Focal Liver Lesions in Budd-Chiari Syndrome: Spectrum of Imaging Findings
Abstract
1. Introduction
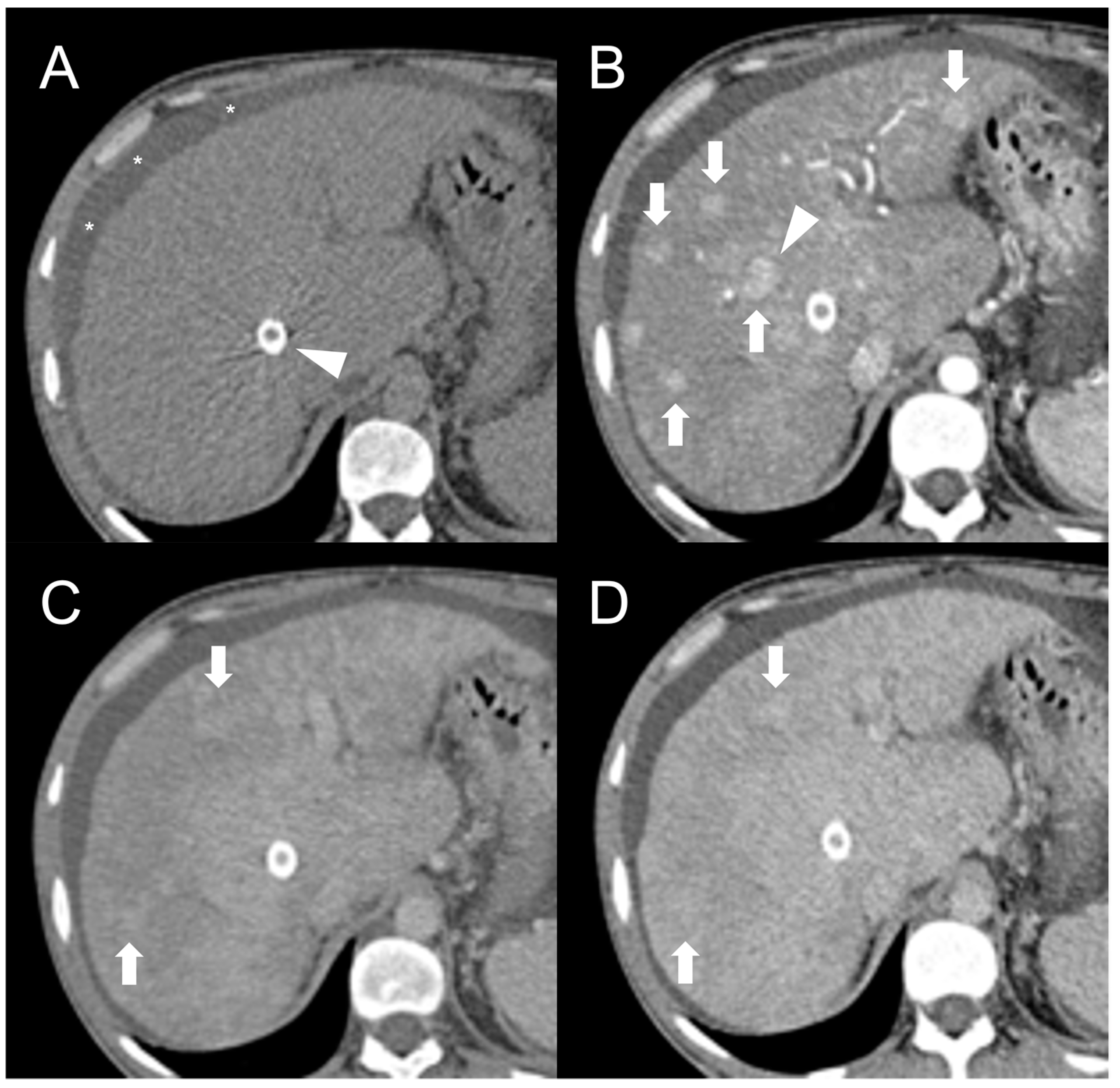
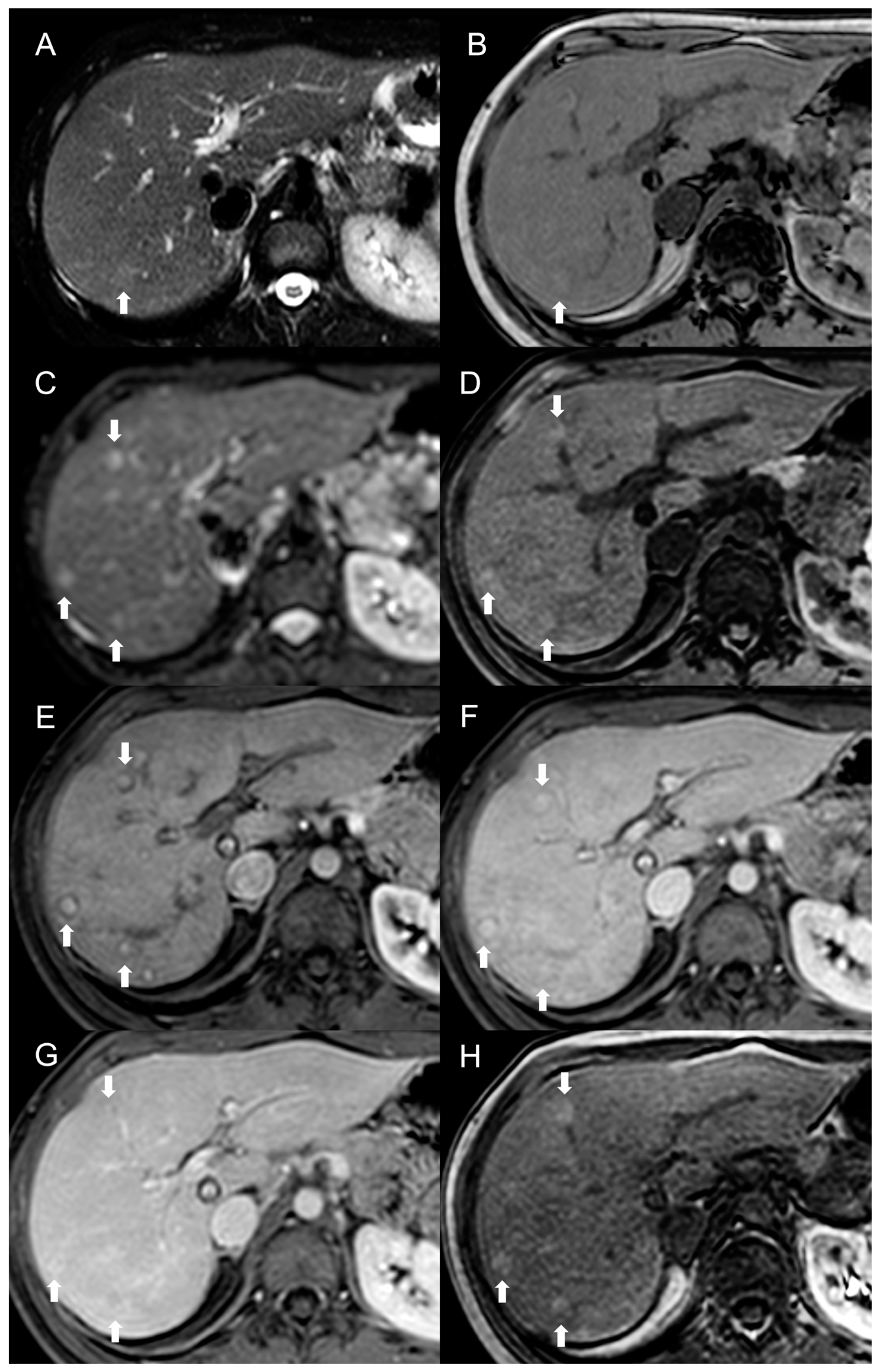
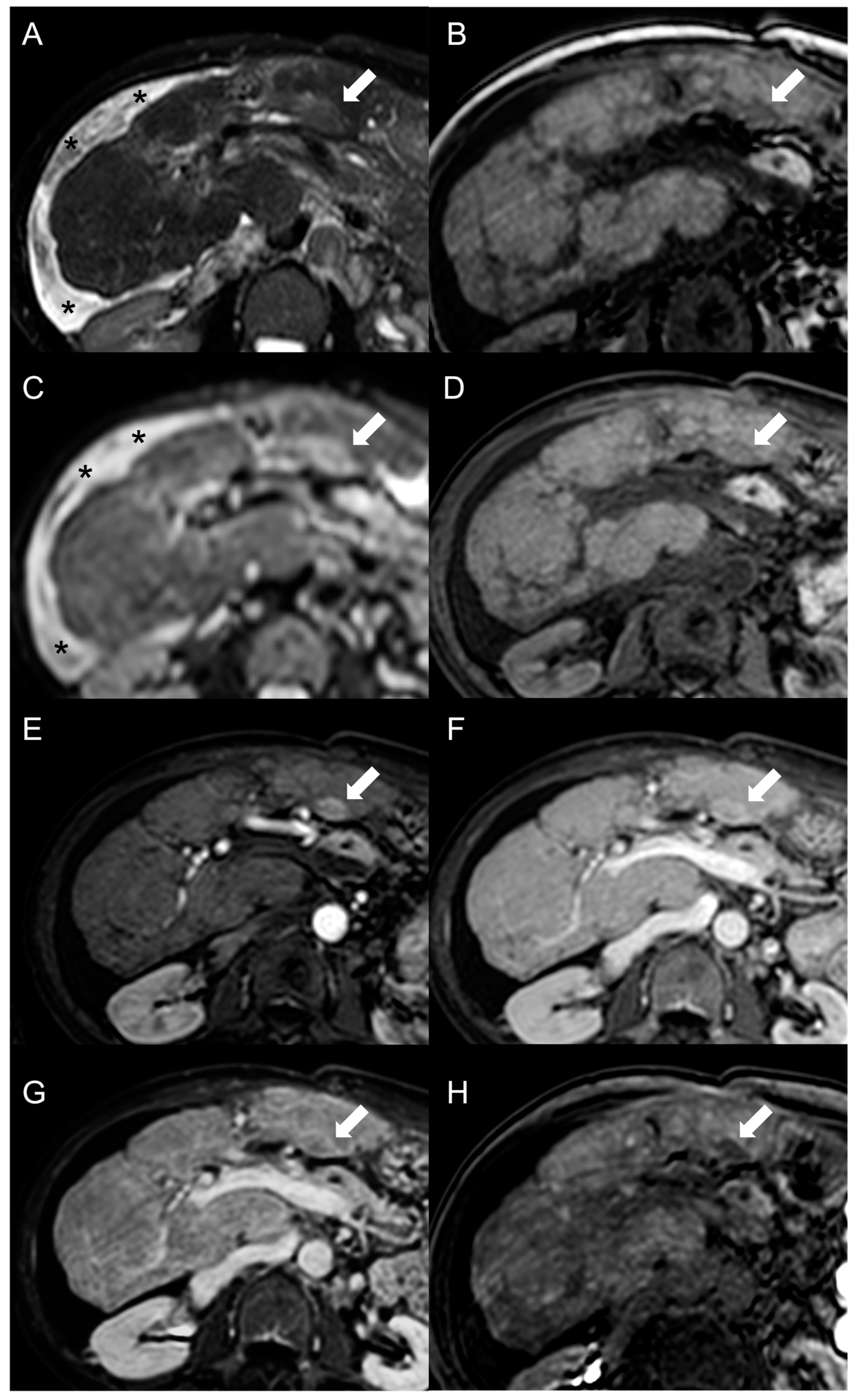
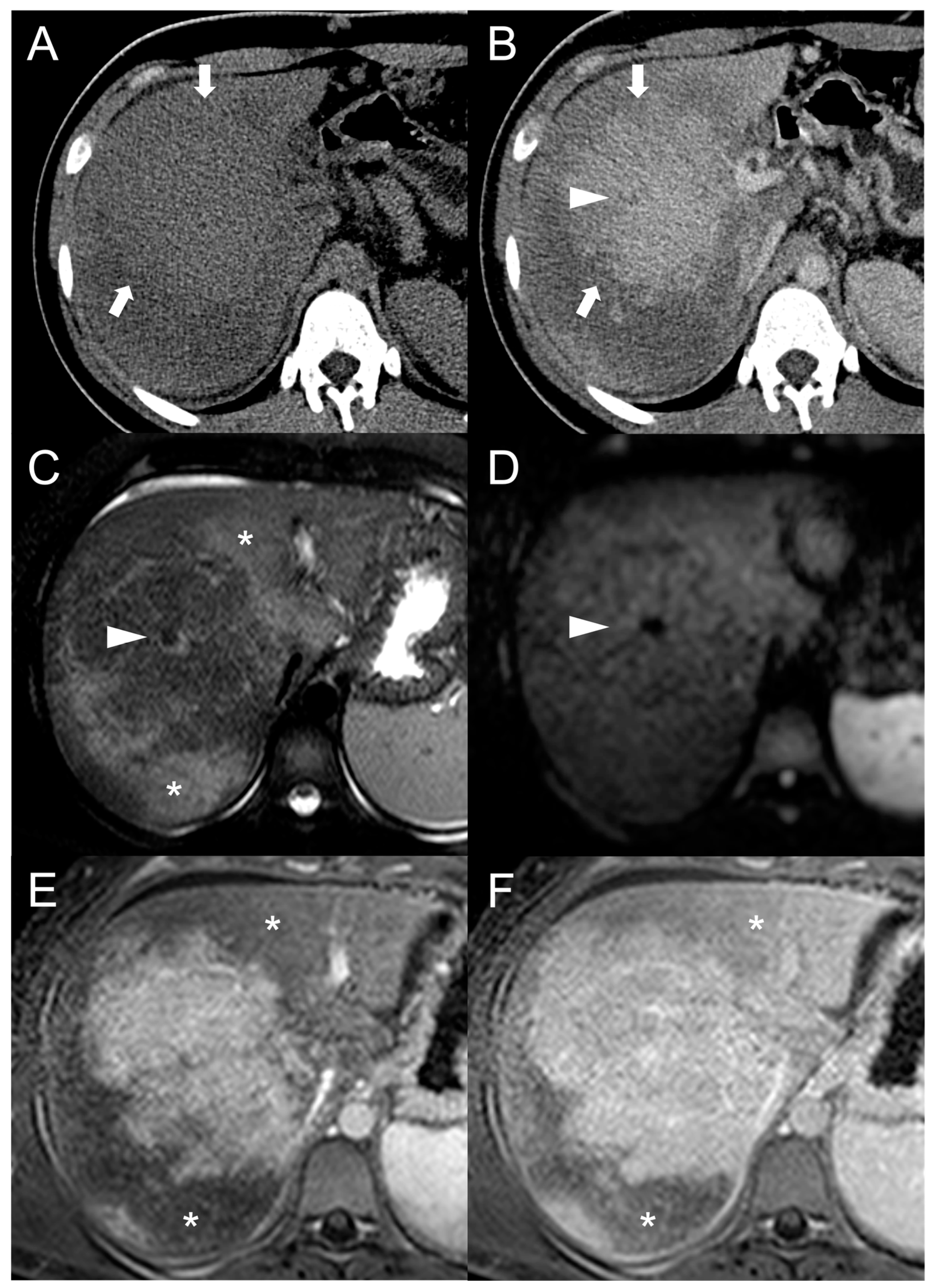
2. Focal Nodular Hyperplasia-like Regenerative Nodules
Imaging Features
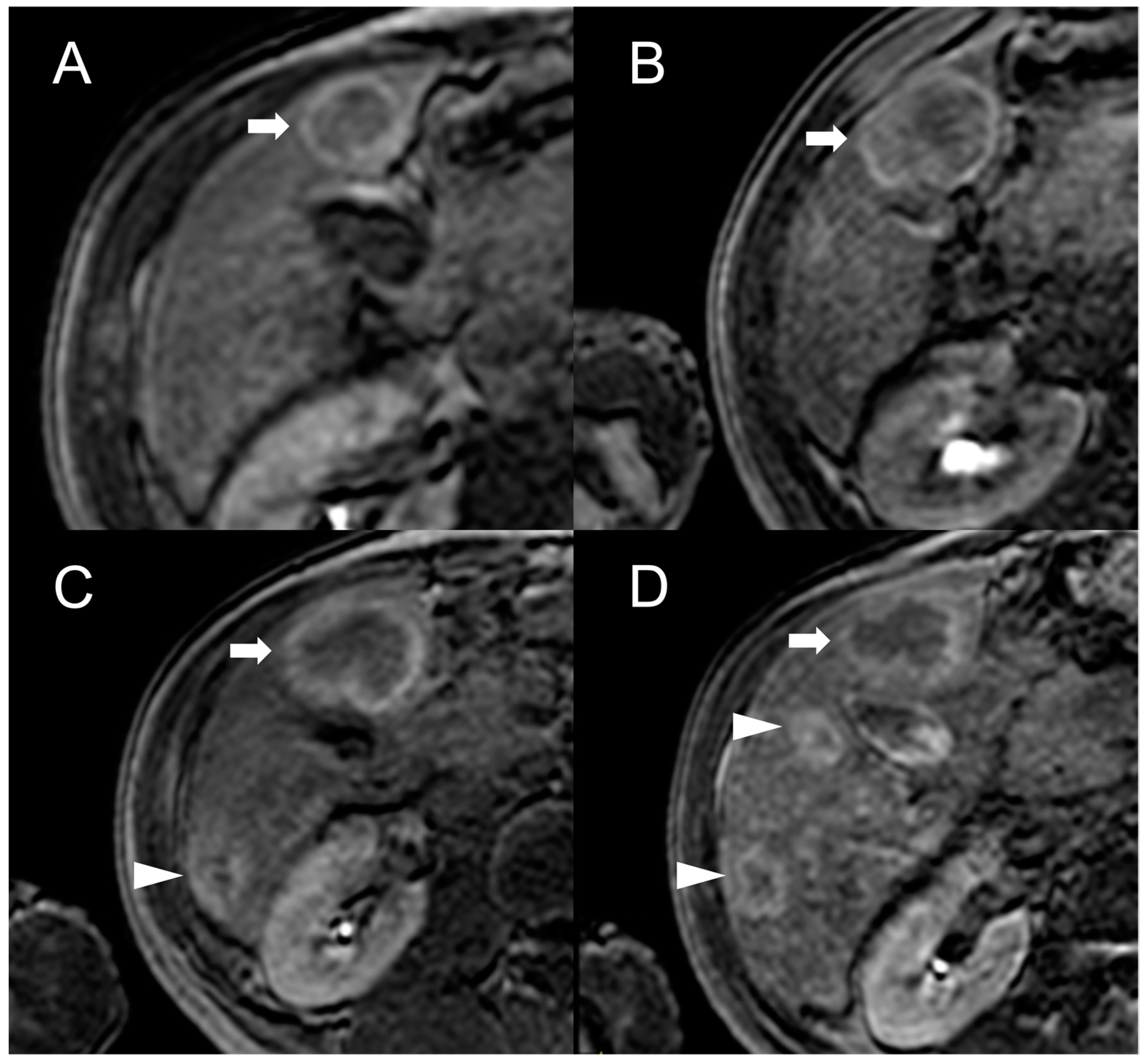
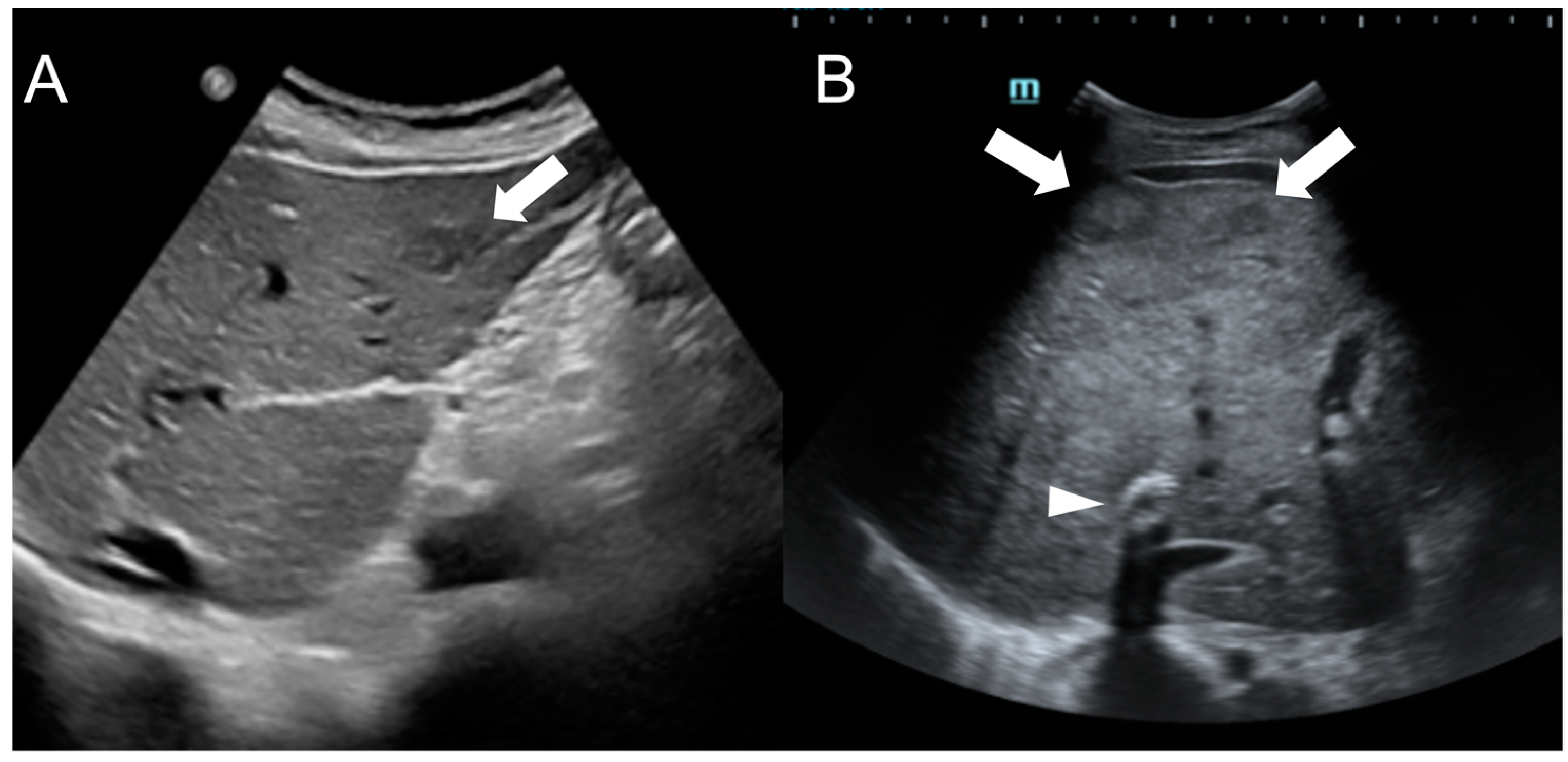
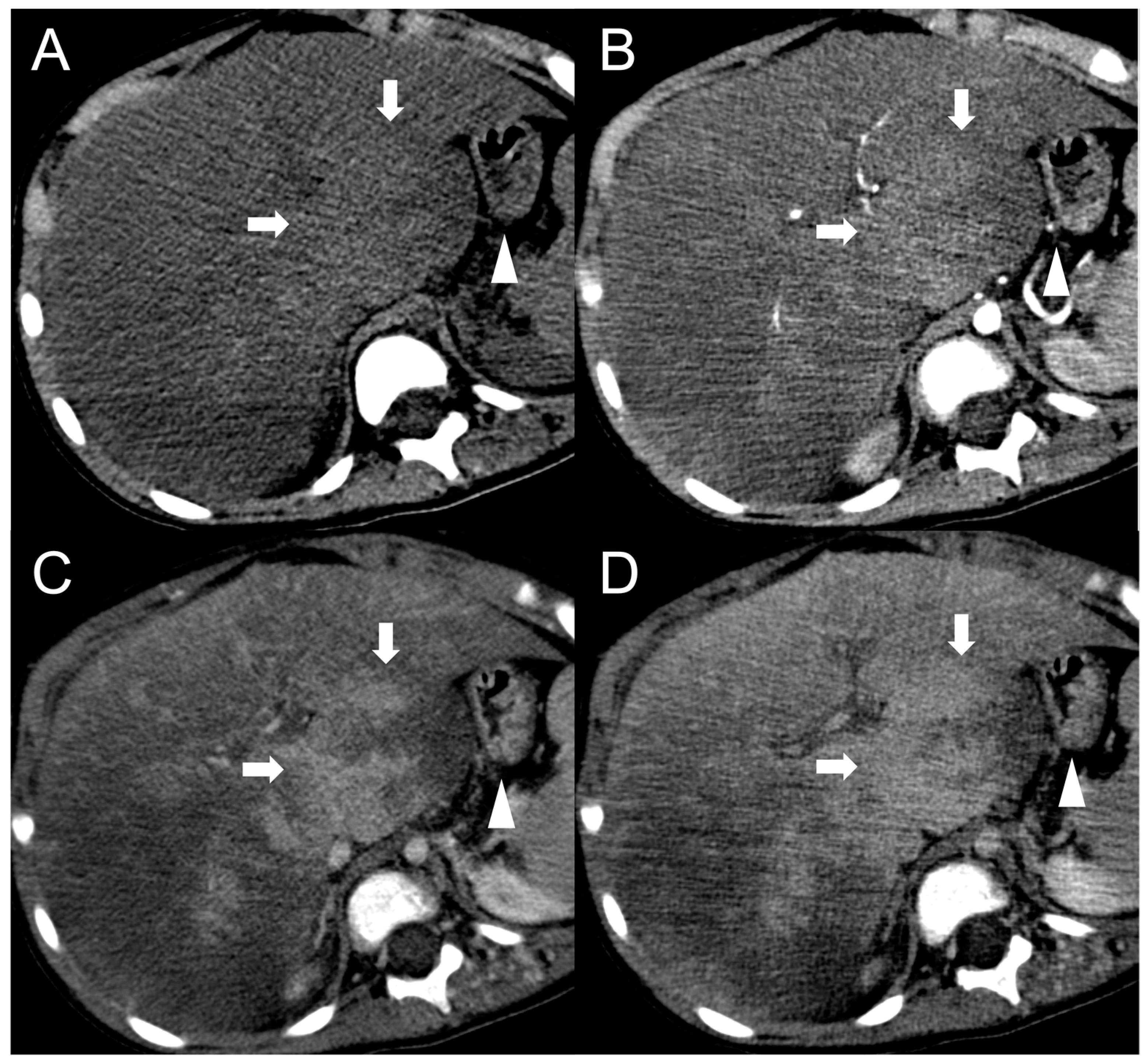
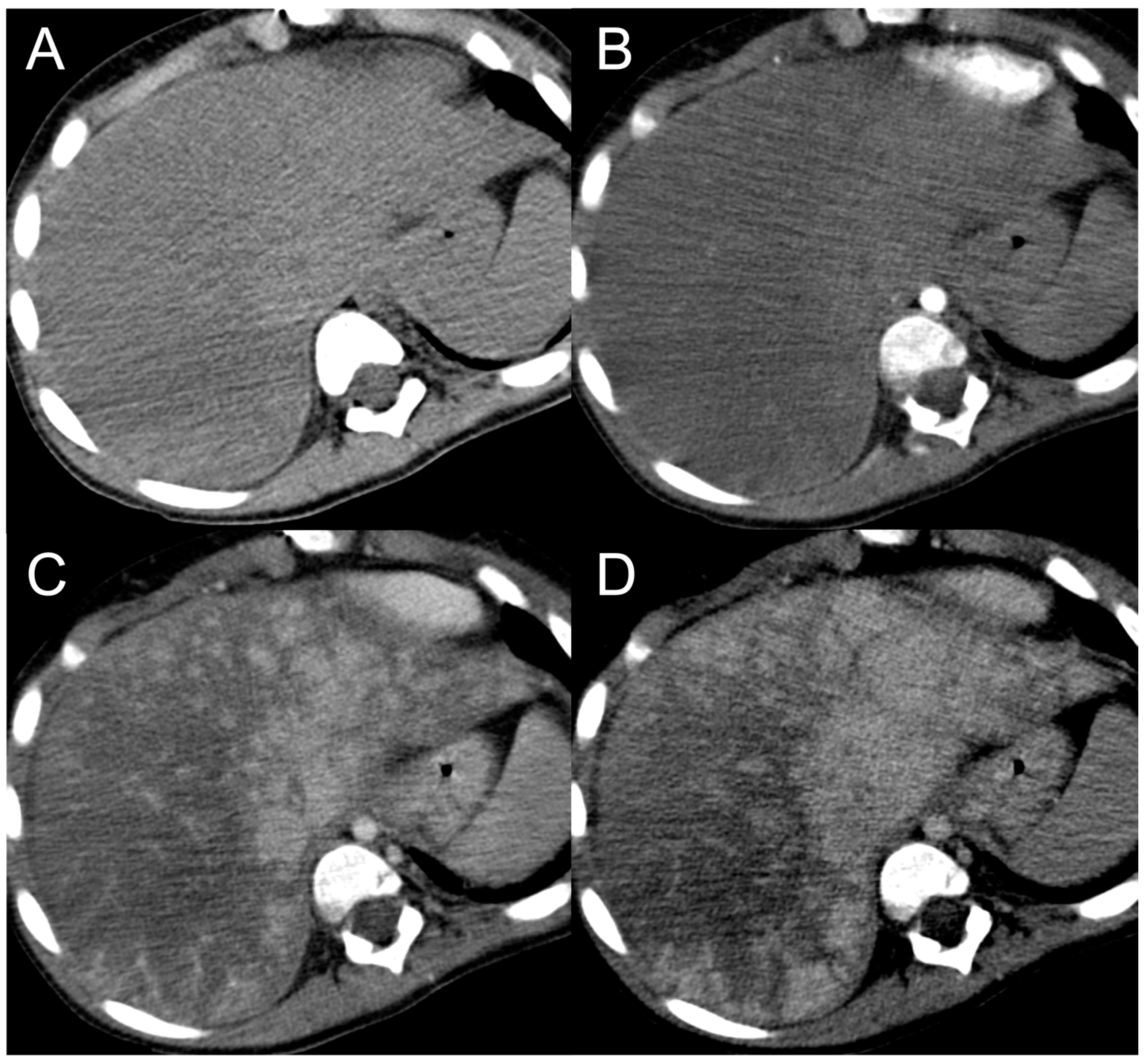
3. Hepatocellular Carcinoma
Imaging Findings
4. Other Focal Liver Lesions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Pagán, J.C.; Valla, D.-C. Primary Budd–Chiari Syndrome. N. Engl. J. Med. 2023, 388, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.M. Liver Cirrhosis in Hepatic Vena Cava Syndrome (or Membranous Obstruction of Inferior Vena Cava). World J. Hepatol. 2015, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Aydinli, M. Budd-Chiari Syndrome: Etiology, Pathogenesis and Diagnosis. World J. Gastroenterol. 2007, 13, 2693. [Google Scholar] [CrossRef] [PubMed]
- Cazals-Hatem, D. Arterial and Portal Circulation and Parenchymal Changes in Budd-Chiari Syndrome: A Study in 17 Explanted Livers. Hepatology 2003, 37, 510–519. [Google Scholar] [CrossRef]
- Vilgrain, V.; Lewin, M.; Vons, C.; Denys, A.; Valla, D.; Flejou, J.F.; Belghiti, J.; Menu, Y. Hepatic Nodules in Budd-Chiari Syndrome: Imaging Features. Radiology 1999, 210, 443–450. [Google Scholar] [CrossRef]
- Panvini, N.; Dioguardi Burgio, M.; Sartoris, R.; Maino, C.; Van Wettere, M.; Plessier, A.; Payancé, A.; Rautou, P.E.; Ladouceur, M.; Vilgrain, V.; et al. MR Imaging Features and Long-Term Evolution of Benign Focal Liver Lesions in Budd-Chiari Syndrome and Fontan-Associated Liver Disease. Diagn. Interv. Imaging 2022, 103, 111–120. [Google Scholar] [CrossRef]
- Tanaka, M.; Wanless, I.R. Pathology of the Liver in Budd-Chiari Syndrome: Portal Vein Thrombosis and the Histogenesis of Veno-Centric Cirrhosis, Veno-Portal Cirrhosis, and Large Regenerative Nodules. Hepatology 1998, 27, 488–496. [Google Scholar] [CrossRef]
- Kim, H.; Nahm, J.H.; Park, Y.N. Budd-Chiari Syndrome with Multiple Large Regenerative Nodules. Clin. Mol. Hepatol. 2015, 21, 89. [Google Scholar] [CrossRef]
- Wanless, I. Regenerative Nodules in Budd Chiari Syndrome. Hepatology 1994, 19, I139. [Google Scholar] [CrossRef]
- Maetani, Y.; Itoh, K.; Egawa, H.; Haga, H.; Sakurai, T.; Nishida, N.; Ametani, F.; Shibata, T.; Kubo, T.; Tanaka, K.; et al. Benign Hepatic Nodules in Budd-Chiari Syndrome: Radiologic-Pathologic Correlation with Emphasis on the Central Scar. AJR Am. J. Roentgenol. 2002, 178, 869–875. [Google Scholar] [CrossRef]
- International Working Party. Terminology of Nodular Hepatocellular Lesions. Hepatology 1995, 22, 983–993. [Google Scholar] [CrossRef]
- Mamone, G.; Carollo, V.; Di Piazza, A.; Cortis, K.; Degiorgio, S.; Miraglia, R. Budd-Chiari Syndrome and Hepatic Regenerative Nodules: Magnetic Resonance Findings with Emphasis of Hepatobiliary Phase. Eur. J. Radiol. 2019, 117, 15–25. [Google Scholar] [CrossRef]
- Ibarrola, C.; Castellano, V.M.; Colina, F. Focal Hyperplastic Hepatocellular Nodules in Hepatic Venous Outflow Obstruction: A Clinicopathological Study of Four Patients and 24 Nodules. Histopathology 2004, 44, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Brancatelli, G.; Federle, M.P.; Grazioli, L.; Golfieri, R.; Lencioni, R. Large Regenerative Nodules in Budd-Chiari Syndrome and Other Vascular Disorders of the Liver: CT and MR Imaging Findings with Clinicopathologic Correlation. AJR Am. J. Roentgenol. 2002, 178, 877–883. [Google Scholar] [CrossRef]
- Flor, N.; Zuin, M.; Brovelli, F.; Maggioni, M.; Tentori, A.; Sardanelli, F.; Cornalba, G.P. Regenerative Nodules in Patients with Chronic Budd-Chiari Syndrome: A Longitudinal Study Using Multiphase Contrast-Enhanced Multidetector CT. Eur. J. Radiol. 2010, 73, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Van Wettere, M.; Purcell, Y.; Bruno, O.; Payancé, A.; Plessier, A.; Rautou, P.E.; Cazals-Hatem, D.; Valla, D.; Vilgrain, V.; Ronot, M. Low Specificity of Washout to Diagnose Hepatocellular Carcinoma in Nodules Showing Arterial Hyperenhancement in Patients with Budd-Chiari Syndrome. J. Hepatol. 2019, 70, 1123–1132. [Google Scholar] [CrossRef]
- Bargalló, X.; Gilabert, R.; Nicolau, C.; García-Pagán, J.C.; Bosch, J.; Brú, C. Sonography of the Caudate Vein: Value in Diagnosing Budd-Chiari Syndrome. Am. J. Roentgenol. 2003, 181, 1641–1645. [Google Scholar] [CrossRef]
- Brancatelli, G.; Federle, M.P.; Grazioli, L.; Golfieri, R.; Lencioni, R. Benign Regenerative Nodules in Budd-Chiari Syndrome and Other Vascular Disorders of the Liver: Radiologic-Pathologic and Clinical Correlation. Radiographics 2002, 22, 847–862. [Google Scholar] [CrossRef]
- de Sousa, J.M.M.; Portmann, B.; Williams, R. Nodular Regenerative Hyperplasia of the Liver and the Budd-Chiari Syndrome. J. Hepatol. 1991, 12, 28–35. [Google Scholar] [CrossRef]
- Faraoun, S.A.; Boudjella, M.E.A.; Debzi, N.; Benidir, N.; Afredj, N.; Guerrache, Y.; Bentabak, K.; Soyer, P.; Bendib, S.E. Budd-Chiari Syndrome: An Update on Imaging Features. Clin. Imaging 2016, 40, 637–646. [Google Scholar] [CrossRef]
- Renzulli, M.; Lucidi, V.; Mosconi, C.; Quarneti, C.; Giampalma, E.; Golferi, R. Large Regenerative Nodules in a Patient with Budd-Chiari Syndrome after TIPS Positioning While on the Liver Transplantation List Diagnosed by Gd-EOB-DTPA MRI. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Van Wettere, M.; Paulatto, L.; Raynaud, L.; Bruno, O.; Payancé, A.; Plessier, A.; Rautou, P.E.; Paradis, V.; Cazals-Hatem, D.; Valla, D.; et al. Hepatobiliary MR Contrast Agents Are Useful to Diagnose Hepatocellular Carcinoma in Patients with Budd-Chiari Syndrome. JHEP Rep. 2020, 2, 100097. [Google Scholar] [CrossRef] [PubMed]
- Soyer, P.; Lacheheb, D.; Caudron, C.; Levesque, M. MRI of Adenomatous Hyperplastic Nodules of the Liver in Budd-Chiari Syndrome. J. Comput. Assist. Tomogr. 1993, 17, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.E.; Lee, M.G.; Lee, Y.S.; Kang, G.H.; Ha, H.K.; Kim, P.N.; Auh, Y.H. Nodular Regenerative Hyperplasia of the Liver in Budd-Chiari Syndrome: CT and MR Features. Abdom. Imaging 2000, 25, 255–258. [Google Scholar] [CrossRef]
- Soler, R.; Rodríguez, E.; Pombo, F.; González, J.; Pombo, S.; Prada, C. Benign Regenerative Nodules with Copper Accumulation in a Case of Chronic Budd-Chiari Syndrome: CT and MR Findings. Abdom. Imaging 2000, 25, 486–489. [Google Scholar] [CrossRef]
- Matsui, O.; Kadoya, M.; Kameyama, T.; Yoshikawa, J.; Takashima, T.; Nakanuma, Y.; Unoura, M.; Kobayashi, K.; Izumi, R.; Ida, M. Benign and Malignant Nodules in Cirrhotic Livers: Distinction Based on Blood Supply. Radiology 1991, 178, 493–497. [Google Scholar] [CrossRef]
- Vilgrain, V.; Paradis, V.; Van Wettere, M.; Valla, D.; Ronot, M.; Rautou, P.E. Benign and Malignant Hepatocellular Lesions in Patients with Vascular Liver Diseases. Abdom. Radiol. 2018, 43, 1968–1977. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Kielar, A.Z.; Elmohr, M.M.; Chernyak, V.; Masch, W.R.; Furlan, A.; Marks, R.M.; Cruite, I.; Fowler, K.J.; Tang, A.; et al. White Paper of the Society of Abdominal Radiology Hepatocellular Carcinoma Diagnosis Disease-Focused Panel on LI-RADS V2018 for CT and MRI. Abdom. Radiol. 2018, 43, 2625–2642. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- De Gottardi, A.; Berzigotti, A.; Buscarini, E.; García Criado, A. Ultrasonography in Liver Vascular Disease. Ultraschall Der Med. Eur. J. Ultrasound 2018, 39, 382–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, S.; Zhou, Y.; Song, Y.; Sun, L. Comparison of Imaging Characteristics between Hepatic Benign Regenerative Nodules and Hepatocellular Carcinomas Associated with Budd-Chiari Syndrome by Contrast Enhanced Ultrasound. Eur. J. Radiol. 2012, 81, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Newerla, C.; Schaeffer, F.; Terracciano, L.; Hohmann, J. Multiple FNH-Like Lesions in a Patient with Chronic Budd-Chiari Syndrome: Gd-EOB-Enhanced MRI and BR1 CEUS Findings. Case Rep. Radiol. 2012, 2012, 685486. [Google Scholar] [CrossRef]
- Takayasu, K.; Muramatsu, Y.; Moriyama, N.; Wakao, F.; Makuuchi, M.; Takayama, T.; Kosuge, T.; Okazaki, N.; Yamada, R. Radiological Study of Idiopathic Budd-Chiari Syndrome Complicated by Hepatocellular Carcinoma. A Report of Four Cases. Am. J. Gastroenterol. 1994, 89, 249–253. [Google Scholar] [PubMed]
- Paul, S.B.; Shalimar, N.; Sreenivas, V.; Gamanagatti, S.R.; Sharma, H.; Dhamija, E.; Acharya, S.K. Incidence and Risk Factors of Hepatocellular Carcinoma in Patients with Hepatic Venous Outflow Tract Obstruction. Aliment. Pharmacol. Ther. 2015, 41, 961–971. [Google Scholar] [CrossRef]
- Ren, W.; Qi, X.; Yang, Z.; Han, G.; Fan, D. Prevalence and Risk Factors of Hepatocellular Carcinoma in Budd-Chiari Syndrome: A Systematic Review. Eur. J. Gastroenterol. Hepatol. 2013, 25, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Wester, A.; Åberg, F.; Rajani, R.; Hagström, H. Minimal Risk of Hepatocellular Carcinoma in Noncirrhotic Budd-Chiari Syndrome: A Three-Decade Population-Based Study. Clin. Gastroenterol. Hepatol. 2022; in press. [Google Scholar] [CrossRef]
- Jang, J.W.; Yoon, S.K.; Bae, S.H.; Choi, J.Y.; Chung, K.W.; Sun, H.S. Rapidly Progressing Budd-Chiari Syndrome Complicated by Hepatocellular Carcinoma. Korean J. Intern. Med. 2003, 18, 191–195. [Google Scholar] [CrossRef]
- Simson, I.W. Membranous Obstruction of the Inferior Vena Cava and Hepatocellular Carcinoma in South Africa. Gastroenterology 1982, 82, 171–178. [Google Scholar] [CrossRef]
- Li, K.-S.; Guo, S.; Chen, Y.-X.; Zhang, Z.-L. Budd-Chiari Syndrome and Its Associated Hepatocellular Carcinoma: Clinical Risk Factors and Potential Immunotherapeutic Benefit Analysis. Front. Oncol. 2022, 12, 1075685. [Google Scholar] [CrossRef]
- Vietti Violi, N.; Lewis, S.; Hectors, S.; Said, D.; Taouli, B. Radiological Diagnosis and Characterization of HCC. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches; Hoshida, Y., Ed.; Molecular and Translational Medicine; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–92. [Google Scholar]
- Legout, J.D.; Bolan, C.; Bowman, A.; Caserta, M.P.; Chen, F.K.; Cox, K.L.; Sanyal, R.; Toskich, B.B.; Lewis, J.T.; Alexander, L.F. Focal Nodular Hyperplasia and Focal Nodular Hyperplasia–like Lesions. Radiographics 2022, 42, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and Significance of Alpha-fetoprotein in Hepatocellular Carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef]
- Moucari, R.; Rautou, P.E.; Cazals-Hatem, D.; Geara, A.; Bureau, C.; Consigny, Y.; Francoz, C.; Denninger, M.H.; Vilgrain, V.; Belghiti, J.; et al. Hepatocellular Carcinoma in Budd-Chiari Syndrome: Characteristics and Risk Factors. Gut 2008, 57, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Shreshtha, A.; Mukund, A.; Bihari, C.; Eapen, C.E.; Han, G.; Deshmukh, H.; Cua, I.H.Y.; Lesmana, C.R.A.; Al Meshtab, M.; et al. Budd-Chiari Syndrome: Consensus Guidance of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol. Int. 2021, 15, 531–567. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Matsui, K.; Shimada, M.; Kubo, S.; Hasegawa, K. Update on Perioperative Management of Patients Undergoing Surgery for Liver Cancer. Ann. Gastroenterol. Surg. 2022, 6, 344. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Martin, D.R.; Saini, S. Imaging of Liver Metastases: MRI. Cancer Imaging 2007, 7, 2–9. [Google Scholar] [CrossRef]
- Elhence, A.; Gamanagatti, S.; Das, P. Shalimar Budd Chiari Syndrome and Intrahepatic Cholangiocarcinoma, An Unusual Combination: Case Report and Review of the Literature. Perm. J. 2020, 24, 204. [Google Scholar] [CrossRef]
- Shah, P.M.; Dhakre, V.W.; Nagral, A. Primary Hepatic Hemangioendothelioma in a Patient with Budd-Chiari Syndrome. BMJ Case Rep. 2017, 2017, bcr2017221103. [Google Scholar] [CrossRef]
- Vilgrain, V.; Lagadec, M.; Ronot, M. Pitfalls in Liver Imaging. Radiology 2016, 278, 34–51. [Google Scholar] [CrossRef]
- Ramanathan, S.; Raghu, V.; Virmani, V.; Sheikh, A.; Al Heidous, M.; Tirumani, S.H. Unveiling the Unreal: Comprehensive Imaging Review of Hepatic Pseudolesions. Clin. Imaging 2021, 80, 439–453. [Google Scholar] [CrossRef]
- Colagrande, S.; Centi, N.; Galdiero, R.; Ragozzino, A. Transient Hepatic Intensity Differences: Part 2, Those Not Associated with Focal Lesions. Am. J. Roentgenol. 2007, 188, 160–166. [Google Scholar] [CrossRef] [PubMed]


| FNH-like RN | HCC | |
|---|---|---|
| CT findings | ||
| Unenhanced scan | Isodense | Heterogenous (commonly iso-hypodense) |
| Arterial phase | Hyperdense (homogenous APHE) | Hyperdense (non-rim APHE) |
| PVP/DP phase | Isodense/slightly hyperdense | Hypodense (early washout—75%) |
| Hypodense perinodular rim | +/− | − |
| Central scar | +/− | − |
| MRI findings | ||
| T1 | Hyperintense or Isointense (75–84%) | Heterogenous (60% hypointense) |
| T2 | Hypointense or isointense (>80%) | Heterogenous (60% hyperintense) |
| DWI | −/(+) | + |
| Arterial phase | Hyperintense (homogeneous APHE) | Hyperintense (non-rim APHE) |
| PVP/DP | Hyperintense or isointense (30–40% hypointense) | Hypointense (early washout—75%) |
| HBP | Hyperintense or isointense | Hypointense |
| Central scar | + | − |
| Hypointense perinodular rim | + | − |
| Enhancing capsule | − | + |
| Ultrasound findings | ||
| Echogenicity | Heterogenous or isoechoic | Heterogenous |
| Doppler flow imaging | (−)/+ | (−)/+ |
| Hypoechoic peripheral rim | −/+ | −/+ |
| CEUS | ||
| Arterial phase | Rapid center-to-periphery filling (30% spoke wheel pattern) | Hyperechoic |
| PVP/DP | Hyperechoic (90%) or isoechoic | Hypoechoic (early washout—80–100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzetto, F.; Rutanni, D.; Carbonaro, L.A.; Vanzulli, A. Focal Liver Lesions in Budd-Chiari Syndrome: Spectrum of Imaging Findings. Diagnostics 2023, 13, 2346. https://doi.org/10.3390/diagnostics13142346
Rizzetto F, Rutanni D, Carbonaro LA, Vanzulli A. Focal Liver Lesions in Budd-Chiari Syndrome: Spectrum of Imaging Findings. Diagnostics. 2023; 13(14):2346. https://doi.org/10.3390/diagnostics13142346
Chicago/Turabian StyleRizzetto, Francesco, Davide Rutanni, Luca Alessandro Carbonaro, and Angelo Vanzulli. 2023. "Focal Liver Lesions in Budd-Chiari Syndrome: Spectrum of Imaging Findings" Diagnostics 13, no. 14: 2346. https://doi.org/10.3390/diagnostics13142346
APA StyleRizzetto, F., Rutanni, D., Carbonaro, L. A., & Vanzulli, A. (2023). Focal Liver Lesions in Budd-Chiari Syndrome: Spectrum of Imaging Findings. Diagnostics, 13(14), 2346. https://doi.org/10.3390/diagnostics13142346






