Immunohistochemical Expression of Epithelial Cell Adhesion Molecule (EpCAM) in Salivary Gland Cancer: Correlation with the Biological Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
- -
- Missing data from the patient’s medical record;
- -
- Short postoperative follow-up (<5 years);
- -
- Tumor type (squamous cell carcinoma, lymphoma, secondary malignancies);
- -
- Poor conditions and amounts of neoplastic tissue; and
- -
- Quality of immunohistochemical staining.
2.2. Immunohistochemical Methods
2.3. Statistical Analysis
3. Results
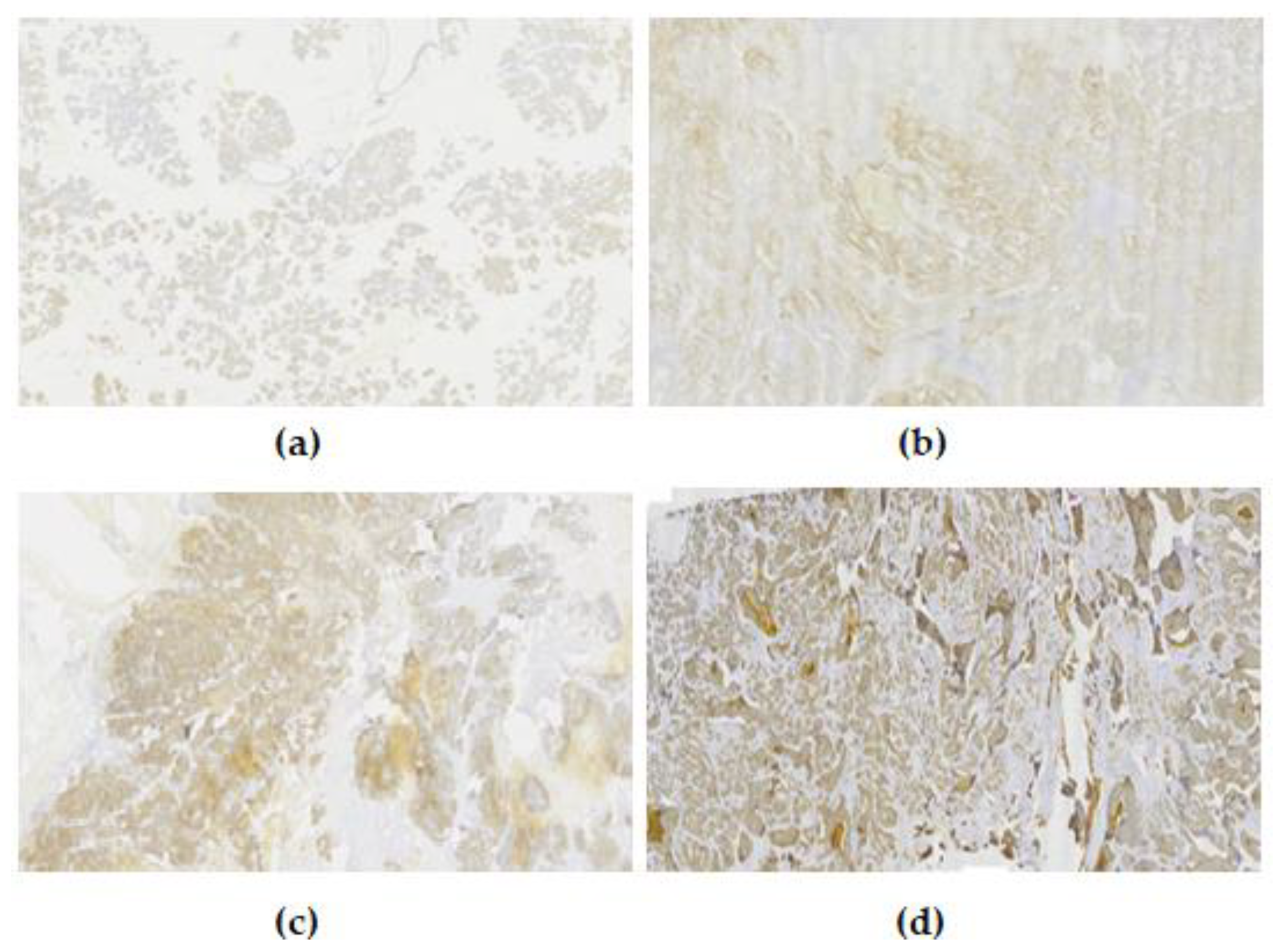
3.1. EpCAM Expression
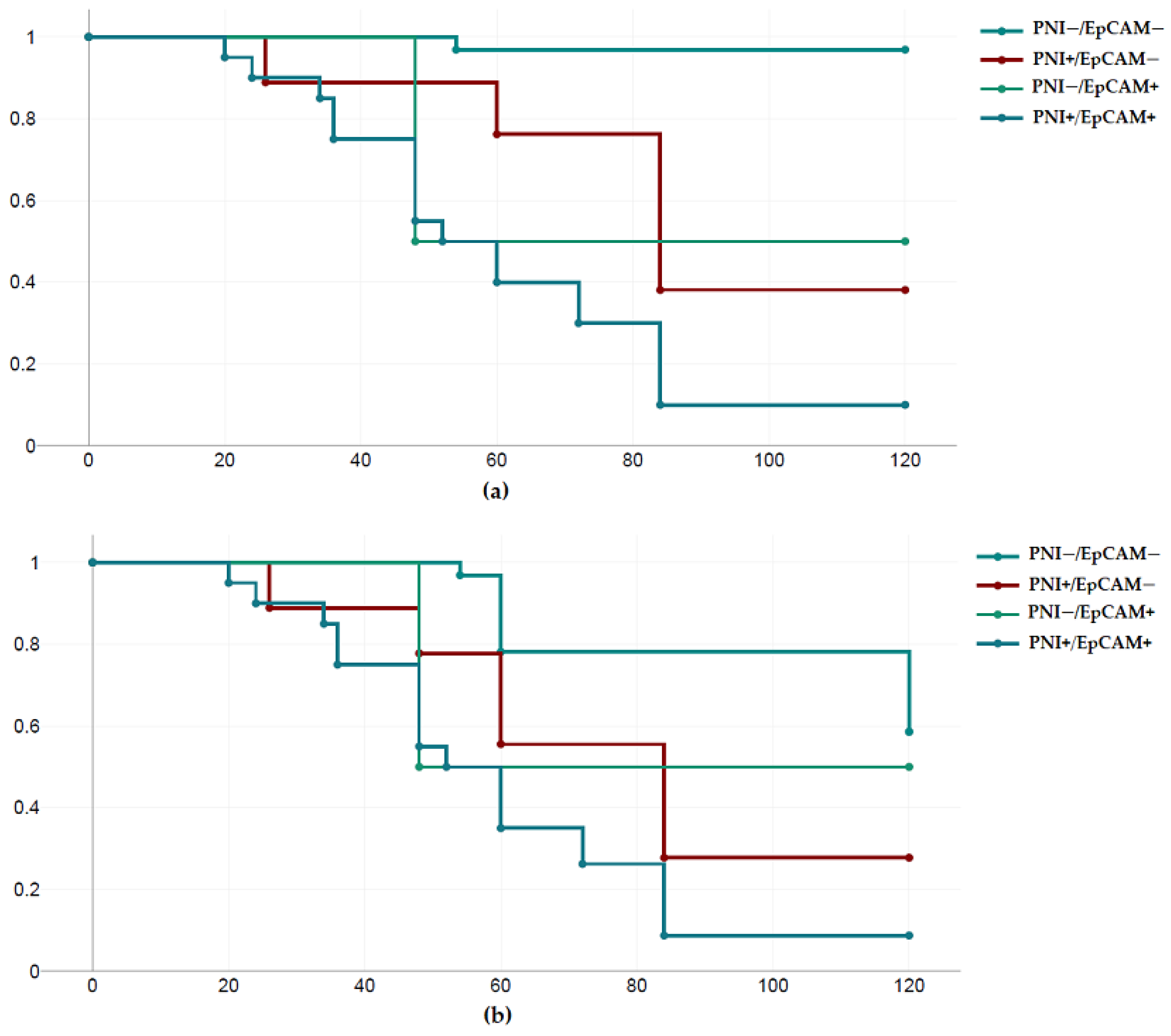
3.2. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kordzińska-Cisek, I.; Grzybowska-Szatkowska, L. Salivary gland cancer—Epidemiology. J. Oncol. 2018, 68, 22–27. [Google Scholar] [CrossRef]
- Ribeiro, A.; Carvalho, A.L.S.H.; Koth, V.S.; Campos, M.M. Salivary Gland Tumors: A Ten-Year Retrospective Analysis in a Brazilian Teaching Hospital. Rev. Bras. Cancerol. 2021, 67, e-041452. [Google Scholar] [CrossRef]
- Speight, P.M.; Barrett, A.W. Prognostic factors in malignant tumours of the salivary glands. Br. J. Oral. Maxillofac. Surg. 2009, 47, 587–593. [Google Scholar] [CrossRef]
- Ellis, G.L.; Auclair, P.L. The normal salivary gland. In Surgical Pathology of the Salivary Glands, 1st ed.; Ellis, G.L., Auclair, P.L., Gnepp, D.R., Eds.; WB Saunders: Philadelphia, PA, USA, 1991; pp. 1–26. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P. (Eds.) Tumours of the salivary glands. In World Health Organisation Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; pp. 159–202. [Google Scholar]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Pavsic, M.; Guncar, G.; Djinovic-Carugo, K.; Lenarcic, B. Crystal structure and its bearing towards an understanding of key biological functions of EpCAM. Nat. Commun. 2014, 5, 4764–4773. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, E.; Reid, L.M. EpCAM expression in normal, non-pathological tissues. Front. Biosci. J. Virtual Libr. 2008, 13, 3096–3100. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- RARECARE—Surveillance of Care Cancers in Europe. Available online: http://dcnapp4.dcn.ed.ac.uk/rcnet/searchpage.aspx (accessed on 6 May 2023).
- van Herpen, C.; Vander Poorten, V.; Skalova, A.; Terhaard, C.; Maroldi, R.; van Engen, A.; Baujat, B.; Locati, L.D.; Jensen, A.D.; Smeele, L.; et al. ESMO Guidelines Committee. Salivary gland cancer: ESMO-European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2022, 7, 100602. [Google Scholar] [CrossRef]
- Spiro, R.H.; Koss, L.G.; Hajdu, S.I.; Strong, E.W. Tumors of minor salivary origin: A clinicopathological study of 492 cases. Cancer 1973, 31, 117–129. [Google Scholar] [CrossRef]
- Tran, L.; Sadeghi, A.; Hanson, D.; Juillard, G.; Mackintosh, R.; Calcaterra, T.C.; Parker, R.G. Major salivary gland tumors: Treatment results and prognostic factors. Laryngoscope 1986, 96, 1139–1144. [Google Scholar] [CrossRef]
- Andersen, L.J.; Therkildsen, M.H.; Ockelmann, H.H.; Bentzen, J.D.; Schiødt, T.; Hansen, H.S. Malignant epithelial tumors in the minor salivary glands, the submandibular gland, and the sublingual gland. Prognostic factors and treatment results. Cancer 1991, 68, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Krutchkoff, D.; Pedersen, C.; Cartun, R.; Berman, M. PLGA of minor salivary glant: A clinicopathologic and comparative immunohiostochemical stydy. Mod. Pathol. 1990, 3, 76–82. [Google Scholar] [PubMed]
- McHugh, C.H.; Roberts, D.B.; El-Naggar, A.K.; Hanna, E.Y.; Garden, A.S.; Kies, M.S.; Weber, R.S.; Kupferman, M.E. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012, 118, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Beesley, L.J.; Russo, N.; Bellile, E.L.; Inglehart, R.C.; Liu, M.; Romanowicz, G.; Wolf, G.T.; Taylor, J.M.G.; D’Silva, N.J. Redefining perineural invasion: Integration of biology. With clinical outcome. Neoplasia 2018, 20, 657–667. [Google Scholar] [CrossRef]
- Shang, J.; Sheng, L.; Wang, K.; Shui, Y.; Wie, Q. Expression of neural cell adhesion molecule in salivary adenoid cystic carcinoma and its correlation with perineural invasion. Oncol. Rep. 2007, 18, 1413–1416. [Google Scholar] [CrossRef]
- Barrett, A.W.; Speight, P.M. Perineural invasion in Adenoid cystic carcinoma of the salivary glands: A valid prognostic indicator? Oral Oncol. 2009, 45, 936–940. [Google Scholar] [CrossRef]
- Huyett, P.; Duvvuri, U.; Ferris, R.L.; Johnson, J.T.; Schaitkin, B.M.; Kim, S. Perineural invasion in parotid gland malignancies. Otolaryngol. Head Neck Surg. 2018, 158, 1035–1041. [Google Scholar] [CrossRef]
- Amit, M.; Binenbaum, Y.; Trejo-Leider, L.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Miles, B.; Yang, X.; Lei, D. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015, 37, 1038–1045. [Google Scholar] [CrossRef]
- Nemzek, W.R.; Hecht, S.; Gandour-Edwards, R.; Donald, P.; McKennan, K. Perineural spread of head and neck tumors: How accurate is MR imaging? AJNR Am. J. Neuroradiol. 1998, 19, 701–706. [Google Scholar]
- Bianchi, B.; Copelli, C.; Cocchi, R.; Ferrari, S.; Pederneschi, N.; Sesenna, E. Adenoid cystic carcinoma of intraoral minor salivary glands. Oral Oncol. 2008, 44, 1026–1031. [Google Scholar] [CrossRef]
- Schwarz, S.; Muller, M.; Ettl, T.; Stockmann, P.; Zenk, J.; Agaimy, A. Morphological heterogeneity of oral salivary gland carcinomas: A Clinicopathologic study of 41 cases with long term follow-up emphasizing the overlapping spectrum of adenoid cystic carcinoma and polymorphous low-grade Adenocarcinoma. Int. J. Clin. Exp. Pathol. 2011, 4, 336–348. [Google Scholar]
- Liu, Y.; Li, H.; Gin, L.; Huang, X.; Su, M.; Han, Z. Prognostic factors in malignant sublingual salivary gland tumors. J. Oral Maxillofac. Surg. 2017, 75, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.N.; Morais, E.F.; Macedo, R.A.; Tinoco, J.M.; Morais, M. Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: A systematic review. Braz. J. Otorhinolaryngol. 2015, 81, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Eran, A.; Billan, S.; Fridman, E.; Na’Ara, S.; Charas, T.; Gil, Z. Perineural spread in noncutaneous head and neck cancer: New insights into an old problem. J. Neurol. Surg. B Skull Base 2016, 77, 86–95. [Google Scholar] [CrossRef]
- Gomez, D.R.; Hoppe, B.S.; Wolden, S.L.; Zhung, J.E.; Patel, S.G.; Kraus, D.H.; Shah, J.P.; Ghossein, R.A.; Lee, N.Y. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: A recent experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Rapidis, A.D.; Givalos, N.; Gakiopoulou, H.; Faratzis, G.; Stavrianos, S.D.; Vilos, G.A.; Douzinas, E.; Patsouris, E. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol. 2005, 41, 328–335. [Google Scholar] [CrossRef]
- Min, R.; Siyi, L.; Wenjun, Y.; Ow, A.; Lizheng, W.; Minjun, D.; Chenping, Z. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: A preliminary study of 62 cases. Int. J. Oral Maxillofac. Surg. 2012, 41, 952–957. [Google Scholar] [CrossRef]
- Garden, A.S.; Weber, R.S.; Morrison, W.H.; Ang, K.; Peters, L.J. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 619–626. [Google Scholar] [CrossRef]
- Chen, A.M.; Garcia, J.; Granchi, P.; Bucci, M.K.; Lee, N.Y. Base of Skull recurrences after treatment of salivary gland cancer with perineural invasion reduced by postoperative radiotherapy. Clin. Otolaryngol. 2009, 34, 539–545. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; A Baeuerle, P. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef] [PubMed]
- van der Fels, C.A.M.; Rosati, S.; de Jong, I.J. EpCAM expression in lymph node metastases of urothelial cell carcinoma of the bladder: A pilot study. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Werner, S.; Pantel, K. Biology and clinical relevance of EpCAM. Cell Stress 2019, 3, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Sun, S.; Chen, Z.; Xiang, S.; Ding, Z.; Huang, Z.; Zhang, B. Understanding the versatile roles and applications of EpCAM in cancers: From bench to bedside. Exp. Hematol. Oncol. 2022, 11, 97. [Google Scholar] [CrossRef]
- Ogura, E.; Senzaki, H.; Yoshizawa, K.; Hioki, K.; Tsubura, A. Immunohistochemical localization of epithelial glycoprotein EGP-2 and carcinoembryonic antigen in normal colonic mucosa and colorectal tumors. Anticancer Res. 1998, 18, 3669–3675. [Google Scholar]
- Xie, X.; Wang, C.Y.; Cao, Y.X.; Wang, W.; Zhuang, R.; Chen, L.-H.; Dang, N.-N.; Fang, L.; Jin, B.-Q. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J. Gastroenterol. 2005, 11, 344–347. [Google Scholar] [CrossRef][Green Version]
- Phattarataratip, E.; Masorn, M.; Jarupoonphol, W.; Supatthanayut, S.; Saeoweiang, P. Differential expression of epithelial cell adhesion molecule in salivary gland neoplasms. Ann. Diagn. Pathol. 2016, 24, 62–67. [Google Scholar] [CrossRef]
- Lee, S.J.; Chung, K.Y.; Kwon, J.E.; Yoon, S.O.; Kim, S.K. Expression of EpCAM in adenoid cystic carcinoma. Pathology 2018, 7, 737–741. [Google Scholar] [CrossRef]
- Ordonez, N.G. Value of the MOC-31 monoclonal antibody in differentiating epithelial pleural mesothelioma from lung adenocarcinoma. Hum. Pathol. 1998, 29, 166–169. [Google Scholar] [CrossRef]
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004, 64, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Yaziji, H.; Battifora, H.; Barry, T.S.; Hwang, H.C.; Bacchi, C.E.; McIntosh, M.W.; Kussick, S.J.; Gown, A.M. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: Identification of a three-antibody immunohistochemical panel with maximal sensitivity and specificity. Mod. Pathol. 2006, 19, 514–523. [Google Scholar] [CrossRef] [PubMed]
| Histopathological Subtypes of Salivary Gland Cancer | n |
|---|---|
| Adenocarcinoma (NOS) | 4 |
| Adenoid cystic carcinoma (AdCC) | 18 |
| Mucoepidermoid carcinoma (MEC) | 20 |
| Polymorphous adenocarcinoma (PAC) | 9 |
| Epithelial–myoepithelial carcinoma | 5 |
| Acinic cell carcinoma (AcCC) | 2 |
| Salivary duct carcinoma | 5 |
| Carcinoma–ex pleomorphic adenoma | 2 |
| Age | |
| ≤50 | 21 |
| >50 | 44 |
| Gender | |
| Male | 30 |
| Female | 35 |
| Site | |
| Major salivary glands | |
| Parotid gland | 24 |
| Submandibular gland | 4 |
| Sublingual gland | 2 |
| Minor salivary glands | |
| Palate | 21 |
| Tongue/floor of mouth | 4 |
| Upper lip | 2 |
| Lower lip | 2 |
| Retromolar mucosa | 3 |
| Buccal mucosa | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaitsidou, I.; Pasteli, N.; Venetis, G.; Poulopoulos, A.; Antoniades, K. Immunohistochemical Expression of Epithelial Cell Adhesion Molecule (EpCAM) in Salivary Gland Cancer: Correlation with the Biological Behavior. Diagnostics 2023, 13, 2652. https://doi.org/10.3390/diagnostics13162652
Kalaitsidou I, Pasteli N, Venetis G, Poulopoulos A, Antoniades K. Immunohistochemical Expression of Epithelial Cell Adhesion Molecule (EpCAM) in Salivary Gland Cancer: Correlation with the Biological Behavior. Diagnostics. 2023; 13(16):2652. https://doi.org/10.3390/diagnostics13162652
Chicago/Turabian StyleKalaitsidou, Ioanna, Nikoleta Pasteli, Gregory Venetis, Athanasios Poulopoulos, and Konstantinos Antoniades. 2023. "Immunohistochemical Expression of Epithelial Cell Adhesion Molecule (EpCAM) in Salivary Gland Cancer: Correlation with the Biological Behavior" Diagnostics 13, no. 16: 2652. https://doi.org/10.3390/diagnostics13162652
APA StyleKalaitsidou, I., Pasteli, N., Venetis, G., Poulopoulos, A., & Antoniades, K. (2023). Immunohistochemical Expression of Epithelial Cell Adhesion Molecule (EpCAM) in Salivary Gland Cancer: Correlation with the Biological Behavior. Diagnostics, 13(16), 2652. https://doi.org/10.3390/diagnostics13162652







