Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study
Abstract
1. Introduction
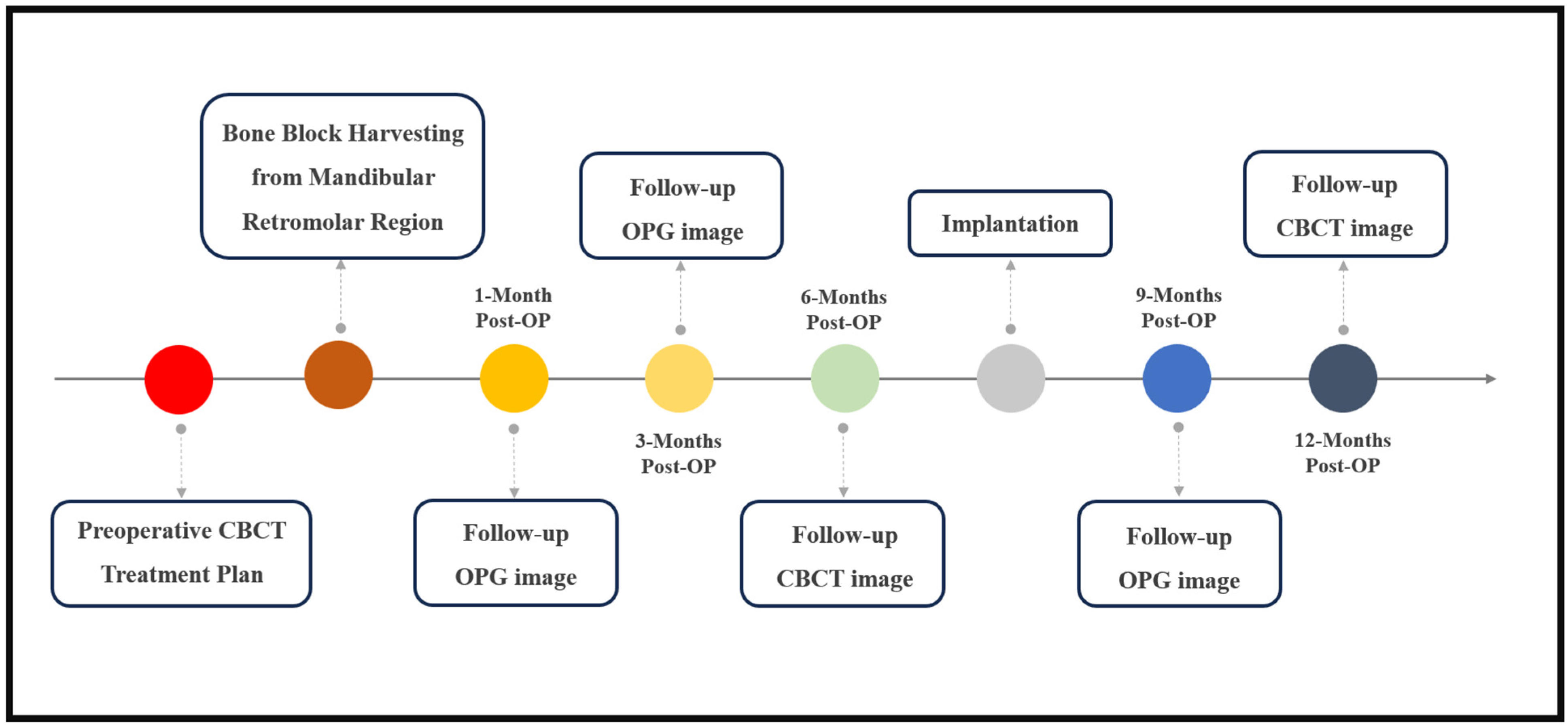
2. Materials and Methods
2.1. Pre-Operative and Follow-Up Radiological Evaluation
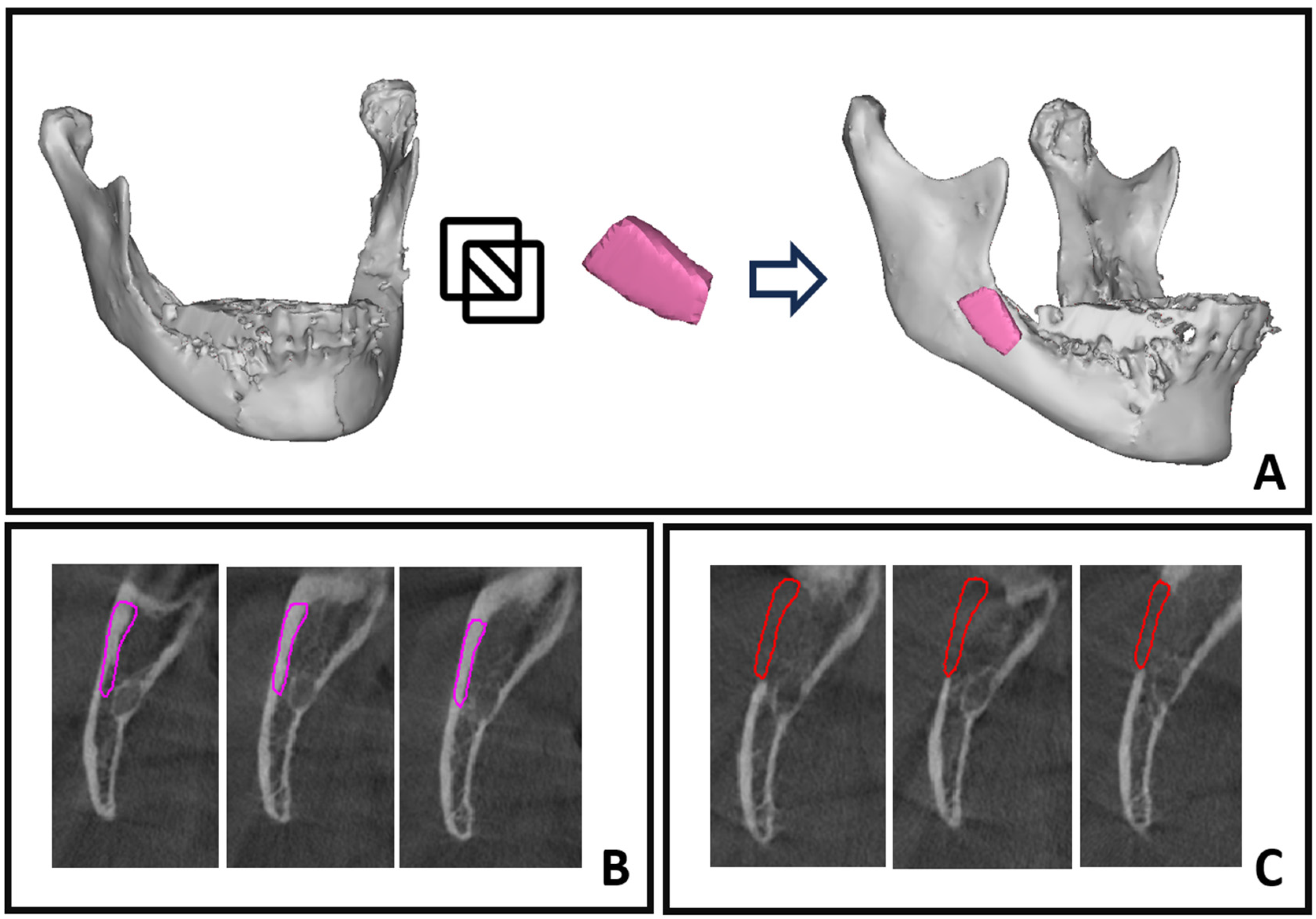
2.2. Surgical Procedure
2.3. Intraoperative Bone Graft Scan
2.4. Computer-Assisted Bone Healing Evaluation
2.5. Segmentation by Thresholding
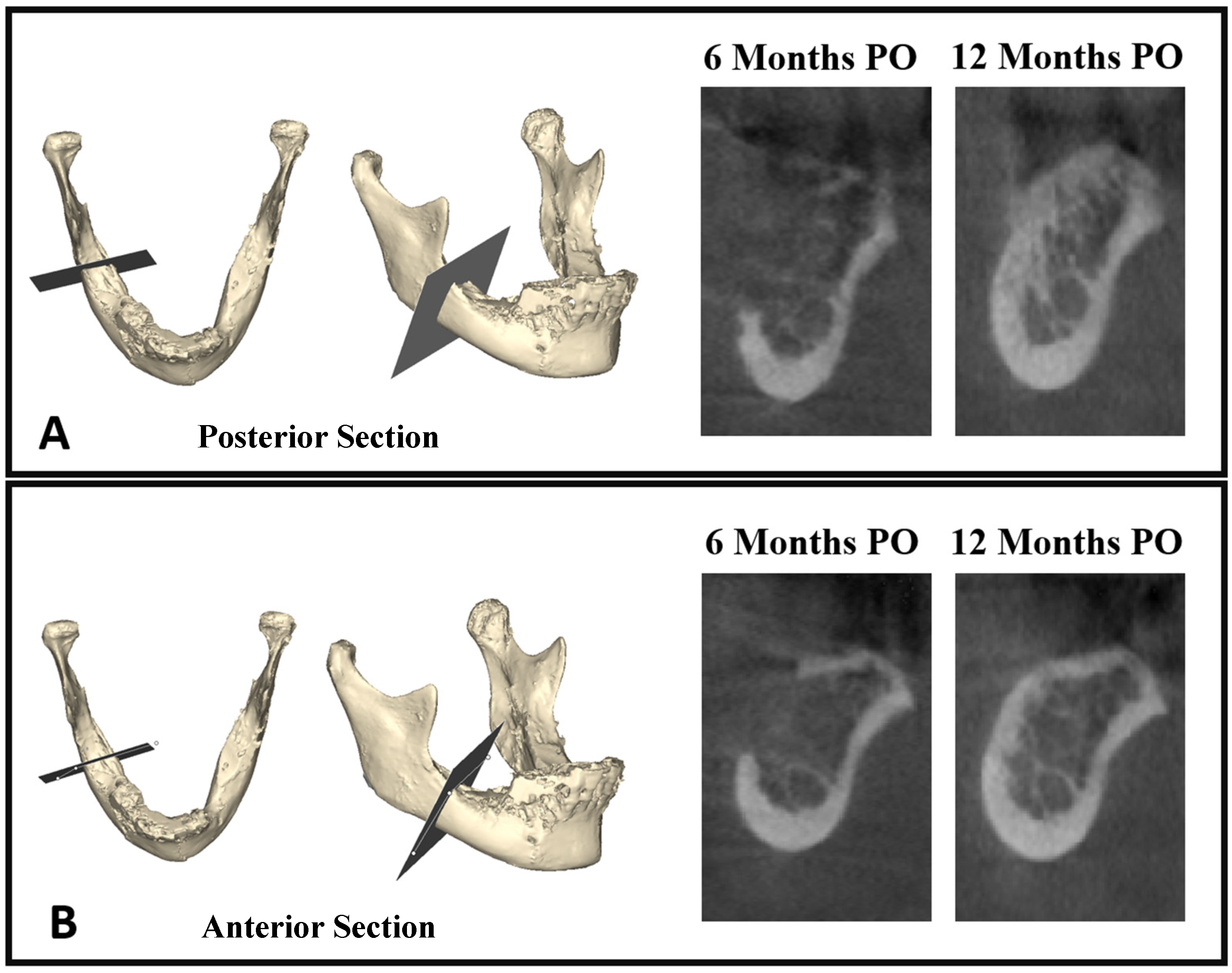
2.6. Superimposition of Objects and Boolean Subtraction
2.7. Hounsfield Unit Averaging
2.8. Statistical Analysis
3. Results
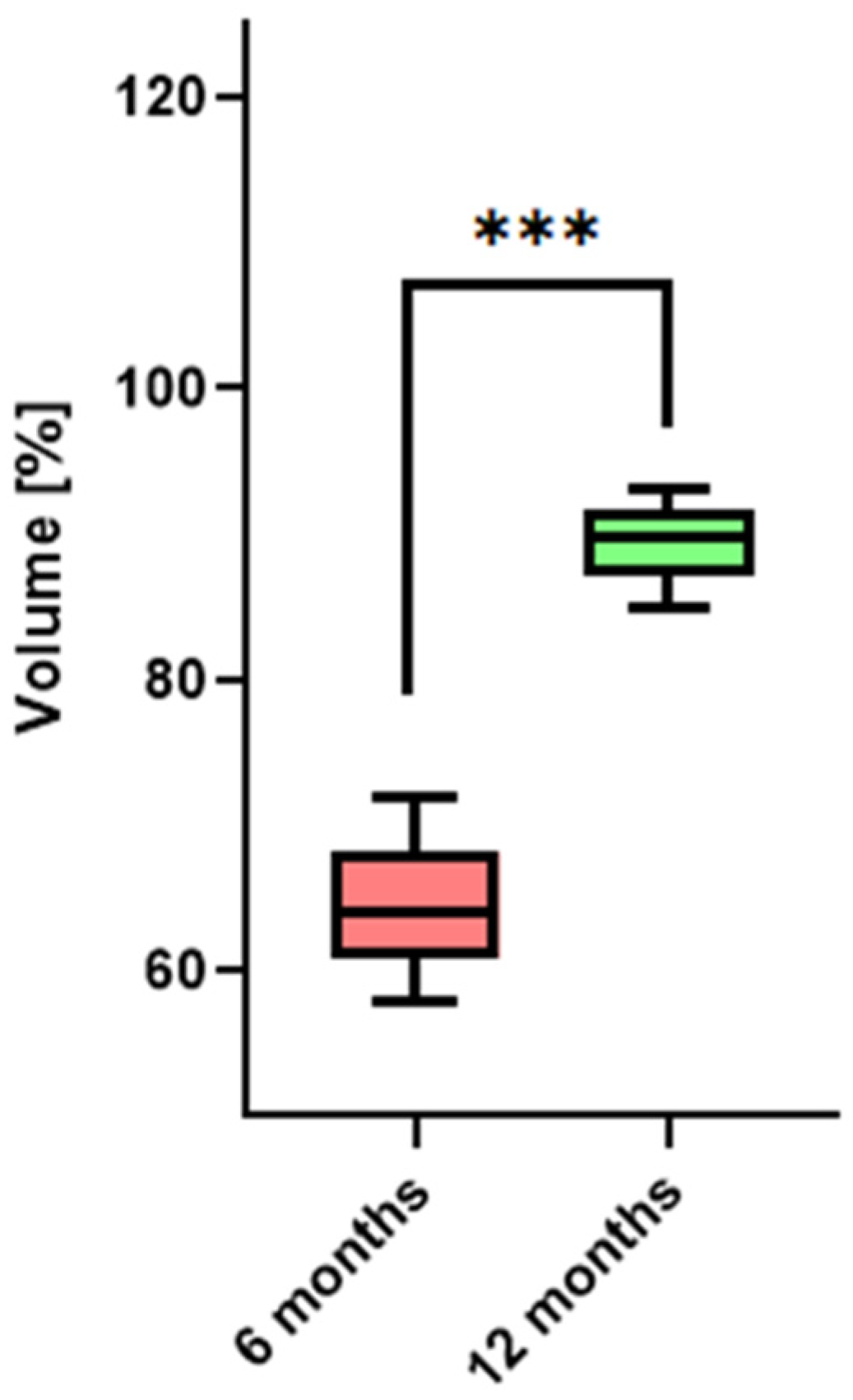
3.1. Bone Healing at Donor Site—Volumetric Evaluation
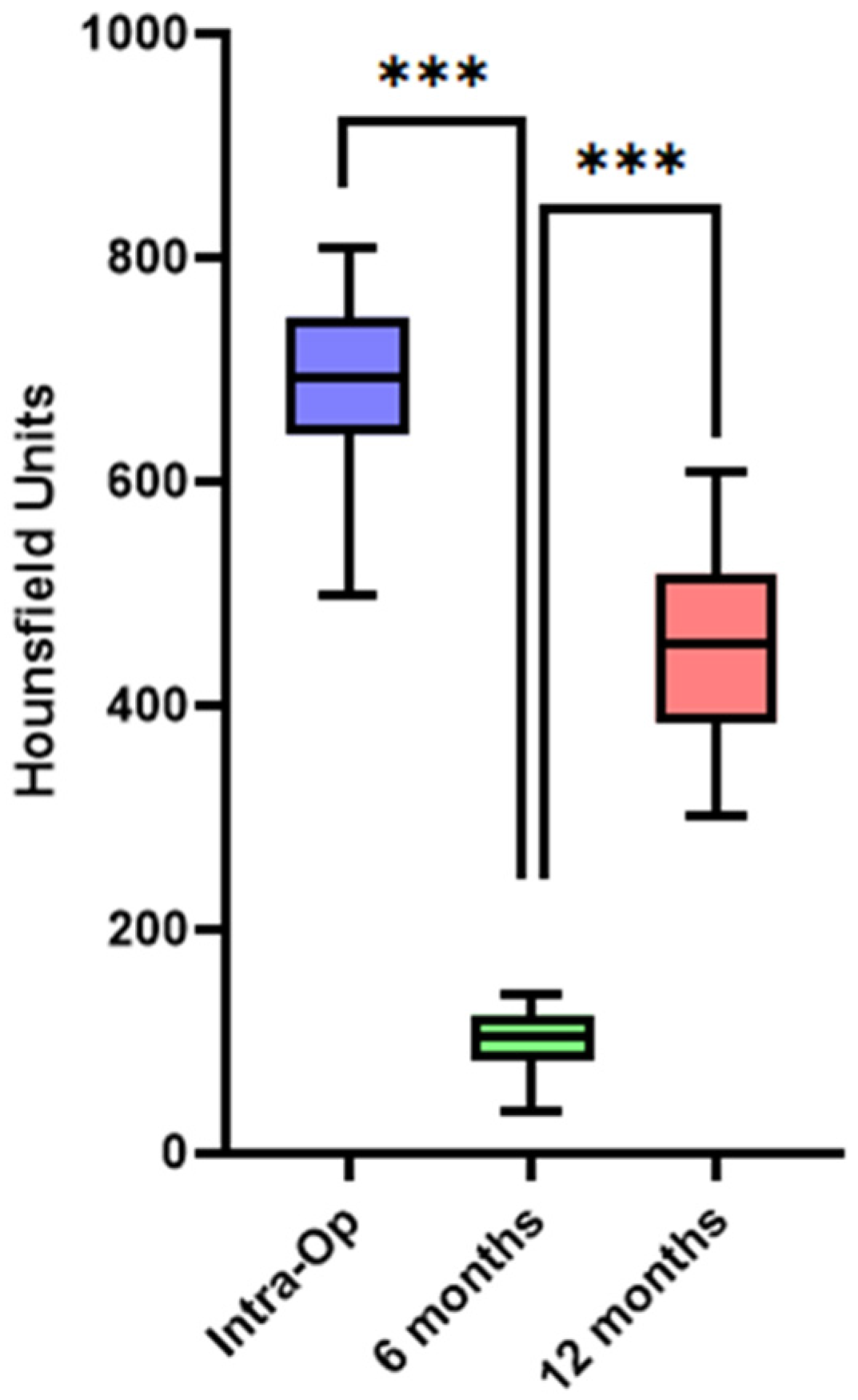
3.2. Bone Healing at Donor Site—Quality/Density Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- French, D.; Ofec, R.; Levin, L. Long term clinical performance of 10,871 dental implants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral. Maxillofac. Implant. 2009, 24, 237–259. [Google Scholar]
- Herford, A.S.; Nguyen, K. Complex bone augmentation in alveolar ridge defects. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 227–244. [Google Scholar] [CrossRef]
- Stern, A.; Barzani, G. Autogenous bone harvest for implant reconstruction. Dent. Clin. N. Am. 2015, 59, 409–420. [Google Scholar] [CrossRef]
- Mertens, C.; Decker, C.; Seeberger, R.; Hoffmann, J.; Sander, A.; Freier, K. Early bone resorption after vertical bone augmentation—A comparison of calvarial and iliac grafts. Clin. Oral Implant. Res. 2013, 24, 820–825. [Google Scholar] [CrossRef]
- Yates, D.M.; Brockhoff, H.C., 2nd; Finn, R.; Phillips, C. Comparison of intraoral harvest sites for corticocancellous bone grafts. J. Oral Maxillofac. Surg. 2013, 71, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sittitavornwong, S.; Gutta, R. Bone graft harvesting from regional sites. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Iezzi, G.; Lizio, G.; Piattelli, A.; Marchetti, C. Reconstruction of atrophied posterior mandible with inlay technique and mandibular ramus block graft for implant prosthetic rehabilitation. J. Oral Maxillofac. Surg. 2009, 67, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Arad, D.; Levin, L.; Sigal, L. Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant. Dent. 2005, 14, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Arad, D.; Levin, L. Symphysis revisited: Clinical and histologic evaluation of newly formed bone and reharvesting potential of previously used symphysial donor sites for onlay bone grafting. J. Periodontol. 2009, 80, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, F.; Simonian, K.; D’Addona, A.; Pontón, J.; Nowzari, H. Human bone repair after mandibular symphysis block harvesting: A clinical and tomographic study. J. Periodontol. 2010, 81, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Pikos, M.A. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2005, 13, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Fontao, F.N.G.K.; Diez, G.F.; Bassi, A.P.F.; Claudino, M. Second Harvest of Mandibular Ramus Blocks in Bone Augmentation Procedures: A Case Letter. J. Oral Implantol. 2014, 40, 397–400. [Google Scholar] [CrossRef]
- Diez, G.F.; Fontão, F.N.; Bassi, A.P.; Gama, J.C.; Claudino, M. Tomographic follow-up of bone regeneration after bone block harvesting from the mandibular ramus. Int. J. Oral Maxillofac. Surg. 2014, 43, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, D.; Fiorino, P.; Corega, C.; Sbricoli, L.; De Santis, D.; Donadello, D.; Ricciardi, G.; Luciano, U.; Bressan, E.; Pardo, A.; et al. Cone-beam volumetric imaging in craniofacial medicine. Minerva Stomatol. 2019, 68, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.-K.; Han, M.; Yongvikul, A.; Huh, J.-K.; Kim, J.-Y. Volumetric analysis of spontaneous bone healing after jaw cyst enucleation. Sci. Rep. 2022, 12, 14953. [Google Scholar] [CrossRef]
- Shapurian, T.; Damoulis, P.D.; Reiser, G.M.; Griffin, T.J.; Rand, W.M. Quantitative evaluation of bone density using the Hounsfield index. Int. J. Oral Maxillofac. Implant. 2006, 21, 290–297. [Google Scholar]
- Fekry, Y.E.; Mahmoud, N.R. Vertical ridge augmentation of atrophic posterior mandible with corticocancellous onlay symphysis graft versus sandwich technique: Clinical and radiographic analysis. Odontology 2023, 111, 993–1002. [Google Scholar] [CrossRef]
- Diz Dios, P.; Monteiro, L.; Pimolbutr, K.; Gobbo, M.; France, K.; Bindakhil, M.; Holmes, H.; Sperotto, F.; Graham, L.; Turati, F.; et al. World Workshop on Oral Medicine VIII: Dentists’ compliance with infective endocarditis prophylaxis guidelines for patients with high-risk cardiac conditions: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 757–771. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Mandibular bone block harvesting from the retromolar region: A 10-year prospective clinical study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med. Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Cevidanes, L.H.; Heymann, G.; Cornelis, M.A.; DeClerck, H.J.; Tulloch, J.F. Superimposition of 3-dimensional cone-beam computed tomography models of growing patients. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 94–99. [Google Scholar] [CrossRef]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. CBCT-based bone quality assessment: Are Hounsfield units applicable? Dentomaxillofac. Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef] [PubMed]
- Khoury, F.; Antoun, A.; Missika, P. Bone Augmentation in Oral Implantology; Quintessenz: Berlin, Germany; London, UK, 2007. [Google Scholar]
- Kablan, F. Wedge Technique; Clinical Innovation Committee 25 Annual Meeting; Academy of Osseointegration: Orlando, FL, USA, 2010. [Google Scholar]
- Kablan, F.; Laster, Z. The use of free fat tissue transfer from the buccal fat pad to obtain and maintain primary closure and to improve soft tissue thickness at bone-augmented sites: Technique presentation and report of case series. Int. J. Oral Maxillofac. Implant. 2014, 29, e220–e231. [Google Scholar] [CrossRef]
- Kablan, F. The Use of Cortical Bone Wedges from the Mandibular Ramus “Wedge Technique” for 3-Dimensional Bone Augmentation of the Atrophic Ridges. In Current Concepts in Dental Implantology-From Science to Clinical Research; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]



| Intraoperative Scanning for Bone Graft Volume | Residual Bony Defect 6 Months Postoperative | Residual Bony Defect 1 Year Postoperative | |
|---|---|---|---|
| 1 | 625.3 | 250.1 | 75.1 |
| 2 | 523.5 | 191.2 | 73.29 |
| 3 | 492.1 | 201.7 | 49.2 |
| 4 | 612.9 | 257.5 | 48.6 |
| 5 | 710.2 | 228.3 | 106.5 |
| 6 | 582.5 | 210.4 | 52.4 |
| 7 | 645.2 | 212.2 | 83.8 |
| 8 | 572.7 | 194.8 | 45.7 |
| 9 | 485.7 | 190.3 | 53.4 |
| 10 | 593.3 | 188.2 | 59.1 |
| 11 | 638.9 | 190.5 | 95.8 |
| 12 | 521.8 | 146.1 | 36.5 |
| 13 | 702.7 | 203.8 | 86.2 |
| 14 | 542.1 | 226.3 | 55.7 |
| 15 | 740.1 | 270.8 | 74.3 |
| 16 | 510.4 | 188.4 | 45.9 |
| 17 | 650.9 | 227.3 | 78.1 |
| 18 | 725.7 | 229.1 | 101.6 |
| 19 | 622.5 | 242.7 | 50.1 |
| 20 | 632.4 | 225.4 | 44.2 |
| Intraoperative Scanning for Bone Graft | CBCT 6 Months Follow-Up Scan | CBCT 12 Months Follow-Up Scan | |
|---|---|---|---|
| 1 | 752 | 124 | 561 |
| 2 | 712 | 56 | 457 |
| 3 | 643 | 113 | 503 |
| 4 | 784 | 88 | 610 |
| 5 | 578 | 110 | 364 |
| 6 | 801 | 78 | 580 |
| 7 | 595 | 38 | 312 |
| 8 | 735 | 140 | 455 |
| 9 | 683 | 124 | 423 |
| 10 | 810 | 133 | 593 |
| 11 | 498 | 98 | 301 |
| 12 | 654 | 128 | 417 |
| 13 | 727 | 141 | 472 |
| 14 | 674 | 89 | 393 |
| 15 | 729 | 122 | 475 |
| 16 | 641 | 76 | 443 |
| 17 | 705 | 99 | 524 |
| 18 | 795 | 117 | 485 |
| 19 | 658 | 81 | 328 |
| 20 | 633 | 96 | 382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoud, S.; Zoabi, A.; Kasem, A.; Totry, A.; Oren, D.; Redenski, I.; Srouji, S.; Kablan, F. Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study. Diagnostics 2024, 14, 504. https://doi.org/10.3390/diagnostics14050504
Daoud S, Zoabi A, Kasem A, Totry A, Oren D, Redenski I, Srouji S, Kablan F. Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study. Diagnostics. 2024; 14(5):504. https://doi.org/10.3390/diagnostics14050504
Chicago/Turabian StyleDaoud, Shadi, Adeeb Zoabi, Adi Kasem, Amir Totry, Daniel Oren, Idan Redenski, Samer Srouji, and Fares Kablan. 2024. "Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study" Diagnostics 14, no. 5: 504. https://doi.org/10.3390/diagnostics14050504
APA StyleDaoud, S., Zoabi, A., Kasem, A., Totry, A., Oren, D., Redenski, I., Srouji, S., & Kablan, F. (2024). Computer-Assisted Evaluation Confirms Spontaneous Healing of Donor Site One Year following Bone Block Harvesting from Mandibular Retromolar Region—A Cohort Study. Diagnostics, 14(5), 504. https://doi.org/10.3390/diagnostics14050504






