The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Clinical Data Collection
2.3. Gut Microbiota Analysis
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Multivariable Logistic Regression Analysis of the Risk Factors for PSSD
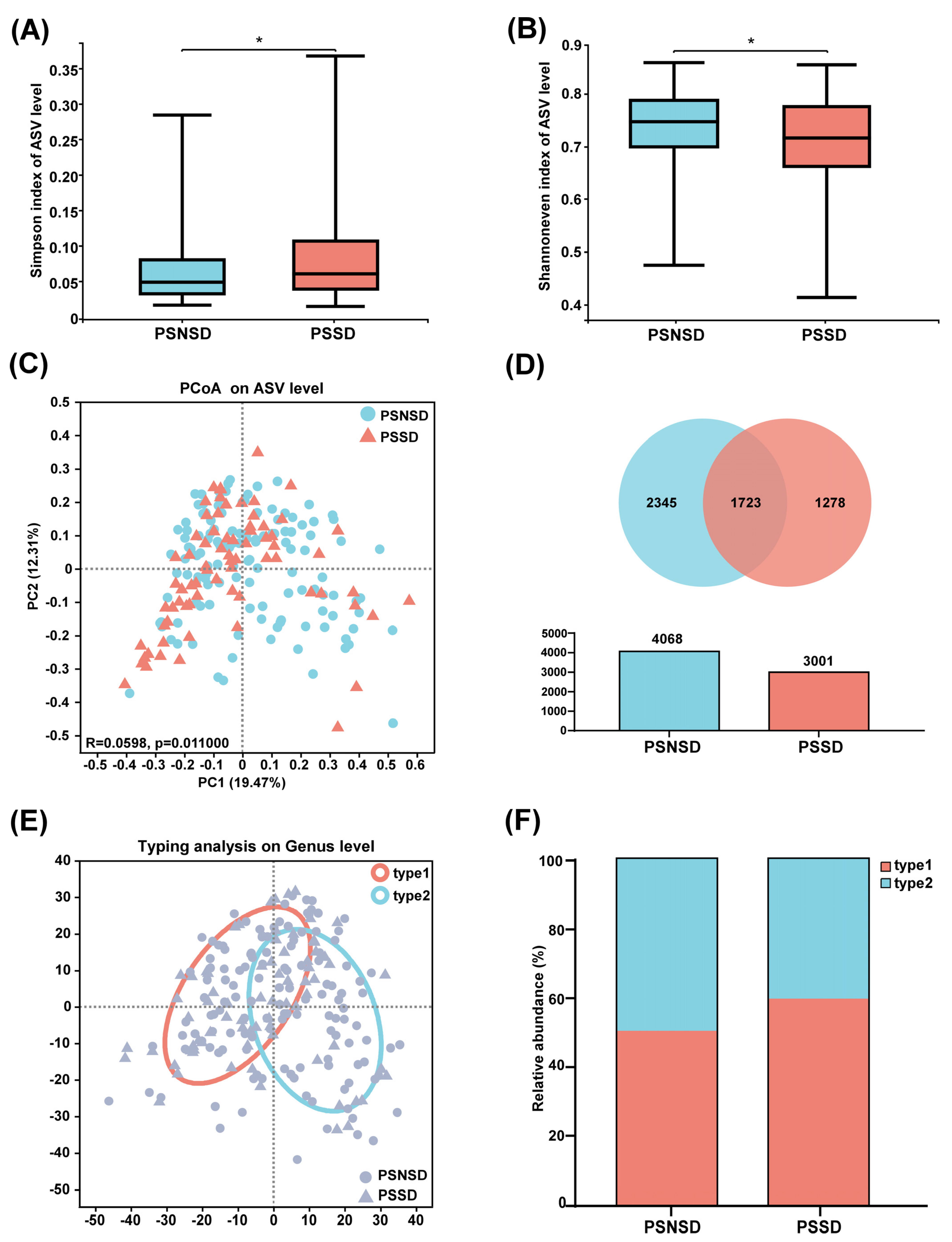
3.3. Changes in Microbiota Diversity and Community Type in Patients with PSSD
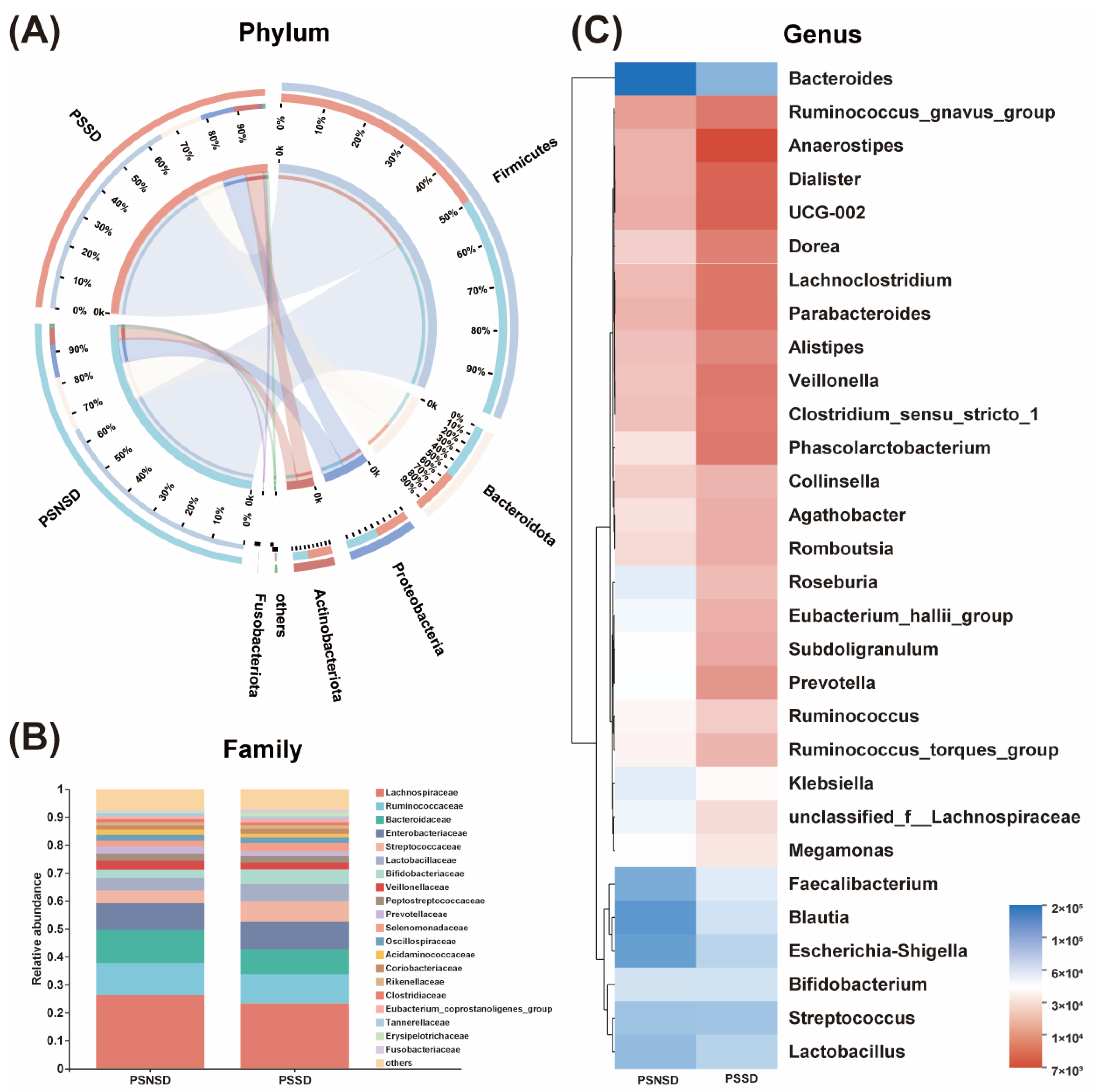
3.4. Changes in the Microbial Composition of PSSD Patients
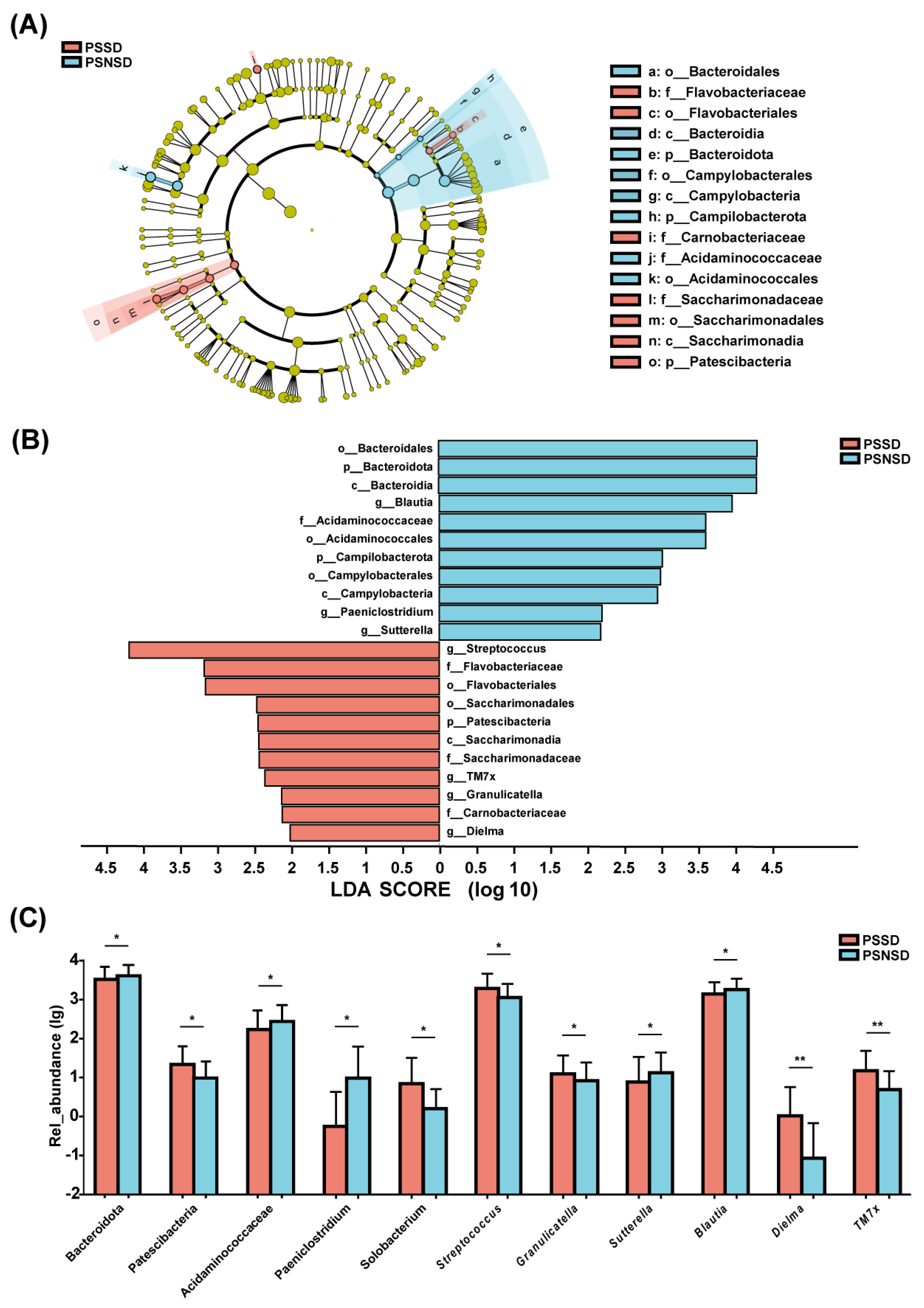
3.5. LEfSe Analysis of the Taxonomic Biomarkers of Gut Microbiota
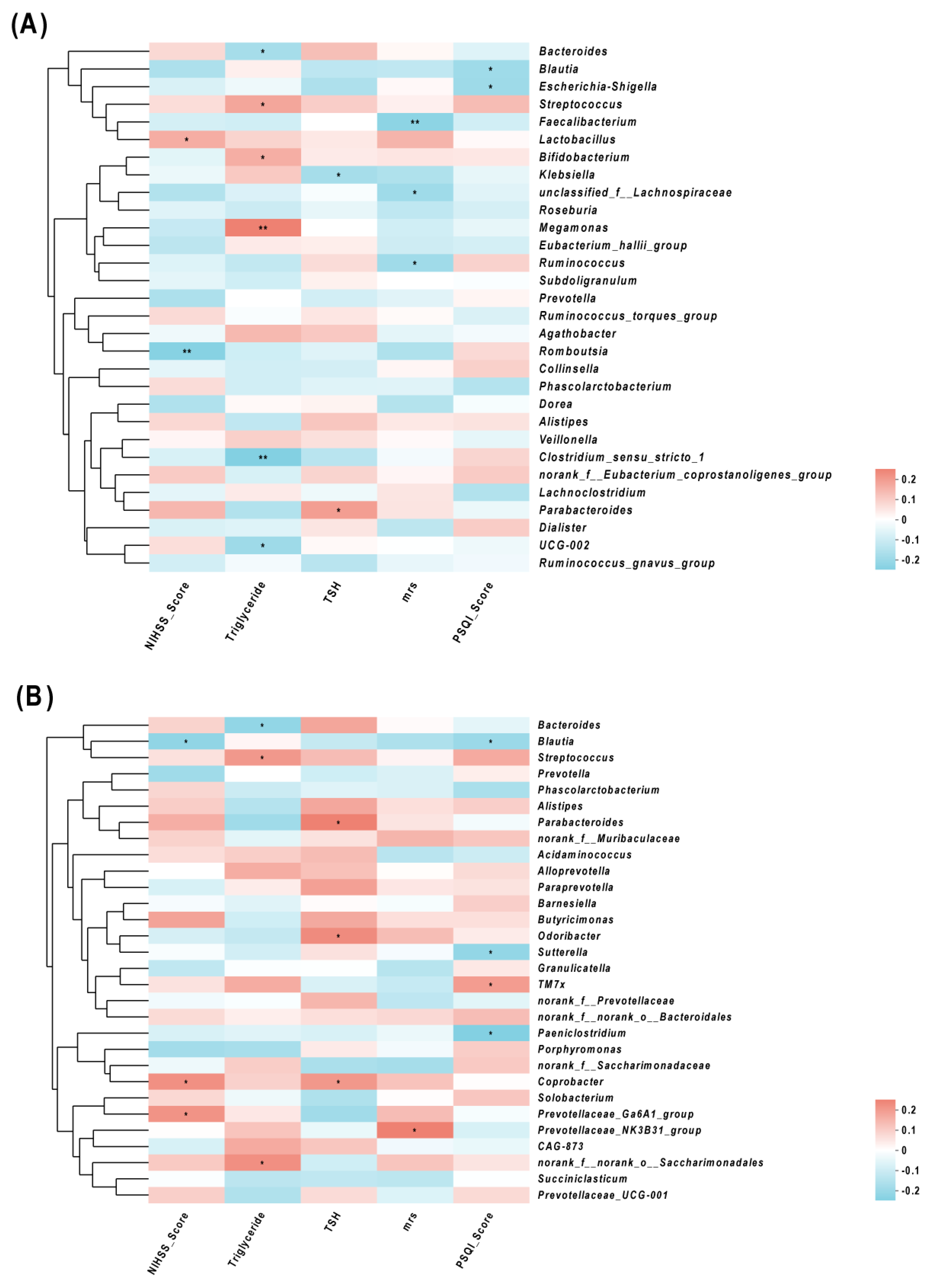
3.6. Correlations of Gut Microbes and Clinical Characteristics
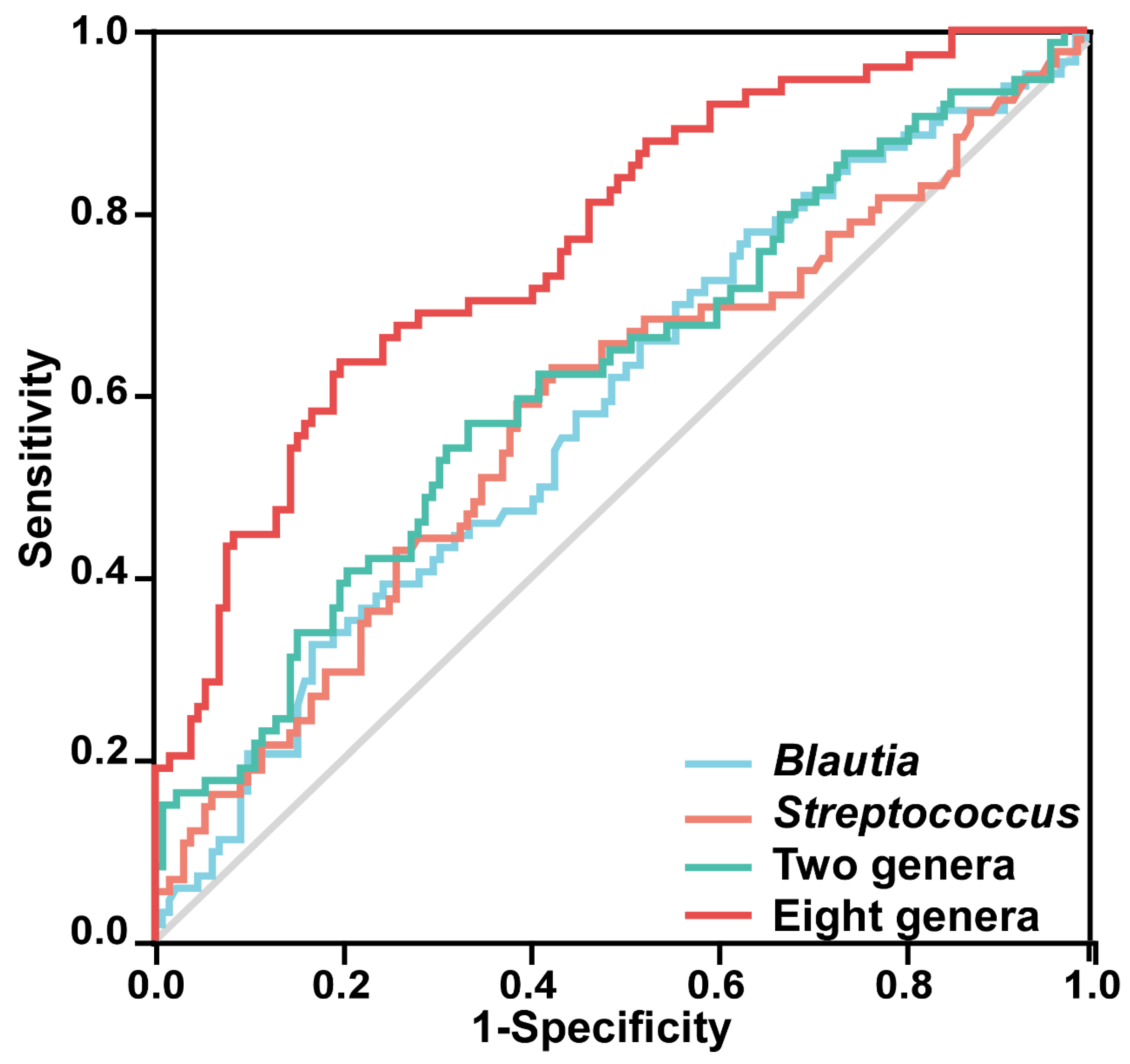
3.7. Predictor Performance of Gut Microbiota
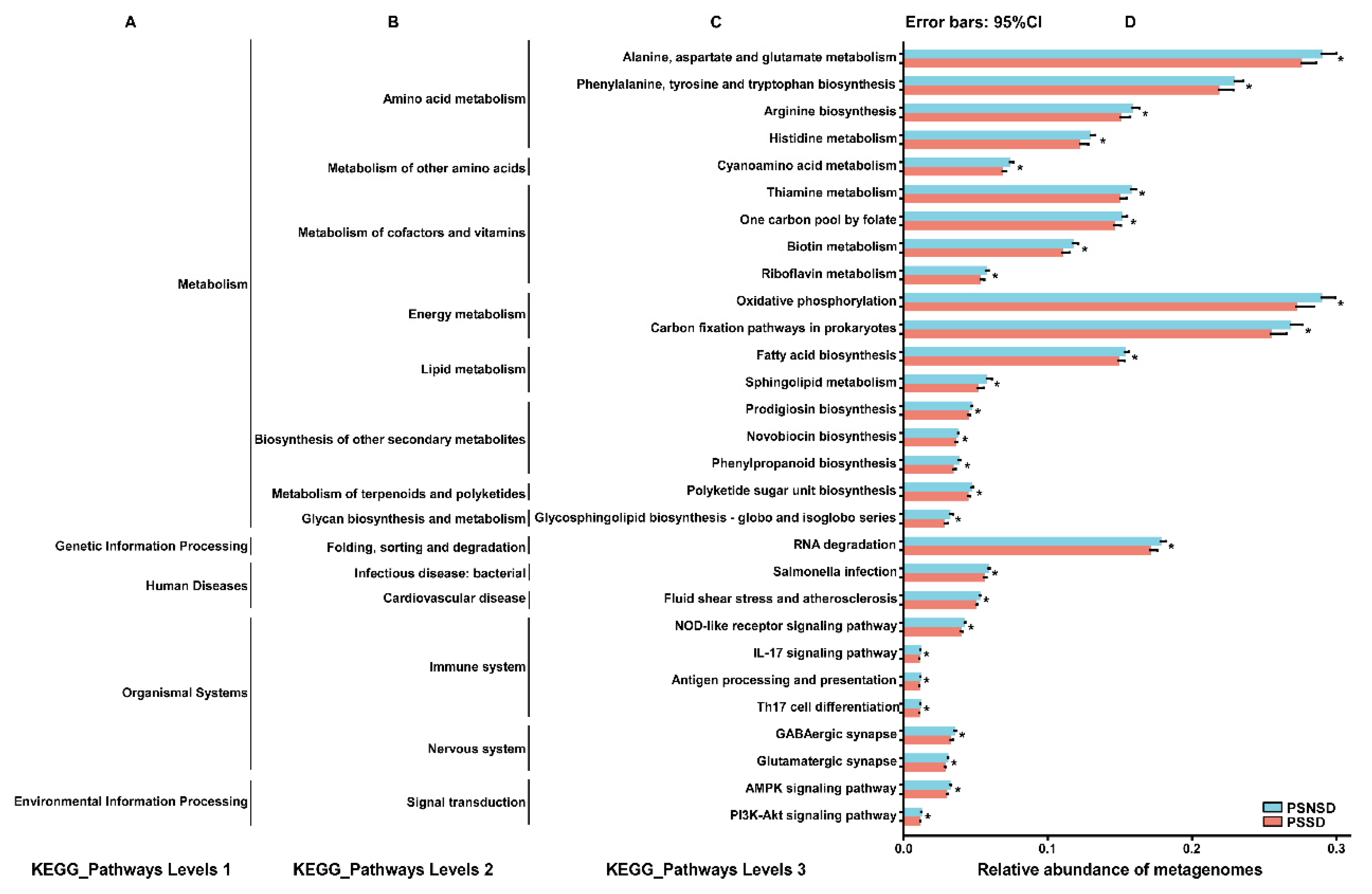
3.8. Gut Microbial Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, H.; Wang, X.P.; Yang, G.Y. Sleep disorders in stroke: An update on management. Aging Dis. 2021, 12, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Duss, S.B.; Seiler, A.; Schmidt, M.H.; Pace, M.; Adamantidis, A.; Müri, R.M.; Bassetti, C.L. The role of sleep in recovery following ischemic stroke: A review of human and animal data. Neurobiol. Sleep Circadian Rhythm. 2017, 2, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Fisse, A.L.; Kemmling, A.; Teuber, A.; Wersching, H.; Young, P.; Dittrich, R.; Ritter, M.; Dziewas, R.; Minnerup, J. The association of lesion location and sleep related breathing disorder in patients with acute ischemic stroke. PLoS ONE 2017, 12, e0171243. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Choi-Kwon, S.; Kim, J.S. Sleep disturbances after cerebral infarction: Role of depression and fatigue. J. Stroke Cerebrovasc. Dis. 2014, 23, 1949–1955. [Google Scholar] [CrossRef]
- Iddagoda, M.T.; Inderjeeth, C.A.; Chan, K.; Raymond, W.D. Post-stroke sleep disturbances and rehabilitation outcomes: A prospective cohort study. Intern. Med. J. 2020, 50, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Xiao, H.; Chen, W. Gut Microbiome: The Cornerstone of Life and Health. Adv. Gut Microbiome Res. 2022, 2022, 9894812. [Google Scholar] [CrossRef]
- Zhu, W.; Romano, K.A.; Li, L.; Buffa, J.A.; Sangwan, N.; Prakash, P.; Tittle, A.N.; Li, X.S.; Fu, X.; Androjna, C.; et al. Gut microbes impact stroke severity via the trimethylamine N-oxide pathway. Cell Host Microbe 2021, 29, 1199–1208.e5. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef]
- Zeng, X.; Gao, X.; Peng, Y.; Wu, Q.; Zhu, J.; Tan, C.; Xia, G.; You, C.; Xu, R.; Pan, S.; et al. Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 2019, 9, 4. [Google Scholar] [CrossRef]
- Houlden, A.; Goldrick, M.; Brough, D.; Vizi, E.S.; Lenart, N.; Martinecz, B.; Roberts, I.S.; Denes, A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav. Immun. 2016, 57, 10–20. [Google Scholar] [CrossRef]
- Peh, A.; O’Donnell, J.A.; Broughton, B.R.S.; Marques, F.Z. Gut microbiota and their metabolites in stroke: A double-edged sword. Stroke 2022, 53, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Wekerle, H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017, 38, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef]
- Nam, H.S. Gut microbiota and ischemic stroke: The role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef]
- Yang, P.Y.; Ho, K.H.; Chen, H.C.; Chien, M.Y. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J. Physiother. 2012, 58, 157–163. [Google Scholar] [CrossRef]
- Zitser, J.; Allen, I.E.; Falgàs, N.; Le, M.M.; Neylan, T.C.; Kramer, J.H.; Walsh, C.M. Pittsburgh Sleep Quality Index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS ONE 2022, 17, e0270095. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and its use in future stroke trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef]
- Park, M.J.; Pilla, R.; Panta, A.; Pandey, S.; Sarawichitr, B.; Suchodolski, J.; Sohrabji, F. Reproductive senescence and ischemic stroke remodel the gut microbiome and modulate the effects of estrogen treatment in female rats. Transl. Stroke Res. 2020, 11, 812–830. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schürmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Zhou, Y.; Wang, D.; Liu, X.; Li, L.; Wang, T.; Zhang, Y.; Jiang, M.; Tang, H.; et al. Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nat. Sci. Sleep 2020, 12, 895–905. [Google Scholar] [CrossRef]
- Talebi, S.; Karimifar, M.; Heidari, Z.; Mohammadi, H.; Askari, G. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2020, 48, 102234. [Google Scholar] [CrossRef]
- Davidsen, N.; Ramhøj, L.; Lykkebo, C.A.; Kugathas, I.; Poulsen, R.; Rosenmai, A.K.; Evrard, B.; Darde, T.A.; Axelstad, M.; Bahl, M.I.; et al. PFOS-induced thyroid hormone system disrupted rats display organ-specific changes in their transcriptomes. Environ. Pollut. 2022, 305, 119340. [Google Scholar] [CrossRef]
- Bruyneel, M.; Veltri, F.; Poppe, K. Prevalence of newly established thyroid disorders in patients with moderate-to-severe obstructive sleep apnea syndrome. Sleep Breath. Schlaf Atm. 2019, 23, 567–573. [Google Scholar] [CrossRef]
- Kadıoğlu, N.; Sert, U.Y.; Sariaslan, S.G.; Mursel, K.; Celen, S. Sleep disorders in pregnancy, influencing factors and quality of life. Z. Geburtshilfe Neonatol. 2022, 226, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sack, D.A.; James, S.P.; Rosenthal, N.E.; Wehr, T.A. Deficient nocturnal surge of TSH secretion during sleep and sleep deprivation in rapid-cycling bipolar illness. Psychiatry Res. 1988, 23, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Chen, G.H.; Li, Z.H.; Jiang, S.; Shen, J. Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: A clinical research. PLoS ONE 2013, 8, e71065. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Hall, J.E.; Klubo-Gwiezdzinska, J. The hypothalamic pituitary thyroid axis and sleep. Curr. Opin. Endocr. Metab. Res. 2021, 17, 8–14. [Google Scholar] [CrossRef]
- Green, M.E.; Bernet, V.; Cheung, J. Thyroid dysfunction and sleep disorders. Front. Endocrinol. 2021, 12, 725829. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, J.; Tang, H.; Wang, Y.; Zhang, W.; Liu, H.; Zhu, L.; Xiao, Z.; Yang, H.; Yu, Y. Elevated thyroid-stimulating hormone levels are associated with poor sleep: A cross-sectional and longitudinal study. Endocrine 2022, 75, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.Y.; Liu, L.L.; Dou, D.Q. Comparative study on medicinal natures (qi) of black ginseng, red ginseng, and ginseng leaves based on typical deficiency-heat syndrome rat model. Evid.-Based Complement. Altern. Med. eCAM 2022, 2022, 5194987. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: A multicenter, randomized, open label clinical trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Kim, S.W.; Lee, S.H. Association between triglyceride glucose index and obstructive sleep apnea risk in Korean adults: A cross-sectional cohort study. Lipids Health Dis. 2020, 19, 182. [Google Scholar] [CrossRef]
- Wrzosek, M.; Wojnar, M.; Sawicka, A.; Tałałaj, M.; Nowicka, G. Insomnia and depressive symptoms in relation to unhealthy eating behaviors in bariatric surgery candidates. BMC Psychiatry 2018, 18, 153. [Google Scholar] [CrossRef]
- Gu, M.; Huang, W.; Li, X.; Liu, Y.; Wang, F.; Fang, C.; Chen, T. Association of hypertriglyceridemic waist phenotype with obstructive sleep apnea: A cross-sectional study. Nat. Sci. Sleep 2021, 13, 2165–2173. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Sun, X.; Yu, Y.; Si, S.; Hou, L.; Yan, R.; Yu, Y.; Li, M.; Li, H.; et al. Genetically predicted insomnia in relation to 14 cardiovascular conditions and 17 cardiometabolic risk factors: A mendelian randomization study. J. Am. Heart Assoc. 2021, 10, e020187. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Valentini, F.; Evangelisti, M.; Arpinelli, M.; Di Nardo, G.; Borro, M.; Simmaco, M.; Villa, M.P. Gut microbiota composition in children with obstructive sleep apnoea syndrome: A pilot study. Sleep. Med. 2020, 76, 140–147. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Hepsomali, P.; Groeger, J.A.; Nishihira, J.; Scholey, A. Effects of Oral Gamma-Aminobutyric Acid (GABA) administration on stress and sleep in humans: A systematic review. Front. Neurosci. 2020, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Doerr, H.M.; Grzelak, A.K.; Busi, S.B.; Jasarevic, E.; Ericsson, A.C.; Bryda, E.C. Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Sci. Rep. 2016, 6, 33726. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 6–20. [Google Scholar] [CrossRef]
- Pava, M.J.; den Hartog, C.R.; Blanco-Centurion, C.; Shiromani, P.J.; Woodward, J.J. Endocannabinoid modulation of cortical up-states and NREM sleep. PLoS ONE 2014, 9, e88672. [Google Scholar] [CrossRef]
- Philip, V.; Newton, D.F.; Oh, H.; Collins, S.M.; Bercik, P.; Sibille, E. Transcriptional markers of excitation-inhibition balance in germ-free mice show region-specific dysregulation and rescue after bacterial colonization. J. Psychiatr. Res. 2021, 135, 248–255. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
| PSSD (n = 74) | PSNSD (n = 131) | p-Value | |
|---|---|---|---|
| Socio-demographic factors | |||
| Male subjects, n (%) | 46 (62.2) | 92 (70.2) | 0.248 |
| Age (years, mean ± SD) | 66 ± 12 | 65 ± 12 | 0.613 |
| Marital status | 0.876 | ||
| Married | 65 (87.8) | 114 (87.0) | |
| Unmarried | 1 (1.4) | 2 (1.5) | |
| Divorced | 8 (10.8) | 15 (11.5) | |
| Education | 0.276 | ||
| Illiterate | 14 (18.9) | 24 (18.3) | |
| Elementary school | 33 (44.6) | 49 (37.4) | |
| Middle school | 20 (27) | 41 (31.3) | |
| High school and above | 7 (9.5) | 17 (12.9) | |
| Health-related behaviors | |||
| Smoking, formerly/currently | 27 (36.5) | 51 (38.9) | 0.731 |
| Drinking, formerly/currently | 28 (37.8) | 49 (37.4) | 0.951 |
| History of disease | |||
| Atrial fibrillation | 7 (9.4) | 10 (7.6) | 0.848 |
| Hypertension | 51 (68.9) | 93 (71) | 0.757 |
| Diabetes mellitus | 28 (37.8) | 45 (34.4) | 0.619 |
| CVD history | 12 (16.2) | 21 (16.0) | 0.972 |
| Hyperlipemia | 32 (43.2) | 53 (40.5) | 0.699 |
| Clinical parameters | |||
| MAP (mmHg) | 108.37 ± 14.14 | 110.22 ± 13.51 | 0.354 |
| ALT (u/L) | 18 (13–25.25) | 17 (12–24) | 0.327 |
| AST (u/L) | 19.5 (16–23) | 18 (15–22) | 0.215 |
| Creatinine (μmol/L) | 63.2 (56.23–73.6) | 62.9 (51.9–76.5) | 0.992 |
| Folate (nmol/L) | 9.29 (6.11–11.49) | 9.61 (7.21–13.47) | 0.191 |
| B12 (pg/mL) | 332 (235.5–406) | 355 (234–515) | 0.327 |
| UA (μmol/L) | 296 (237.25–356.75) | 314 (246–364) | 0.400 |
| Hcy (μmol/L) | 10.7 (9.3–13.1) | 11.1 (9.1–13.7) | 0.582 |
| hs-CRP (mg/L) | 1.675 (0.78–4.72) | 1.23 (0.61–3.42) | 0.271 |
| TG (mmol/L) | 1.66 (1.28–2.1) | 1.45 (1.08–1.78) | 0.003 * |
| TC (mmol/L) | 4.46 ± 1.04 | 4.37 ± 1.13 | 0.590 |
| LDL (mmol/L) | 2.87 ± 0.95 | 2.84 ± 1.07 | 0.880 |
| HDL (mmol/L) | 1.07 (0.84–1.27) | 1 (0.84–1.23) | 0.348 |
| FPG (mmol/L) | 5.41 (4.93–6.71) | 5.49 (4.85–6.33) | 0.650 |
| HbA1C (%) | 6.08 (5.62–7.00) | 5.9 (5.46–7.19) | 0.618 |
| FT3 (pg/mL) | 2.85 (2.64–3.13) | 2.92 (2.67–3.21) | 0.234 |
| FT4 (ng/dL) | 1.13 (1.13–1,13) | 1.14 (1.04–1.27) | 0.224 |
| TSH (μIU) | 2.58 (2.56–2.58) | 1.66 (1.02–2.66) | <0.001 * |
| NHISS in | 2 (1–4) | 2 (1–3) | 0.250 |
| PSQI in | 2 (2–3) | 2 (1–3) | 0.480 |
| PSQI out | 8 (7–10) | 3 (2–4) | <0.001 * |
| mRS out | 1 (1–2) | 1 (0–2) | 0.194 |
| Variable | Multivariate | |||
|---|---|---|---|---|
| B (SE) | OR | 95% CI | p | |
| TG (mmol/L) | 0.391 (0.205) | 1.478 | 0.989–2.208 | 0.057 |
| TSH (μIU) | 0.243 (0.111) | 1.275 | 1.026–1.585 | 0.028 * |
| NHISS | 0.139 (0.062) | 1.149 | 1.017–1.298 | 0.026 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Chen, J.; Chen, Q.; Zhao, Y.; Liu, J.; Sun, J.; Hu, X. The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders. Diagnostics 2023, 13, 2970. https://doi.org/10.3390/diagnostics13182970
Xie H, Chen J, Chen Q, Zhao Y, Liu J, Sun J, Hu X. The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders. Diagnostics. 2023; 13(18):2970. https://doi.org/10.3390/diagnostics13182970
Chicago/Turabian StyleXie, Huijia, Jiaxin Chen, Qionglei Chen, Yiting Zhao, Jiaming Liu, Jing Sun, and Xuezhen Hu. 2023. "The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders" Diagnostics 13, no. 18: 2970. https://doi.org/10.3390/diagnostics13182970
APA StyleXie, H., Chen, J., Chen, Q., Zhao, Y., Liu, J., Sun, J., & Hu, X. (2023). The Diagnostic Value of Gut Microbiota Analysis for Post-Stroke Sleep Disorders. Diagnostics, 13(18), 2970. https://doi.org/10.3390/diagnostics13182970




