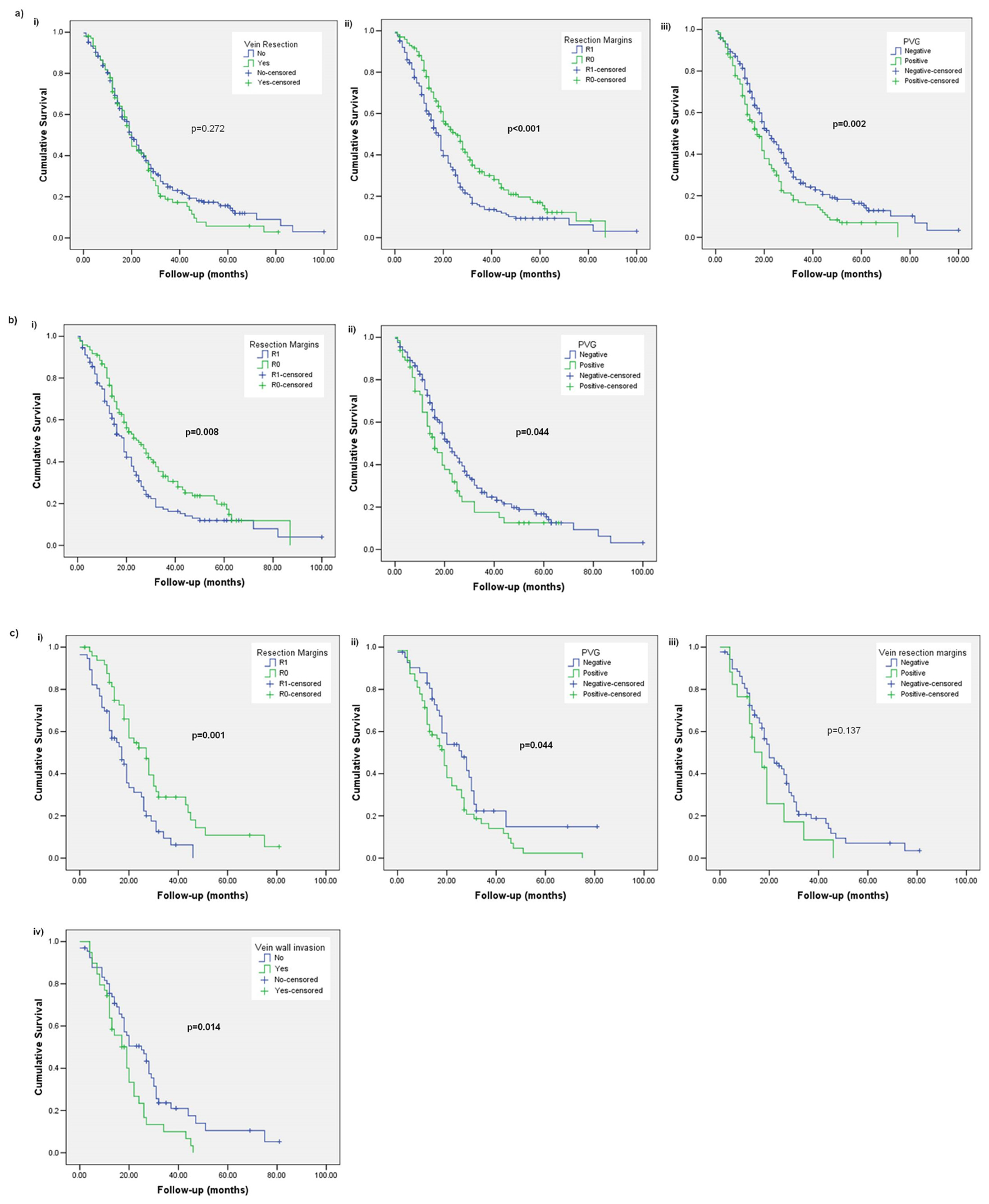
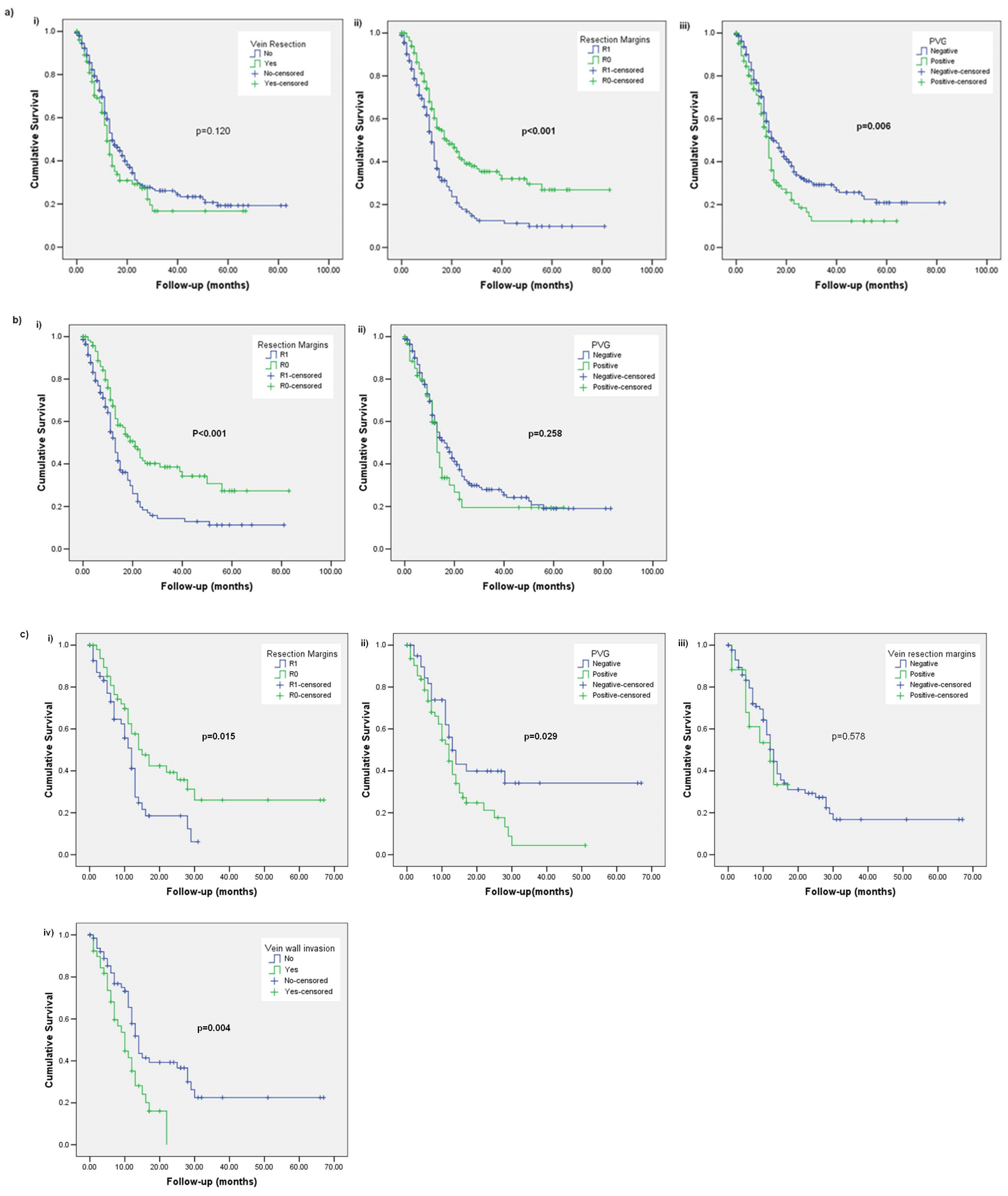
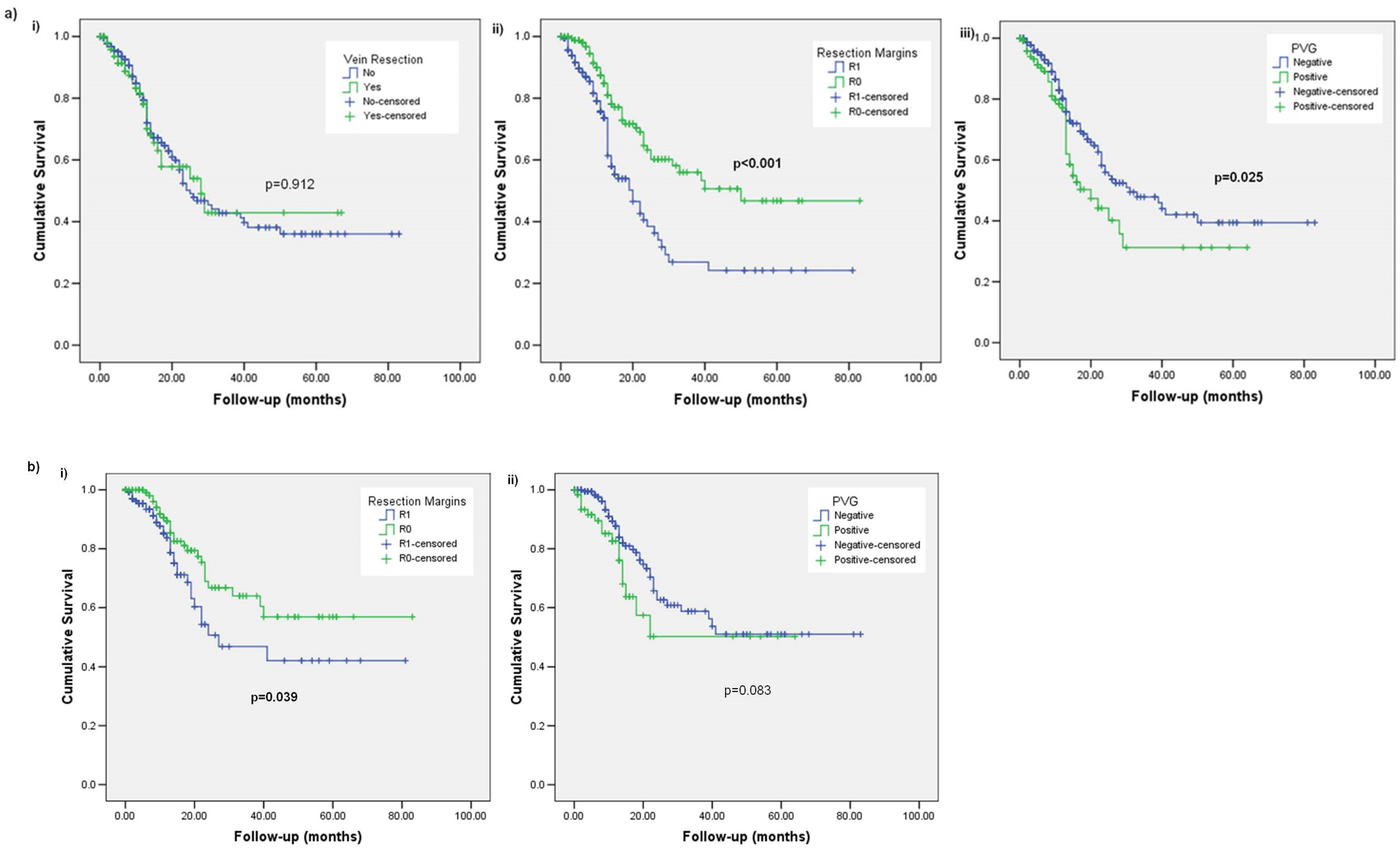
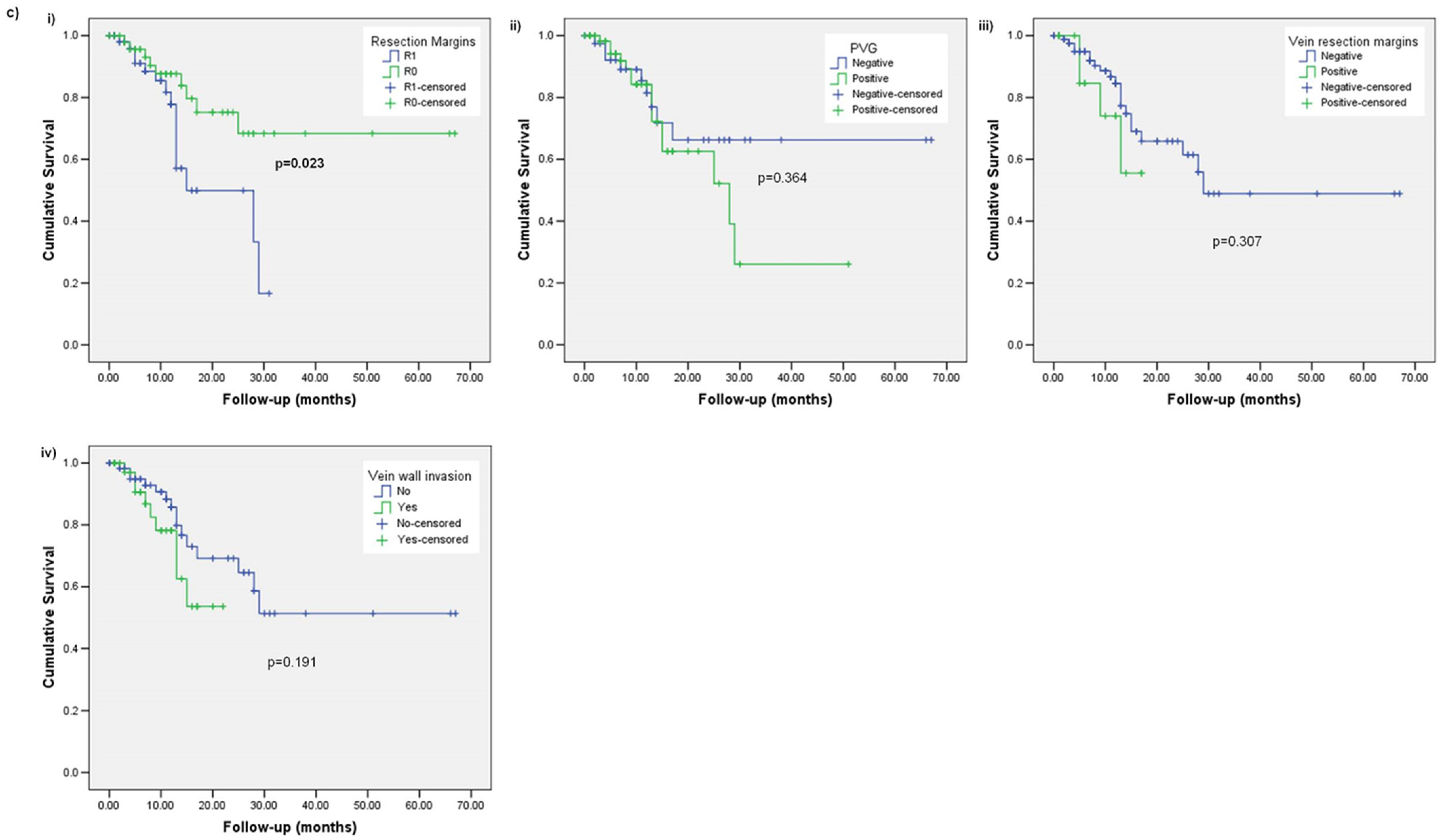
3. Results
3.1. Subsection Overall Survival (OS)
3.2. Disease-Free Survival (DFS)
3.3. Local Recurrence (LR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartwig, W.; Werner, J.; Jäger, D.; Debus, J.; Büchler, M.W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013, 14, e476–e485. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Hackert, T.; Niesen, W.; Hinz, U.; Tjaden, C.; Strobel, O.; Ulrich, A.; Michalski, C.W.; Büchler, M.W. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 358–363. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally advanced pancreatic cancer: Neoadjuvant therapy with FOLFIRINOX results in resectability in 2/3 of the patients. Pancreatology 2016, 264, 457–461. [Google Scholar] [CrossRef]
- Hartwig, W.; Gluth, A.; Hinz, U.; Koliogiannis, D.; Strobel, O.; Hackert, T.; Werner, J.; Büchler, M.W. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br. J. Surg. 2016, 103, 1683–1694. [Google Scholar] [CrossRef]
- Hartwig, W.; Hackert, T.; Hinz, U.; Hassenpflug, M.; Strobel, O.; Büchler, M.W.; Werner, J. Multivisceral resection for pancreatic malignancies: Risk-analysis and long-term outcome. Ann. Surg. 2009, 250, 81–87. [Google Scholar] [CrossRef]
- Attard, J.A.; Farrugia, A.; Pathanki, A.; Roberts, K.J.; Dasari, B.; Isaac, J.; Ma, Y.T.; Chatzizacharias, N.A. Treatment Strategies for the Optimal Management of Locally Advanced Pancreatic Adenocarcinoma with Curative Intent: A Systematic Review. Pancreas 2020, 49, 1264–1275. [Google Scholar] [CrossRef]
- Chatzizacharias, N.A.; Tsai, S.; Griffin, M.; Tolat, P.; Ritch, P.; George, B.; Barnes, C.; Aldakkak, M.; Khan, A.H.; Hall, W.; et al. Locally advanced pancreas cancer: Staging and goals of therapy. Surgery 2018, 163, 1053–1062. [Google Scholar] [CrossRef]
- Attard, J.A.; Isaac, J.; Roberts, K.; Faulkner, T.; Chatzizacharias, N.A. Resection of Replaced Common Hepatic Artery in Locally Advanced Pancreatic Cancer: A Case Report and Analysis of Technical and Oncological Implications. Pancreas 2020, 49, E31–E33. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef]
- Kalisvaart, M.; Broadhurst, D.; Marcon, F.; Pande, R.; Schlegel, A.; Sutcliffe, R.; Marudanayagam, R.; Mirza, D.; Chatzizacharias, N.; Abradelo, M.; et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: Systematic review and a single-centre retrospective study. HPB 2020, 22, 1240–1249. [Google Scholar] [CrossRef]
- Fortner, J.G.; Kim, D.K.; Cubilla, A.; Turnbull, A.; Pahnke, L.D.; Shils, M.E. Regional pancreatectomy: En bloc pancreatic, portal vein and lymph node resection. Ann. Surg. 1977, 186, 42–50. [Google Scholar] [CrossRef]
- Worni, M.; Castleberry, A.W.; Clary, B.M.; Gloor, B.; Carvalho, E.; Jacobs, D.O.; Pietrobon, R.; Scarborough, J.E.; White, R.R. Concomitant vascular reconstruction during pancreatectomy for malignant disease: A propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surg. 2013, 148, 331–338. [Google Scholar] [CrossRef]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef]
- Raptis, D.A.M.; Sánchez-Velázquez, P.M.; Machairas, N.; Sauvanet, A.; de Leon, A.R.; Oba, A.; Koerkamp, B.G.; Lovasik, B.; Chan, C.; Yeo, C.J.; et al. Defining Benchmark Outcomes for Pancreatoduodenectomy with Portomesenteric Venous Resection. Ann. Surg. 2020, 272, 731. [Google Scholar] [CrossRef]
- Groen, J.V.; Michiels, N.; van Roessel, S.; Besselink, M.G.; Bosscha, K.; Busch, O.R.; van Dam, R.; van Eijck, C.H.J.; Koerkamp, B.G.; van der Harst, E.; et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: Impact on short- and long-term outcomes in a nationwide cohort analysis. Br. J. Surg. 2021, 109, 96–104. [Google Scholar] [CrossRef]
- Giovinazzo, F.; Turri, G.; Katz, M.H.; Heaton, N.; Ahmed, I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br. J. Surg. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Ravikumar, R.; Sabin, C.; Abu Hilal, M.; Al-Hilli, A.; Aroori, S.; Bond-Smith, G.; Bramhall, S.; Coldham, C.; Hammond, J.; Hutchins, R.; et al. Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br. J. Surg. 2017, 104, 1539–1548. [Google Scholar] [CrossRef]
- Verbeke, C.; Webster, F.; Brosens, L.; Campbell, F.; Del Chiaro, M.; Esposito, I.; Feakins, R.M.; Fukushima, N.; Gill, A.J.; Kakar, S.; et al. Dataset for the reporting of carcinoma of the exocrine pancreas: Recommendations from the International Collaboration on Cancer Reporting (ICCR). Histopathology 2021, 79, 902–912. [Google Scholar] [CrossRef]
- Campbell, F.; Foulis, A.; Verbeke, C. Dataset for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. Pathology 2010, 2010, 1–27. [Google Scholar]
- American College of Pathologists. 2021. Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (accessed on 1 February 2023).
- van Roest, M.H.; Gouw, A.S.; Peeters, P.M.; Porte, R.J.; Slooff, M.J.; Fidler, V.; de Jong, K.P. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: Perineural growth more important prognostic factor than tumor localization. Ann. Surg. 2008, 248, 97–103. [Google Scholar] [CrossRef]
- Tseng, J.F.; Tamm, E.P.; Lee, J.E.; Pisters, P.W.; Evans, D.B. Venous resection in pancreatic cancer surgery. Best. Pr. Res. Clin. Gastroenterol. 2006, 20, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.J.; Prasad, P.; Steele, Y.; Marcon, F.; Faulkner, T.; Cilliers, H.; Dasari, B.; Abradelo, M.; Marudanayagam, R.; Sutcliffe, R.P.; et al. A reduced time to surgery within a ‘fast track’ pathway for periampullary malignancy is associated with an increased rate of pancreatoduodenectomy. HPB 2017, 19, 713–720. [Google Scholar] [CrossRef]
- NICE. Pancreatic Cancer in Adults: Diagnosis and Ancreatic Cancer In adults: Diagnosis and Management Management NICE Guideline: Your Responsibility our Responsibility. 2018. Available online: https://www.nice.org.uk/guidance/ng85/resources/pancreatic-cancer-in-adults-diagnosis-and-management-pdf-1837696373701 (accessed on 1 February 2023).
- National Comprehensive Cancer Network. 2020. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 1 February 2023).
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Katz, M.H.G.; Prakash, L.; Varadhachary, G.R.; Wolff, R.A.; Shroff, R.T.; Javle, M.; Fogelman, D.; Overman, M.; Crane, C.H.; et al. Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: A 25-Year Single-Institution Experience. J. Gastrointest. Surg. 2017, 21, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015, 262, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sekigami, Y.; Michelakos, T.; Castillo, C.F.-D.; Kontos, F.; Qadan, M.; Wo, J.Y.; Harrison, J.; Deshpande, V.; Catalano, O.; Lillemoe, K.D.; et al. Intraoperative Radiation Mitigates the Effect of Microscopically Positive Tumor Margins on Survival Among Pancreatic Adenocarcinoma Patients Treated with Neoadjuvant FOLFIRINOX and Chemoradiation. Ann. Surg. Oncol. 2021, 28, 4592–4601. [Google Scholar] [CrossRef]
- Butler, J.R.; Ahmad, S.A.; Katz, M.H.; Cioffi, J.L.; Zyromski, N.J. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB 2016, 18, 305–311. [Google Scholar] [CrossRef]
- Cooper, C. Cancer of the Exocrine Pancreas, Ampulla of Vater and, Distal Common Bile Duct: Structure Reporting Protocol; Royal College of Pathologists of Australasia: Sydney, Australia, 2020. [Google Scholar]
- Isaji, S.; Murata, Y.; Kishiwada, M. New Japanese Classification of Pancreatic Cancer. Pancreatic Cancer; Neoptolemos, J., Urrutia, R., Abbruzzese, J., Büchler, M., Eds.; Springer: New York, NY, USA, 2018. [Google Scholar]
- Kleive, D.; Labori, K.J.; Line, P.-D.; Gladhaug, I.P.; Verbeke, C.S. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB 2020, 22, 50–57. [Google Scholar] [CrossRef]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic cancer surgery: The new R-status counts. Pancreatology 2017, 265, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Büchler, M.W. Most Pancreatic Cancer Resections are R1 Resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, C.S.; Knapp, J.; Gladhaug, I.P. Tumour growth is more dispersed in pancreatic head cancers than in rectal cancer: Implications for resection margin assessment. Histopathology 2011, 59, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.A.; Smith, R.; Whelan, P.; Sutton, R.; Raraty, M.; Neoptolemos, J.; Ghaneh, P. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009, 55, 277–283. [Google Scholar] [CrossRef]
- Chandrasegaram, M.D.; Goldstein, D.; Simes, J.; Gebski, V.; Kench, J.G.; Gill, A.J.; Samra, J.S.; Merrett, N.D.; Richardson, A.J.; Barbour, A.P. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br. J. Surg. 2015, 102, 1459–1472. [Google Scholar] [CrossRef]
- Kolbeinsson, H.; Hoppe, A.; Bayat, A.; Kogelschatz, B.; Mbanugo, C.; Chung, M.; Wolf, A.; Assifi, M.M.; Wright, G.P. Recurrence patterns and postrecurrence survival after curative intent resection for pancreatic ductal adenocarcinoma. Surgery 2021, 169, 649–654. [Google Scholar] [CrossRef]
- Malleo, G.; Maggino, L.; Marchegiani, G.; Feriani, G.; Esposito, A.; Landoni, L.; Casetti, L.; Paiella, S.; Baggio, E.; Lipari, G.; et al. Pancreatectomy with venous resection for pT3 head adenocarcinoma: Perioperative outcomes, recurrence pattern and prognostic implications of histologically confirmed vascular infiltration. Pancreatology 2017, 17, 847–857. [Google Scholar] [CrossRef]
- Weyhe, D.; Obonyo, D.; Uslar, V.N.; Stricker, I.; Tannapfel, A. Predictive factors for long-term survival after surgery for pancreatic ductal adenocarcinoma: Making a case for standardized reporting of the resection margin using certified cancer center data. PLoS ONE 2021, 16, e0248633. [Google Scholar] [CrossRef]
- Hernandez, J.; Mullinax, J.; Clark, W.; Toomey, P.; Villadolid, D.; Morton, C.; Ross, S.; Rosemurgy, A. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann. Surg. 2009, 250, 76–80. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Behrman, S.W.; Benson, A.B.; Casper, E.S.; Chiorean, E.G.; Chung, V.; Cohen, S.J.; Czito, B.; Engebretson, A.; et al. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2014, 12, 1083–1093. [Google Scholar] [CrossRef]
- Nelson, D.W.; Blanchard, T.H.; Causey, M.W.; Homann, J.F.; Brown, T.A. Examining the accuracy and clinical usefulness of intraoperative frozen section analysis in the management of pancreatic lesions. Am. J. Surg. 2013, 205, 613. [Google Scholar] [CrossRef] [PubMed]
- van Roessel, S.; Kasumova, G.G.; Tabatabaie, O.; Ng, S.C.; van Rijssen, L.B.; Verheij, J.; Najarian, R.M.; van Gulik, T.M.; Besselink, M.G.; Busch, O.R.; et al. Pathological Margin Clearance and Survival After Pancreaticoduodenectomy in a US and European Pancreatic Center. Ann. Surg. Oncol. 2018, 25, 1760. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Oussoultzoglou, E.; Bachellier, P.; Rosso, E.; Nakano, H.; Audet, M.; Jaeck, D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch. Surg. 2007, 142, 172. [Google Scholar] [CrossRef] [PubMed]
- Yekebas, E.F.; Bogoevski, D.; Cataldegirmen, G.; Kunze, C.; Marx, A.; Vashist, Y.K.; Schurr, P.G.; Liebl, L.; Thieltges, S.; Gawad, K.A.; et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: Perioperative outcome and long-term survival in 136 patients. Ann. Surg. 2008, 247, 300–309. [Google Scholar] [CrossRef]
- Müller, S.A.; Hartel, M.; Mehrabi, A.; Welsch, T.; Martin, D.J.; Hinz, U.; Schmied, B.M.; Büchler, M.W. Vascular Resection in Pancreatic Cancer Surgery: Survival Determinants. J. Gastrointest. Surg. 2009, 13, 784–792. [Google Scholar] [CrossRef]
- Wang, J.; Estrella, J.S.; Peng, L.; Rashid, A.; Varadhachary, G.R.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Vauthey, J.-N.; Katz, M.H.; et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012, 118, 3801–3811. [Google Scholar] [CrossRef]
- Tseng, J.F.; Raut, C.P.; Lee, J.E.; Pisters, P.W.; Vauthey, J.-N.; Abdalla, E.K.; Gomez, H.F.; Sun, C.C.; Crane, C.H.; Wolff, R.A.; et al. Pancreaticoduodenectomy with vascular resection: Margin status and survival duration. J. Gastrointest. Surg. 2004, 8, 935–950. [Google Scholar] [CrossRef]
- Nakagohri, T.; Kinoshita, T.; Konishi, M.; Inoue, K.; Takahashi, S. Survival benefits of portal vein resection for pancreatic cancer. Am. J. Surg. 2003, 186, 149–153. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Liu, Y.; Li, B.; Xu, D. Pancreatectomy combined with superior mesenteric vein–portal vein resection for pancreatic cancer: A meta-analysis. World J. Surg. 2012, 36, 884–891. [Google Scholar] [CrossRef]
- Han, S.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Han, I.W.; You, Y.H. Long-term outcomes following en bloc resection for pancreatic ductal adenocarcinoma of the head with portomesenteric venous invasion. Asian J. Surg. 2021, 44, 313–320. [Google Scholar] [CrossRef]
| Complete Cohort (n = 372) | No Vein Resection (n = 267) | Vein Resection (n = 105) | p | |
|---|---|---|---|---|
| Age (median and range; years) | 69 (34–85) | 69 (34–85) | 71 (48–82) | 0.025 |
| Sex (male:female) | 193:179 (52%:48%) | 148:119 (55%:45%) | 45:60 (43%:57%) | 0.038 |
| Pre-operative stage on imaging | <0.001 | |||
| Resectable | 279 (75%) | 248 (93%) | 31 (30%) | |
| BR-V | 93 (25%) | 19 (7%) | 74 (70%) | |
| Pre-operative CA19-9 (median and range) | 200 (2–36,000) | 193 (2–36,000) | 237 (2–10,000) | 0.929 |
| Vein resection | - | |||
| Yes | 105 (28%) | N/A | 105 (100%) | |
| No | 267 (72%) | |||
| Vein Reconstruction | - | |||
| Primary | 85 (23%) | N/A | 85 (81%) | |
| Interposition graft | 20 (5%) | 20 (19%) | ||
| pT | 0.430 | |||
| pT1 | 35 (9%) | 28 (10%) | 7 (7%) | |
| pT2 | 308 (83%) | 218 (82%) | 90 (86%) | |
| pT3 | 29 (8%) | 21 (8%) | 8 (7%) | |
| pN | 0.172 | |||
| pN0 | 44 (12%) | 38 (14%) | 6 (5%) | |
| pN1 | 185 (39%) | 101 (38%) | 44 (42%) | |
| pN2 | 183 (49%) | 128 (48%) | 56 (53%) | |
| Peri-neural invasion (PNI) | 344 (93%) | 245 (92%) | 99 (94%) | 0.515 |
| Intra-vascular invasion (VI) | 334 (90%) | 239 (90%) | 95 (91%) | 1.000 |
| Surgical margin status | 0.818 | |||
| Negative (R0) | 170 (46%) | 121 (45%) | 45 (46%) | |
| Positive (R1) | 202 (54%) | 146 (55%) | 53 (54%) | |
| Specific margin/surface positivity | ||||
| Anterior | 21 (6%) | 17 (6%) | 4 (4%) | 0.457 |
| Posterior | 31 (8%) | 27 (10%) | 4 (4%) | 0.059 |
| Vein groove | 128 (34%) | 54 (24%) | 63 (60%) | <0.001 |
| Vein transection | 17 (5%) | - | 17 (16%) | N/A |
| SMA | 65 (18%) | 47 (18%) | 18 (17%) | 1.000 |
| Pancreas | 53 (14%) | 31 (12%) | 22 (21%) | 0.022 |
| BD | 11 (3%) | 9 (3%) | 2 (2%) | 0.527 |
| Duodenum | 14 (4%) | 10 (4%) | 3 (3%) | 0.766 |
| Vein wall invasion | 39 (11%) | N/A | 39 (37%) | - |
| Post-operative complications | 154 (42%) | 108 (41%) | 46 (44%) | 0.640 |
| Comprehensive Complications Index (median and range) | 0 (0–100) | 0 (0–100) | 0 (0–100) | 0.887 |
| Length of stay (median and range; days) | 9 (2–200) | 9 (2–84) | 9 (4–200) | 0.032 |
| Adjuvant chemotherapy | 267 (73%) | 193 (73%) | 74 (73%) | 1.000 |
| Follow-up (median and range; months) | 18 (0–100) | 17 (0–100) | 18 (0–81) | 0.444 |
| Disease recurrence | 226 (61%) | 159 (60%) | 67 (64%) | 0.639 |
| Local | 84 (23%) | 60 (23%) | 24 (23%) | |
| Distant | 107 (29%) | 70 (26%) | 37 (35%) | |
| Both | 35 (9%) | 29 (11%) | 6 (6%) |
| Univariable Analysis p; HR (95% CI) | |||
|---|---|---|---|
| Complete Cohort | No Vein Resection Subgroup | Vein Resection Subgroup | |
| Age | 0.791; 1.002 (0.989–1.015) | 0.779; 1.002 (0.987–1.018) | 0.886; 0.998 (0.975–1.022) |
| Sex | 0.579; 0.936 (0.740–1.183) | 0.539; 0.915 (0.690–1.214) | 0.735; 0.927 (0.598–1.437) |
| Pre-operative CA19–9 | 0.948; 1.000 (1.000–1.000) | 0.915; 1.000 (1.000–1.000) | 0.946; 1.000 (1.000–1.000) |
| Pre-operative stage | 0.075; 1.270 (0.976–1.654) | 0.745; 0.900 (0.476–1.701) | 0.054; 1.671 (0.990–2.818) |
| Vein resection | 0.282; 1.151 (0.891–1.488) | - | - |
| pT | 0.010; 1.473 (1.097–1.977) | 0.048; 1.389 (1.004–1.922) | 0.109; 1.808 (0.876–3.732) |
| pN | <0.001; 1.679 (1.401–2.013) | <0.001; 1.715 (1.389–2.117) | 0.018; 1.558 (1.078–2.252) |
| Surgical margin status | <0.001; 1.565 (1.234–1.983) | 0.009; 1454 (1.096–1.929) | 0.002; 2.041 (1.299–3.206) |
| Anterior surface | 0.949; 1.017 (0.613–1.685) | 0.830; 1.064 (0.605–1.869) | 0.872; 0.980 (0.308–3.111) |
| Posterior surface | 0.141; 1.342 (0.907–1.986) | 0.210; 1.315 (0.857–2.018) | 0.178; 2.003 (0.729–5.503) |
| Vein groove | 0.002; 1.466 (1.148–1.872) | 0.049; 1.389 (1.001–1.928) | 0.050; 1.572 (1.000–2.471) |
| Vein transection margin | 0.442; 0.949 (0.829–1.085) | N/A | 0.148; 1.535 (0.859–2.741) |
| SMA margin | 0.002; 1.585 (1.181–2.128) | 0.004; 1.667 (1.175–2.365) | 0.232; 1.398 (0.807–2.421) |
| Pancreas margin | 0.085; 1.366 (0.958–1.947) | 0.821; 1.058 (0.648–1729) | 0.011; 1.998 (1.169–3.415) |
| BD margin | 0.061; 1.829 (0.971–3.444) | 0.046; 2.058 (1.012–4.185) | 0.678; 1.348 (0.329–5.518) |
| Duodenal/gastric margin | 0.997; 1.001 (0.532–1.884) | 0.847; 0.928 (0.436–1.976) | 0.751; 1.205 (0.379–3.831) |
| Vein wall invasion | 0.730; 0.975 (0.842–1.128) | N/A | 0.017; 1.728 (1.103–2.706) |
| PNI | 0.844; 0.954 (0.598–1.521) | 0.698; 1.114 (0.646–1.920) | 0.024; 0.345 (0.137–0.868) |
| VI | 0.414; 1.187 (0.787–1.791) | 0.626; 1.129 (0.694–1.835) | 0.389; 1.408 (0.646–3.065) |
| Post-operative complications | 0.885; 1.018; (0.801–1.293) | 0.646; 0.935 (0.701–1.247) | 0.238; 1.296 (0.842–1.996) |
| Comprehensive complications index | 0.001; 1.013 (1.005–1.020) | 0.005; 1.012 (1.003–1.020) | 0.032; 1.015 (1.001–1.030) |
| Adjuvant chemotherapy | <0.001; 0.393 (0.304–0.509) | <0.001; 0.331 (0.243–0.451) | 0.030; 0.590 (0.366–0.951) |
| (A) Multivariable models for total margin status p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| Pre-operative stage Resectable as reference | NS | Not included as p > 0.200 in univariable analysis | 0.063; 1.678 (0.972–2.897) |
| pT pT1 as reference pT2 pT3 | 0.018 | NS | 0.009 |
| 0.921; 1.021 (0.673–1.550) | 0.881; 1.076 (0.413–2.802) | ||
| 0.030; 1.863 (1.061–3.270) | 0.029; 3.663 (1.145–11.723) | ||
| pN pN0 as reference pN1 pN2 | <0.001 | <0.001 | 0.063 |
| <0.001; 2.591 (1.595–4.209) | <0.001; 4.388 (2.465–7.811) | 0.911; 0.942 (0.330–2.688) | |
| <0.001; 3.736 (2.318–6.019) | <0.001; 5.817 (3.339–10.137) | 0.340; 1.635 (0.595–4.494) | |
| Surgical margin status R0 as reference | 0.015; 1.351 (1.059–1.723) | NS | 0.009; 1.942 (1.181–3.195) |
| Vein wall invasion Negative as reference | Not included as p > 0.200 in univariable analysis | Not included as p > 0.200 in univariable analysis | 0.031; 1.708 (1.051–2.775) |
| PNI Negative as reference | Not included as p > 0.200 in univariable analysis | Not included as p > 0.200 in univariable analysis | 0.022; 0.318 (0.119–0.851) |
| Adjuvant chemotherapy No chemotherapy as reference | <0.001; 2.909 (2.235–3.787) | <0.001; 4.571 (3.260–6.408) | NS |
| (B) Multivariable models for individual surgical margins p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| pT pT1 as reference pT2 pT3 | 0.009 | NS | 0.002 |
| 0.813; 1.052 (0.691–1.601) | 0.390; 0.667 (0.265–1.680) | ||
| 0.015; 2.030 (1.145–3.599) | 0.084; 2.695 (0.874–8.308) | ||
| pN pN0 as reference pN1 pN2 | <0.001 | <0.001 | NS |
| <0.001; 2.505 (1.542–4.068) | <0.001; 4.384 (2.463–7.801) | ||
| <0.001; 3.695 (2.298–5.941) | <0.001; 5.712 (3.274–9.966) | ||
| PVG Negative as reference | 0.055; 1.280 (0.995–1.646) | NS | NS |
| BD margin Negative as reference | NS | 0.048; 2.052 (1.005–4.189) | Not included as p > 0.200 in univariable analysis |
| Pancreas margin Negative as reference | NS | Not included | 0.001; 2.465 (1.415–4.293) |
| Vein wall invasion Negative as reference | Not included as p > 0.200 in univariable analysis | N/A | 0.005; 1.947 (1.228–3.089) |
| Comprehensive complications index | 0.084; 1.007 (0.999–1.015) | NS | NS |
| Adjuvant chemotherapy No chemotherapy as reference | <0.001; 2.672 (2.027–3.523) | <0.001; 4.639 (3.308–6.507) | NS |
| Univariable Analysis p; HR (95% CI) | |||
|---|---|---|---|
| Complete Cohort | No Vein Resection Subgroup | Vein Resection Subgroup | |
| Age | 0.449; 0.994 (0.980–1.009) | 0.773; 0.997 (0.980–1.015) | 0.189; 0.983 (0.957–1.009) |
| Sex | 0.380; 0.889 (0.684–1.155) | 0.391; 0.871 (0.636–1.194) | 0.653; 0.895 (0.553–1.449) |
| Pre-operative CA19–9 | 0.515; 1.000 (1.000–1.000) | 0.438; 1.000 (1.000–1.000) | 0.990; 1.000 (1.000–1.000) |
| Pre-operative stage | 0.010; 1.467 (1.095–1.966) | 0.294; 1.390 (0.751–2.572) | 0.110; 1.600 (0.900–2.846) |
| Vein resection | 0.130; 1.248 (0.937–1.661) | - | - |
| pT | 0.007; 1.607 (1.142–2.262) | 0.003; 1.780 (1.215–2.608) | 0.965; 1.019 (0.447–2.323) |
| pN | <0.001; 1.647 (1.348–2.012) | <0.001; 1.751 (1.389–2.208) | 0.227; 1.286 (0.855–1.933) |
| Surgical margin status | <0.001; 1.813 (1.388–2.370) | <0.001; 1.807 (1.313–2.485) | 0.019; 1.805 (1.101–2.957) |
| Anterior surface | 0.809; 0.931 (0.520–1.666) | 0.660; 0.860 (0.439–1.685) | 0.498; 1.497 (0.466–4.812) |
| Posterior surface | 0.086; 1.470 (0.947–2.284) | 0.213; 1.366 (0.836–2.233) | 0.030; 3.127 (1.118–8.748) |
| PVG | 0.008; 1.447 (1.101–1.903) | 0.270; 1.231 (0.850–1.783) | 0.036; 1.734 (1.037–2.901) |
| Vein transection margin | 0.180; 0.903 (0.778–1.048) | N/A | 0.589; 1.216 (0.598–2.474) |
| SMA margin | <0.001; 1.932 (1.397–2.674) | <0.001; 2.384 (1.633–3.480) | 0.656; 1.159 (0.605–2.219) |
| Pancreas margin | 0.445; 1.168 (0.785–1.738) | 0.793; 1.074 (0.630–1.831) | 0.619; 1.168 (0.634–2.151) |
| BD margin | 0.052; 2.023 (0.995–4.113) | 0.004; 3.047 (1.414–6.568) | 0.549; 0.546 (0.076–3.943) |
| Duodenal/gastric margin | 0.378; 1.330 (0.705–2.508) | 0.670; 1.179 (0.552–2.518) | 0.288; 1.883 (0.586–6.049) |
| Vein wall invasion | 0.597; 0.957 (0.813–1.126) | N/A | 0.006; 2.019 (1.219–3.345) |
| PNI | 0.101; 1.700 (0.901–3.207) | 0.106; 1.799 (0.882–3.667) | 0.921; 1.074 (0.262–4.399) |
| VI | 0.063; 1.563 (0.976–2.503) | 0.236; 1.394 (0.805–2.415) | 0.125; 2.051 (0.820–5.130) |
| Post-operative complications | 0.152; 0.820 (0.624–1.076) | 0.280; 0.836 (0.604–1.157) | 0.269; 0.754 (0.458–1.244) |
| Comprehensive complications index | 0.145; 0.993 (0.983–1.002) | 0.245; 0.993 (0.982–1.005) | 0.300; 0.991 (0.974–1.008) |
| Adjuvant chemotherapy | 0.003; 0.613 (0.445–0.846) | 0.012; 0.604 (0.408–0.894) | 0.130; 0.646 (0.367–1.138) |
| (A) Multivariable models for total margin status p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| Pre-operative stage Resectable as reference | 0.027; 1.398 (1.039–1.883) | Not included as p > 0.200 on univariable analysis | NS |
| pT pT1 as reference pT2 pT3 | 0.014 | 0.015 | Not included as p > 0.200 on univariable analysis |
| 0.925; 0.977 (0.598–1.595) | 0.648; 1.144 (0.642–2.038) | ||
| 0.040; 1.966 (1.030–3.753) | 0.019; 2.444 (1.160–5.146) | ||
| pN pN0 as reference pN1 pN2 | <0.001 | <0.001 | Not included as p > 0.200 in univariable analysis |
| <0.001; 2.964 (1.708–5.144) | <0.001; 3.450 (1.860–6.399) | ||
| <0.001; 3.970 (2.289–6.885) | <0.001; 4.536 (2.459–8.367) | ||
| Surgical margin status R0 as reference | <0.001; 1.734 (1.321–2.276) | 0.002; 1.693 (1.218–2.352) | 0.044; 1.684 (1.013–2.799) |
| Vein wall invasion Negative as reference | Not included as p > 0.200 on univariable analysis | N/A | 0.016; 1.894 (1.127–3.182) |
| Post-operative complications Negative as reference | 0.015; 0.703 (0.530–0.933) | Not included as p > 0.200 in univariable analysis | Not included as p > 0.200 in univariable analysis |
| Adjuvant chemotherapy No chemotherapy as reference | <0.001; 2.167 (1.532–3.065) | <0.001; 2.653 (1.728–4.075) | NS |
| (B) Multivariable models for individual surgical margins p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| Pre-operative stage Resectable as reference | 0.011; 1.476 (1.092–1.993) | Not included as p < 0.200 in univariable analysis | NS |
| pT pT0 as reference pT1 pT2 | 0.038 | 0.038 | Not included as p > 0.200 in univariable analysis |
| 0.680; 0.901 (0.550–1.476) | 0.776; 1.088 (0.608–1.949) | ||
| 0.126; 1.672 (0.865–3.234) | 0.047; 2.145 (1.009–4.558) | ||
| pN pN0 as reference pN1 pN2 | <0.001 | <0.001 | Not included as p > 0.200 in univariable analysis |
| <0.001; 2.887 (1.665–5.006) | <0.001; 3.339 (1.795–6.209) | ||
| <0.001; 3.898 (2.248–6.760) | <0.001; 4.371 (2.346–8.144) | ||
| Posterior margin Negative as reference | 0.051; 1.585 (0.998–2.516) | Not included as p > 0.200 in univariable analysis | 0.024; 3.295 (1.173–9.254) |
| SMA margin Negative as reference | 0.002; 1.688 (1.208–2.357) | <0.001; 2.197 (1.480–3.262) | Not included as p > 0.200 in univariable analysis |
| BD margin Negative as reference | NS | 0.002; 3.441 (1.580–7.494) | Not included as p > 0.200 in univariable analysis |
| Vein wall invasion Negative as reference | Not included as p > 0.200 in univariable analysis | N/A | 0.003; 2.169 (1.306–3.602) |
| Post-operative complications Negative as reference | 0.009; 0.682 (0.513–0.907) | Not included as p > 0.200 in univariable analysis | Not included as p > 0.200 in univariable analysis |
| Adjuvant chemotherapy No chemotherapy as reference | <0.001; 2.127 (1.508–3.001) | <0.001; 2.827 (1.841–4.340) | NS |
| Univariable Analysis p; HR (95% CI) | |||
|---|---|---|---|
| Complete Cohort | No Vein Resection Subgroup | Vein Resection Subgroup | |
| Age | 0.844; 1.002 (0.981–1.023) | 0.966; 1.001 (0.976–1.025) | 0.800; 1.006 (0.963–1.050) |
| Sex | 0.668; 1.083 (0.751–1.562) | 0.525; 1.147 (0.751–1.752) | 0.875; 0.943 (0.453–1.963) |
| Pre-operative CA19–9 | 0.243; 1.000 (1.000–1.000) | 0.322; 1.000 (1.000–1.000) | 0.705; 1.000 (1.000–1.000) |
| Pre-operative stage | 0.266; 1.276 (0.831–1.958) | 0.668; 1.219 (0.492–3.021) | 0.206; 1.787 (0.726–4.399) |
| Vein resection | 0.914; 1.024 (0.671–1.562) | N/A | N/A |
| pT | 0.016; 1.820 (1.121–2.957) | 0.023; 1.839 (1.088–3.108) | 0.337; 1.871 (0.521–6.716) |
| pN | 0.000; 1.673 (1.269–2.206) | <0.001; 1.856 (1.357–2.540) | 0.739; 1.108 (0.607–2.022) |
| Surgical margin status | <0.001; 2.135 (1.464–3.115) | 0.002; 1.997 (1.294–3.083) | 0.022; 2.452 (1.140–5.273) |
| Anterior surface | 0.705; 1.159 (0.540–2.492) | 0.743; 1.149 (0.501–2.636) | 0.810; 1.279 (0.171–9.557) |
| Posterior surface | 0.064; 1.762 (0.968–3.207) | 0.090; 1.731 (0.918–3.261) | 0.445; 2.201 (0.290–16.702) |
| PVG | 0.028; 1.538 (1.048–2.257) | 0.079; 1.540 (0.952–2.492) | 0.199; 1.659 (0.767–3.591) |
| Vein transection margin | 0.978; 1.003 (0.805–1.250) | N/A | 0.603; 1.328 (0.456–3.872) |
| SMA margin | 0.004; 1.997 (1.248–3.197) | 0.001; 2.432 (1.413–4.185) | 0.646; 1.255 (0.476–3.314) |
| Pancreas margin | 0.203; 1.412 (0.830–2.404) | 0.305; 1.414 (0.729–2.741) | 0.529; 1.339 (0.539–3.326) |
| BD margin | 0.003; 3.588 (1.561–8.248) | 0.001; 5.117 (2.028–12.913) | 0.798; 1.298 (0.176–9.605) |
| Duodenal/gastric margin | 0.281; 1.571 (0.690–3.576) | 0.693; 1.224 (0.448–3.342) | 0.111; 3.277 (0.762–14.094) |
| Vein wall invasion | 0.552; 1.076 (0.845–1.371) | N/A | 0.030; 2.365 (1.089–5.133) |
| PNI | 0.030; 4.697 (1.160–19.030) | 0.043; 4.246 (1.043–17.292) | 0.517; 21.287 (0.002–222017.1) |
| VI | 0.101; 1.769 (0.895–3.497) | 0.260; 1.560 (0.720–3.383) | 0.223; 2.454 (0.579–10.403) |
| Post-operative complications | 0.173; 0.766 (0.523–1.124) | 0.259; 0.775 (0.497–1.207) | 0.356; 0.697 (0.324–1.500) |
| Comprehensive complications index | 0.056; 0.986 (0.971–1.000) | 0.075; 0.984 (0.967–1.002) | 0.390; 0.988 (0.962–1.015) |
| Adjuvant chemotherapy | 0.388; 1.244 (0.758–2.040) | 0.706; 0.889 (0.481–1.642) | 0.324; 1.538 (0.654–3.618) |
| (A) Multivariable models for total margin status p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| pT pT1 as reference pT2 pT3 | 0.005 | 0.027 | Not included as p > 0.200 in univariable analysis |
| 0.993; 1.003 (0.518–1.941) | 0.719; 1.146 (0.547–2.399) | ||
| 0.020; 2.756 (1.174–6.473) | 0.028; 2.949 (1.121–7.755) | ||
| pN pN0 as reference pN1 pN2 | 0.014 | 0.003 | Not included as p > 0.200 in univariable analysis |
| 0.112; 1.728 (0.880–3.391) | 0.114; 1.794 (0.8168–3.707) | ||
| 0.007; 2.516 (1.288–4.916) | 0.002; 3.082 (1.517–6.259) | ||
| Surgical margin statusR0 as reference | <0.001; 2.041 (1.391–2.996) | 0.007; 1.845 (1.183–2.877) | 0.022; 2.452 (1.140–5.273) |
| (B) Multivariable models for individual surgical margins p; HR (95% CI) | |||
| Complete cohort | No vein resection subgroup | Vein resection subgroup | |
| pT pT1 as reference pT2 pT3 | 0.010 | NS | Not included as p > 0.200 in univariable analysis |
| 0.826; 0.928 (0.476–1.809) | |||
| 0.045; 2.398 (1.020–5.638) | |||
| pN pN0 as reference pN1 pN2 | 0.014 | 0.004 | Not included as p > 0.200 in univariable analysis |
| 0.133; 1.684 (0.853–3.326) | 0.134; 1.741 (0.843–3.597) | ||
| 0.008; 2.498 (1.269–4.917) | 0.003; 2.981 (1.466–6.060) | ||
| SMA margin Negative as reference | 0.012; 1.854 (1.145–3.001) | 0.006; 2.172 (1.245–3.788) | Not included as p > 0.200 in univariable analysis |
| BD margin Negative as reference | 0.005; 3.342 (1.431–7.808) | 0.001; 5.276 (2.067–13.468) | Not included as p > 0.200 in univariable analysis |
| PNI Negative as reference | 0.99; 3.277 (0.799–13.441) | NS | Not included as p > 0.200 in univariable analysis |
| Vein wall invasion Negative as reference | Not included as p > 0.200 in univariable analysis | N/A | 0.030; 2.365 (1.089–5.133) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuja, M.; Pandé, R.; Chugtai, S.; Brown, R.M.; Cain, O.; Bartlett, D.C.; Dasari, B.V.M.; Marudanayagam, R.; Roberts, K.J.; Isaac, J.; et al. Vein Wall Invasion Is a More Reliable Predictor of Oncological Outcomes than Vein-Related Margins after Pancreaticoduodenectomy for Early Stages of Pancreatic Ductal Adenocarcinoma. Diagnostics 2023, 13, 3465. https://doi.org/10.3390/diagnostics13223465
Ahuja M, Pandé R, Chugtai S, Brown RM, Cain O, Bartlett DC, Dasari BVM, Marudanayagam R, Roberts KJ, Isaac J, et al. Vein Wall Invasion Is a More Reliable Predictor of Oncological Outcomes than Vein-Related Margins after Pancreaticoduodenectomy for Early Stages of Pancreatic Ductal Adenocarcinoma. Diagnostics. 2023; 13(22):3465. https://doi.org/10.3390/diagnostics13223465
Chicago/Turabian StyleAhuja, Manish, Rupaly Pandé, Shafiq Chugtai, Rachel M. Brown, Owen Cain, David C. Bartlett, Bobby V. M. Dasari, Ravi Marudanayagam, Keith J. Roberts, John Isaac, and et al. 2023. "Vein Wall Invasion Is a More Reliable Predictor of Oncological Outcomes than Vein-Related Margins after Pancreaticoduodenectomy for Early Stages of Pancreatic Ductal Adenocarcinoma" Diagnostics 13, no. 22: 3465. https://doi.org/10.3390/diagnostics13223465
APA StyleAhuja, M., Pandé, R., Chugtai, S., Brown, R. M., Cain, O., Bartlett, D. C., Dasari, B. V. M., Marudanayagam, R., Roberts, K. J., Isaac, J., Sutcliffe, R. P., & Chatzizacharias, N. (2023). Vein Wall Invasion Is a More Reliable Predictor of Oncological Outcomes than Vein-Related Margins after Pancreaticoduodenectomy for Early Stages of Pancreatic Ductal Adenocarcinoma. Diagnostics, 13(22), 3465. https://doi.org/10.3390/diagnostics13223465







