Comparative Effectiveness of Two Models of Point-of-Care Ultrasound for Detection of Post-Void Residual Urine during Acute Ischemic Stroke: Preliminary Findings of Real-World Clinical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Instruments and Measurements
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics
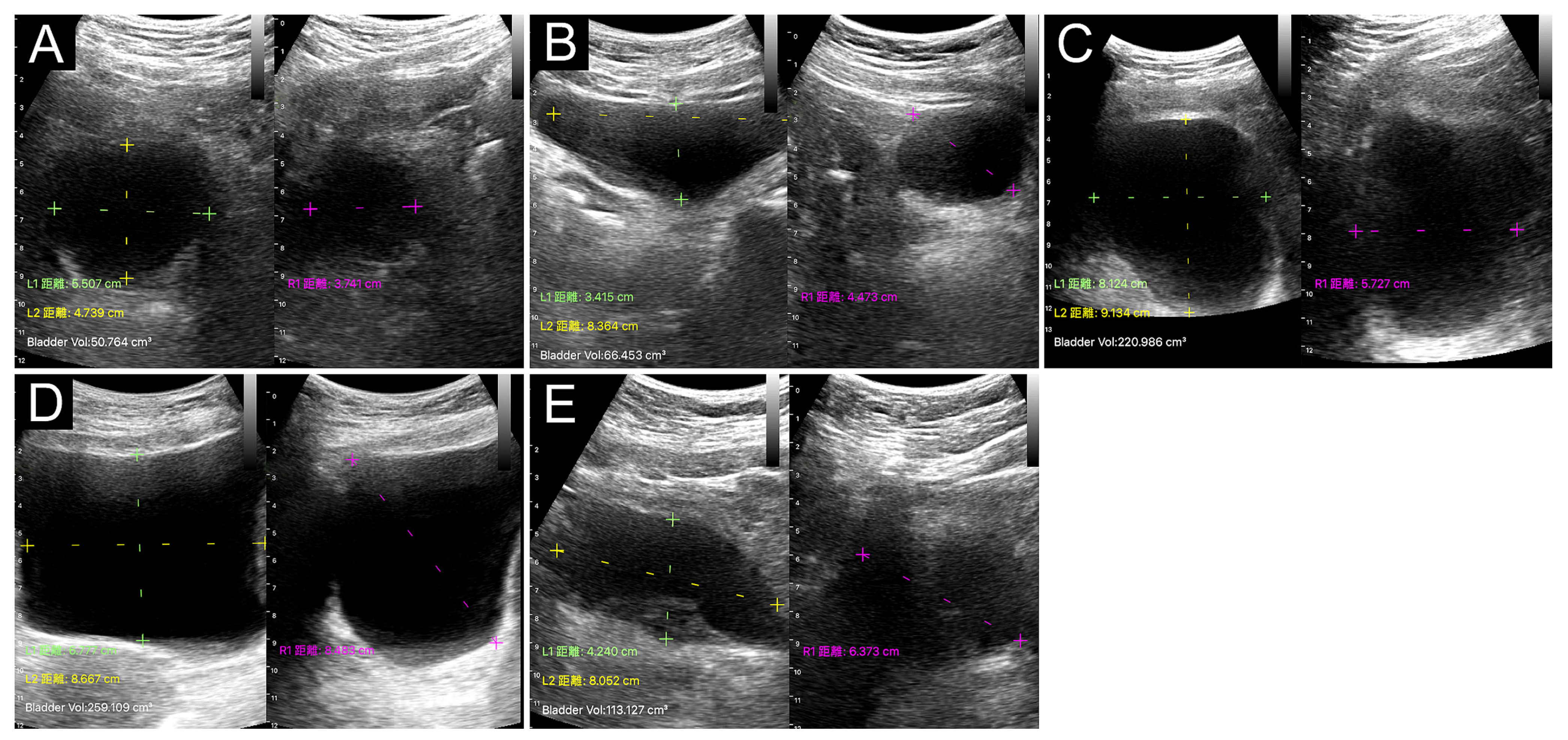
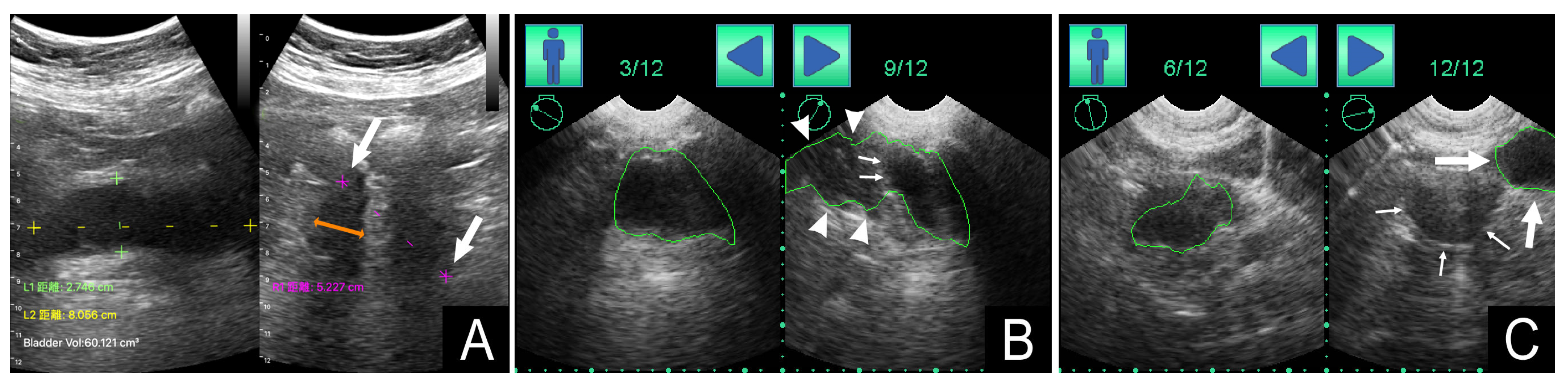
3.2. Bladder POCUS Findings
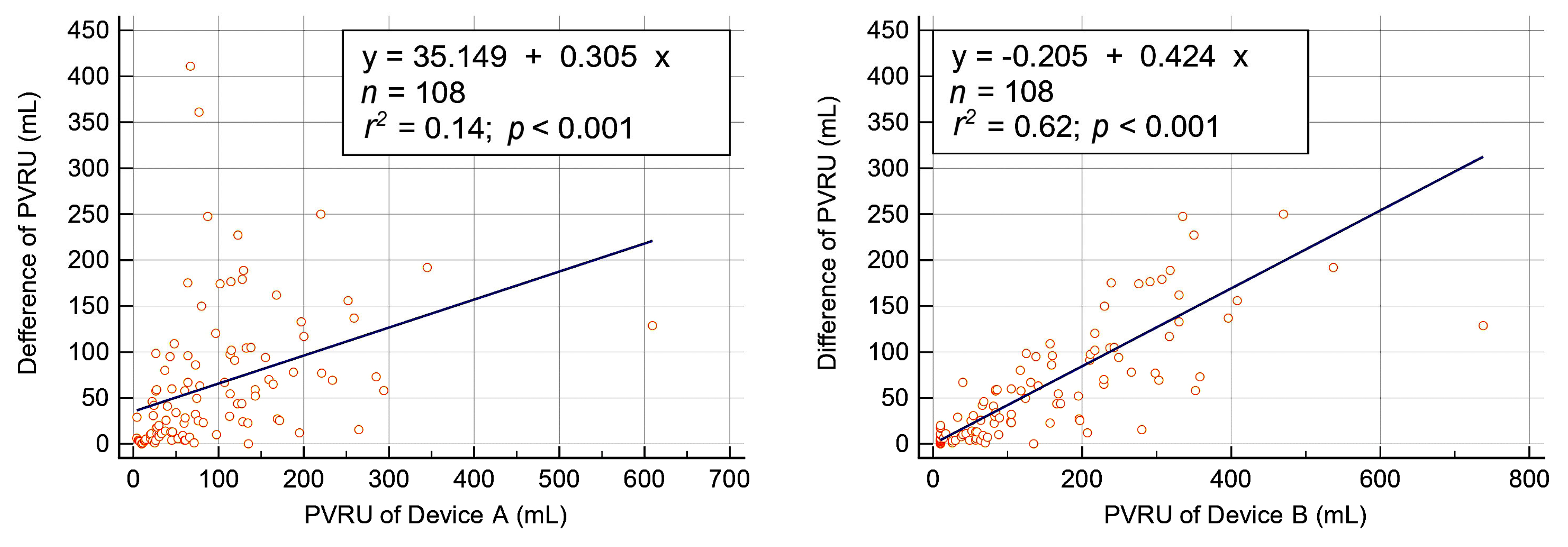
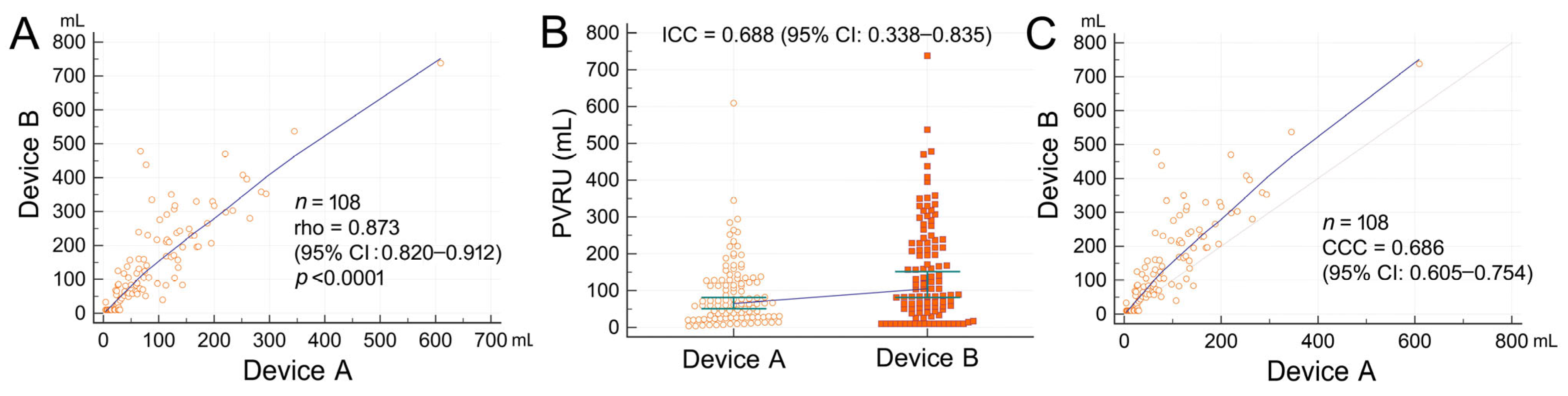
3.3. Comparison of PVRU Volumes Measured with POCUS and Catheterization
3.4. Measurement Deviations of Bladder POCUS
3.5. Correlation between Bladder Shape and PVRU Volume
3.6. Questionnaire Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arboix, A.; Massons, J.; García-Eroles, L.; Targa, C.; Oliveres, M.; Comes, E. Clinical predictors of prolonged hospital stay after acute stroke: Relevance of medical complications. Int. J. Clin. Med. 2012, 3, 502–507. [Google Scholar] [CrossRef]
- Burney, T.L.; Senapati, M.; Desai, S.; Choudhary, S.T.; Badlani, G.H. Acute cerebrovascular accident and lower urinary tract dysfunction: A prospective correlation of the site of brain injury with urodynamic findings. J. Urol. 1996, 156, 1748–1750. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.H.; Lo, S.F.; Chou, L.W.; Yang, P.Y.; Chang, C.H.; Chou, E.C. Incomplete bladder emptying in patients with stroke: Is detrusor external sphincter dyssynergia a potential cause? Arch. Phys. Med. Rehab. 2010, 91, 1105–1109. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, P.Y.; Chen, G.C.; Chuang, S.Y.; Tzeng, I.S.; Lin, S.K. Portable bladder ultrasound reduces incidence of urinary tract infection and shortens hospital length of stay in patients with acute ischemic stroke. J. Cardiovasc. Nurs. 2018, 33, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.H.; Cross, S.; Barrett, J.; French, B.; Leathley, M.; Sutton, C.J.; Watkins, C. Treatment of urinary incontinence after stroke in adults. Cochrane Database Syst. Rev. 2008, 23, CD004462. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Young, S. Incidence and outcome of poststroke urinary retention: A prospective study. Arch. Phys. Med. Rehab. 2000, 81, 1464–1467. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Mayo, P.H.; Koenig, S.J. Point-of care ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef]
- Lewis, N.A. Implementing a bladder ultrasound program. Rehabil. Nurs. 1995, 204, 215–217. [Google Scholar] [CrossRef]
- Erasmie, U.; Lidefelt, K.J. Accuracy of ultrasonic assessment of residual urine in children. Pediatr. Radiol. 1989, 19, 388–390. [Google Scholar] [CrossRef]
- Dicuio, M.; Pomara, G.; Menchini Fabris, F.; Ales, V.; Dahlstrand, C.; Morelli, G. Measurements of urinary bladder volume: Comparison of five ultrasound calculation methods in volunteers. Arch. Ital. Urol. Androl. 2005, 77, 60–62. [Google Scholar]
- Chang, S.J.; Chiang, I.N.; Hsieh, C.H.; Lin, C.D.; Yang, S.D. Age- and gender-specific nomograms for single and dual post-void residual urine in healthy children. Neurourol. Urodynam. 2013, 32, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Bih, L.I.; Ho, C.C.; Tsai, S.J.; Lai, Y.C.; Chow, W. Bladder shape impact on the accuracy of ultrasonic estimation of bladder volume. Arch. Phys. Med. Rehabil. 1998, 79, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Palese, A.; Buchini, S.; Deroma, L.; Barbone, F. The effectiveness of the ultrasound bladder scanner in reducing urinary tract infections: A meta-analysis. J. Clin. Nurs. 2010, 19, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- Hvarness, H.; Skjoldbye, B.; Jakobsen, H. Urinary bladder volume measurements: Comparison of three ultrasound calculation methods. Scand. J. Urol. Nephrol. 2002, 36, 177–181. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Peters, P.C. Can transabdominal ultrasound estimation of postvoiding residual (PVR) replace catheterization? Urology 1998, 18, 445–449. [Google Scholar] [CrossRef]
- Kuzmić, A.C.; Brkljacić, B.; Ivanković, D. The impact of bladder shape on the ultrasonographic measurement of bladder volume in children. Pediatr. Radiol. 2003, 33, 530–534. [Google Scholar] [CrossRef]
- Cho, H.; Song, I.; Jang, J.; Yoo, Y. A lightweight deep learning network on a system-on-chip for wearable ultrasound bladder volume measurement systems: Preliminary study. Bioengineering 2023, 10, 525. [Google Scholar] [CrossRef]
- Teng, C.H.; Huang, Y.H.; Kuo, B.J.; Bih, L.I. Application of portable ultrasound scanners in the measurement of post-void residual urine. J. Nurs. Res. 2005, 13, 216–224. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tsay, W.L.; Lou, M.F.; Dai, Y.T. The effectiveness of implementing a bladder ultrasound programme in neurosurgical units. J. Adv. Nurs. 2007, 57, 192–200. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Chambers, S.K.; Rutherford, T.J.; Foster, H.E., Jr. Cystic pelvic pathology presenting as falsely elevated post-void residual urine measured by portable ultrasound bladder scanning: Report of 3 cases and review of the literature. Urology 2000, 55, 590. [Google Scholar] [CrossRef]
- Sullivan, R.; Baston, C.M. When not to trust the bladder scanner. The use of point-of-care ultrasound to estimate urinary bladder volume. Ann. Am. Thorac. Soc. 2019, 16, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, T.A.; van den Boogaard, C.; van Roon, E.N.; Kalkman, C.J.; Veeger, N. Non-invasive bladder volume measurement for the prevention of postoperative urinary retention: Validation of two ultrasound devices in a clinical setting. J. Clin. Monit. Comput. 2018, 32, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.J.; Kirkland, M.; Lovett, J.; Watson, A.R. Clinical agreement between automated and calculated ultrasound measurements of bladder volume. Br. J. Radiol. 2003, 76, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Prentice, D.M.; Sona, C.; Wessman, B.T.; Ablordeppey, E.A.; Isakow, W.; Arroyo, C.; Schallom, M. Discrepancies in measuring bladder volumes with bedside ultrasound and bladder scanning in the intensive care unit: A pilot study. J. Intensive Care. Soc. 2018, 19, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Yabunaka, K.; Yoshida, M.; Nakagami, G.; Sanada, H. Validity assessment of two bladder volume estimation methods using hand-held ultrasonography devices: Verification with a small amount of bladder urine. J. Nurs. Sci. Eng. 2019, 6, 22–32. [Google Scholar] [CrossRef]
- Small, D.R.; Watson, A.; McConnachie, A. A quantitative comparison of four current portable ultrasound bladder scanners. Br. J. Med. Surg. Urol. 2008, 1, 35–40. [Google Scholar] [CrossRef]
- Nagle, A.S.; Bernardo, R.J.; Varghese, J.; Carucci, L.R.; Klausner, A.P.; Speich, J.E. Comparison of 2D and 3D ultrasound methods to measure serial bladder volumes during filling: Steps toward development of non-invasive ultrasound urodynamics. Bladder 2018, 5, e32. [Google Scholar] [CrossRef]

| Characteristics | Total (n = 55) | Men (n = 23) | Women (n = 32) | p Value |
|---|---|---|---|---|
| Age (years) | 74 (65–85) | 80 (70–84) | 71 (63–85) | 0.194 |
| Body mass index | 23.4 (21.4–26.7) | 24.7 (22.8–27.3) | 23.2 (20.9–24.9) | 0.031 |
| AGN3 score | 2 (1–2) | 1 (1–2) | 2 (1–3) | 0.102 |
| Number of POCUS measurements | 108 | 44 | 64 | |
| TOAST classification | ||||
| Small artery occlusion | 28 | 10 | 18 | |
| Large artery atherosclerosis | 16 | 9 | 8 | |
| Cardioembolism | 9 | 4 | 4 | |
| Other determined etiology | 2 | 0 | 2 |
| Device | Total (n = 108) | Men (n = 43) | Women (n = 65) | p Value 1 |
|---|---|---|---|---|
| Device A (mL) | 65 (28–129) | 113 (46–158) | 52 (26–105) | 0.013 |
| Device B (mL) | 105 (42–230) | 159 (82–237) | 73 (26–220) | 0.042 |
| p value 2 | <0.001 | <0.001 | <0.001 | |
| Device A PVRU > 100 mL | 40 (37%) | 23 (53%) | 17 (26%) | 0.005 |
| Device B PVRU > 100 mL | 56 (52%) | 28 (65%) | 28 (43%) | 0.031 |
| Device A | Device B | |||||
|---|---|---|---|---|---|---|
| Characteristics | Deviations (n = 11; 10%) | No Deviations (n = 97; 90%) | p Value | Deviations (n = 43; 40%) | No Deviations (n = 65; 60%) | p Value |
| Age (years) | 83 (73–87) | 71 (65–84) | 0.099 | 75 (66–84) | 72 (64–85) | 0.702 |
| Female sex | 5 (45%) | 60 (62%) | 0.339 | 25 (58%) | 40 (62%) | 0.841 |
| BMI | 27.3 (22.8–27.6) | 23.3 (21.2–26.1) | 0.101 | 24.1 (21.7–27.3) | 23.2 (21.1–24.9) | 0.139 |
| PVRU (mL) | 97 (65–133) | 64 (26–129) | 0.089 | 105 (71–215) | 89 (10–253) | 0.344 |
| PVRU Volume (mL) | Measurement Deviations | Gender | ||||
|---|---|---|---|---|---|---|
| Bladder Shape | Device A (n = 108) | Device B (n = 108) | Device A (n = 11) | Device B (n = 43) | Women (n = 64) | Men (n = 44) |
| Spherical (n = 32) | 26 (12–46) | 26 (10–67) | 1 (3%) | 13 (41%) | 23 (72%) | 9 (28%) |
| Triangular (n = 24) | 43 (26–74) | 71 (46–105) | 2 (8%) | 9 (38%) | 16 (67%) | 8 (33%) |
| Undefined (n = 11) | 61 (40–91) | 89 (49–152) | 4 (36%) | 6 (55%) | 6 (55%) | 5 (45%) |
| Ellipsoid (n = 18) | 135 (114–159) | 204 (141–276) | 3 (17%) | 7 (39%) | 5 (28%) | 13 (72%) |
| Cuboid (n = 23) | 188 (124–257) | 317 (244–386) | 1 (4%) | 8 (35%) | 15 (65%) | 8 (35%) |
| p value | <0.001 * | <0.001 * | 0.019 ** | 0.860 ** | 0.033 ** | |
| PVRU (mL) | Difference (%) | Agreement | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Mean | Median | ICC | CCC | ||
| Total measurements (n = 60) | |||||||
| Device B | 135 ± 135 | 68 (10–237) | |||||
| Device A | |||||||
| 0.52 | 89 ± 87 | 61 (21–135) | 34 ± 22 | 30 (17–52) | 0.762 | 0.759 | |
| 0.66 | 113 ± 110 | 78 (27–171) | 29 ± 23 | 23 (9–48) | 0.872 | 0.870 | |
| 0.72 | 124 ± 121 | 85 (29–187) | 30 ± 23 | 25 (10–47) | 0.902 | 0.901 | |
| 0.81 | 139 ± 136 | 95 (33–210) | 32 ± 21 | 29 (15–49) | 0.894 | 0.892 | |
| Measurement with PVRU < 100 mL (n = 31) | |||||||
| Device B | 27 ± 22 | 10 (10–47) | |||||
| Device A | |||||||
| 0.52 | 26 ± 21 | 22 (12–30) | 33 ± 23 | 29 (12–56) | 0.545 | 0.537 | |
| 0.66 | 33 ± 26 | 28 (15–38) | 34 ± 24 | 29 (11–57) | 0.528 | 0.519 | |
| 0.72 | 36 ± 28 | 30 (16–42) | 36 ± 24 | 33 (18–54) | 0.505 | 0.497 | |
| 0.81 | 40 ± 32 | 34 (18–47) | 42 ± 21 | 38 (27–54) | 0.464 | 0.458 | |
| Measurement with PVRU 100–200 mL (n = 12) | |||||||
| Device B | 158 ± 160 | 160 (132–189) | |||||
| Device A | |||||||
| 0.52 | 116 ± 41 | 127 (73–141) | 27 ± 18 | 26 (14–38) | 0.460 | 0.437 | |
| 0.66 | 148 ± 52 | 162 (93–179) | 17 ± 15 | 10 (7–23) | 0.691 | 0.671 | |
| 0.72 | 161 ± 56 | 176 (101–195) | 17 ± 14 | 16 (3–25) | 0.683 | 0.662 | |
| 0.81 | 181 ± 63 | 198 (114–220) | 21 ± 11 | 24 (13–26) | 0.579 | 0.555 | |
| Measurement with PVRU > 200 mL (n = 17) | |||||||
| Device B | 317 ± 77 | 318 (262–351) | |||||
| Device A | |||||||
| 0.52 | 188 ± 83 | 188 (120–255) | 41 ± 21 | 38 (25–61) | 0.297 | 0.282 | |
| 0.66 | 239 ± 105 | 238 (153–324) | 29 ± 22 | 22 (9–50) | 0.479 | 0.308 | |
| 0.72 | 261 ± 115 | 260 (167–333) | 26 ± 21 | 17 (11–46) | 0.543 | 0.342 | |
| 0.81 | 293 ± 129 | 293 (188–397) | 24 ± 19 | 17 (9–39) | 0.591 | 0.576 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-L.; Lai, S.-H.; Cheng, C.-F.; Chiu, V.; Lin, S.-K. Comparative Effectiveness of Two Models of Point-of-Care Ultrasound for Detection of Post-Void Residual Urine during Acute Ischemic Stroke: Preliminary Findings of Real-World Clinical Application. Diagnostics 2023, 13, 2599. https://doi.org/10.3390/diagnostics13152599
Chang W-L, Lai S-H, Cheng C-F, Chiu V, Lin S-K. Comparative Effectiveness of Two Models of Point-of-Care Ultrasound for Detection of Post-Void Residual Urine during Acute Ischemic Stroke: Preliminary Findings of Real-World Clinical Application. Diagnostics. 2023; 13(15):2599. https://doi.org/10.3390/diagnostics13152599
Chicago/Turabian StyleChang, Wan-Ling, Shu-Hui Lai, Chu-Fang Cheng, Valeria Chiu, and Shinn-Kuang Lin. 2023. "Comparative Effectiveness of Two Models of Point-of-Care Ultrasound for Detection of Post-Void Residual Urine during Acute Ischemic Stroke: Preliminary Findings of Real-World Clinical Application" Diagnostics 13, no. 15: 2599. https://doi.org/10.3390/diagnostics13152599
APA StyleChang, W.-L., Lai, S.-H., Cheng, C.-F., Chiu, V., & Lin, S.-K. (2023). Comparative Effectiveness of Two Models of Point-of-Care Ultrasound for Detection of Post-Void Residual Urine during Acute Ischemic Stroke: Preliminary Findings of Real-World Clinical Application. Diagnostics, 13(15), 2599. https://doi.org/10.3390/diagnostics13152599






