Hyperpolarized Carbon-13 MRI in Breast Cancer
Abstract
:1. Background
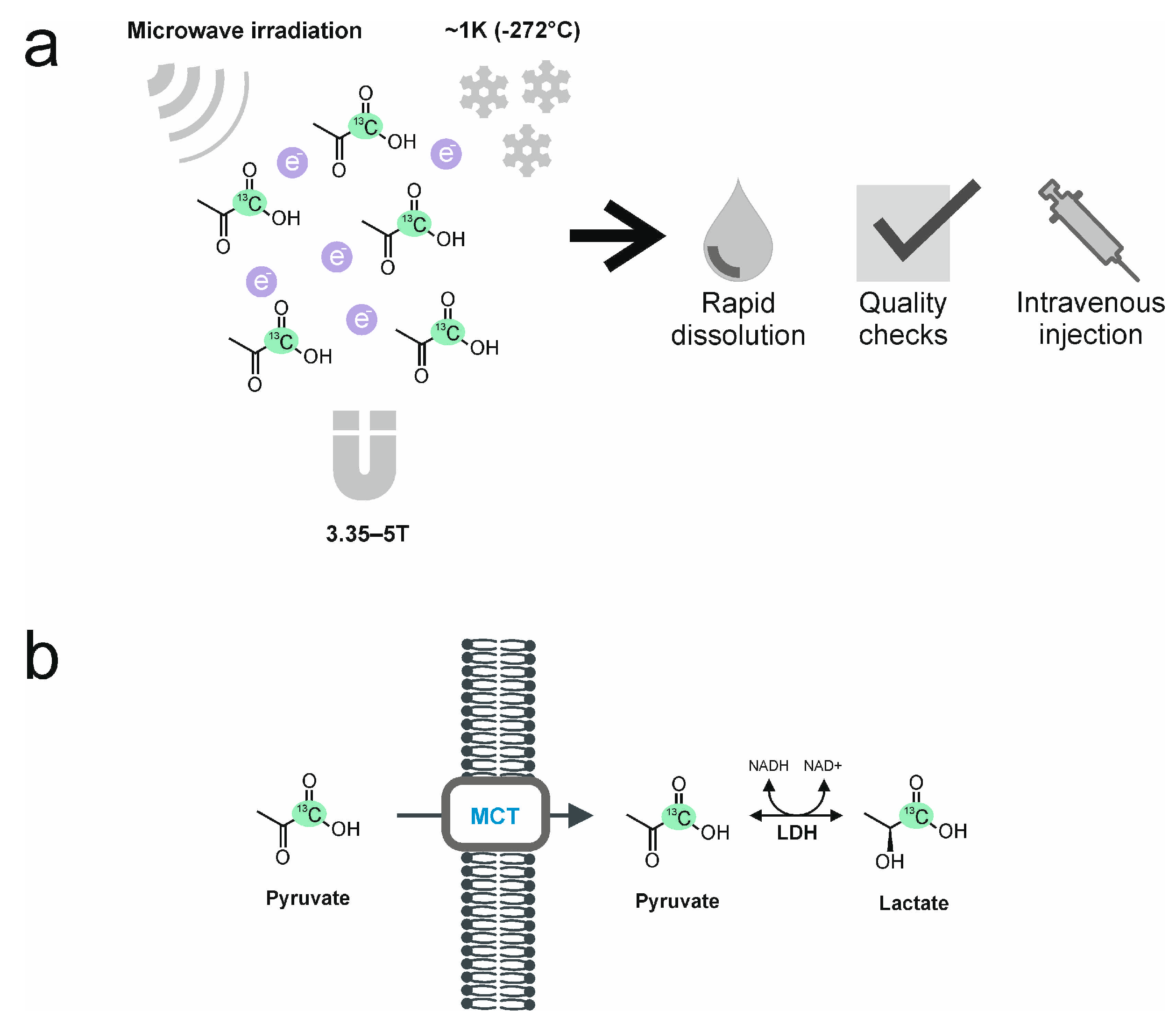
2. The Process of Hyperpolarization
3. Hyperpolarized [1-13C]pyruvate
4. In Vitro and In Vivo Experiments with HP 13C-MRI in Breast Cancer
5. Outlook for New Substrates for Clinical Translation
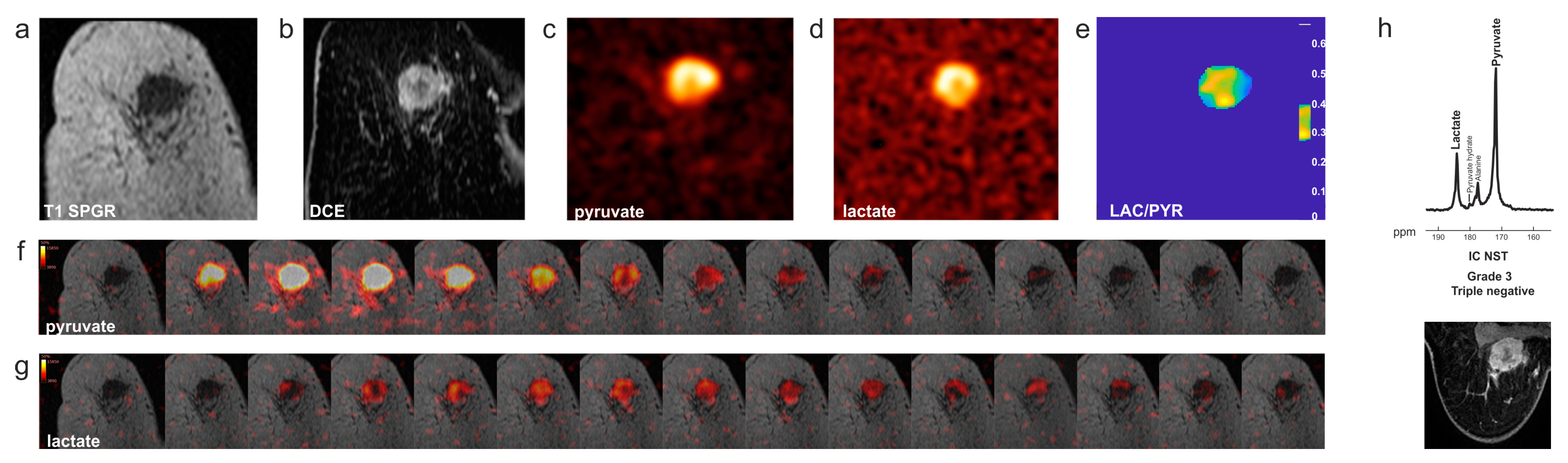
6. Clinical Hyperpolarized 13C-MRI in Patients with Breast Cancer
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebech, A.M.; Gaardsting, A.; Loft, A.; Graff, J.; Markova, E.; Bertelsen, A.K.; Madsen, J.L.; Andersen, K.F.; Von Benzon, E.; Helms, M.; et al. Whole-Body 18F-FDG PET/CT Is Superior to CT as First-Line Diagnostic Imaging in Patients Referred with Serious Nonspecific Symptoms or Signs of Cancer: A Randomized Prospective Study of 200 Patients. J. Nucl. Med. 2017, 58, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Gong, W.; Zhang, Z.; Tu, G.; Li, J.; Xiong, F.; Hou, H.; Zhang, Y.; Wu, M.; Zhang, L. Comparing the Diagnostic Value of 18 F-FDG-PET/CT versus CT for Differentiating Benign and Malignant Solitary Pulmonary Nodules: A Meta-Analysis. J. Thorac. Dis. 2019, 11, 2082–2098. [Google Scholar] [CrossRef]
- Bennani-Baiti, B.; Bennani-Baiti, N.; Baltzer, P.A. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0160346. [Google Scholar] [CrossRef]
- Gadian, D.G. NMR and Its Application to Living Systems, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1995. [Google Scholar]
- O’Flynn, E.A.M.; DeSouza, N.M. Functional Magnetic Resonance: Biomarkers of Response in Breast Cancer. Breast Cancer Res. 2011, 13, 204. [Google Scholar] [CrossRef]
- Cheng, M.; Bhujwalla, Z.M.; Glunde, K. Targeting Phospholipid Metabolism in Cancer. Front. Oncol. 2016, 6, 266. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, X.J.; Song, H.S.; Chen, L.H. 1H-MRS Evaluation of Breast Lesions by Using Total Choline Signal-to-Noise Ratio as an Indicator of Malignancy: A Meta-Analysis. Med. Oncol. 2015, 32, 160. [Google Scholar] [CrossRef]
- Baltzer, P.A.T.; Dietzel, M. Breast Lesions: Diagnosis by Using Proton MR Spectroscopy at 1.5 and 3.0 T—Systematic Review and Meta-Analysis. Radiology 2013, 267, 735–746. [Google Scholar] [CrossRef]
- Meisamy, S.; Bolan, P.J.; Baker, E.H.; Bliss, R.L.; Gulbahce, E.; Everson, L.I.; Nelson, M.T.; Emory, T.H.; Tuttle, T.M.; Yee, D.; et al. Neoadjuvant Chemotherapy of Locally Advanced Breast Cancer: Predicting Response with in Vivo 1H MR Spectroscopy—A Pilot Study at 4 T. Radiology 2004, 233, 424–431. [Google Scholar] [CrossRef]
- Bolan, P.J.; Kim, E.; Herman, B.A.; Newstead, G.M.; Rosen, M.A.; Schnall, M.D.; Pisano, E.D.; Weatherall, P.T.; Morris, E.A.; Lehman, C.D.; et al. MR Spectroscopy of Breast Cancer for Assessing Early Treatment Response: Results from the ACRIN 6657 MRS Trial. J. Magn. Reson. Imaging 2017, 46, 290–302. [Google Scholar] [CrossRef]
- Witney, T.H.; Kettunen, M.I.; Day, S.E.; Hu, D.; Neves, A.A.; Gallagher, F.A.; Fulton, S.M.; Brindle, K.M. A Comparison between Radiolabeled Fluorodeoxyglucose Uptake and Hyperpolarized 13C-Labeled Pyruvate Utilization as Methods for Detecting Tumor Response to Treatment. Neoplasia 2009, 11, 574–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witney, T.H.; Kettunen, M.I.; Hu, D.E.; Gallagher, F.A.; Bohndiek, S.E.; Napolitano, R.; Brindle, K.M. Detecting Treatment Response in a Model of Human Breast Adenocarcinoma Using Hyperpolarised [1-13C]Pyruvate and [1,4-13C2]Fumarate. Br. J. Cancer 2010, 103, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.E.; Ardenkjær-Larsen, J.H.; In ’T Zandt, R.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic Resonance Imaging of PH in Vivo Using Hyperpolarized 13C-Labelled Bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Brindle, K.M.; Bohndiek, S.E.; Gallagher, F.A.; Kettunen, M.I. Tumor Imaging Using Hyperpolarized 13C Magnetic Resonance Spectroscopy. Magn. Reson. Med. 2011, 66, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Henry, P.G.; Adriany, G.; Deelchand, D.; Gruetter, R.; Marjanska, M.; Öz, G.; Seaquist, E.R.; Shestov, A.; Uǧurbil, K. In Vivo 13C NMR Spectroscopy and Metabolic Modeling in the Brain: A Practical Perspective. Magn. Reson. Imaging 2006, 24, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Golman, K.; Ardenkjær-Larsen, J.H.; Petersson, J.S.; Månsson, S.; Leunbach, I. Molecular Imaging with Endogenous Substances. Proc. Natl. Acad. Sci. USA 2003, 100, 10435–10439. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [Green Version]
- Ardenkjær-Larsen, J.H.; Bowen, S.; Petersen, J.R.; Rybalko, O.; Vinding, M.S.; Ullisch, M.; Nielsen, N.C. Cryogen-free Dissolution Dynamic Nuclear Polarization Polarizer Operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med. 2019, 81, 2184–2194. [Google Scholar] [CrossRef] [Green Version]
- Keshari, K.R.; Wilson, D.M. Chemistry and Biochemistry of 13 C Hyperpolarized Magnetic Resonance Using Dynamic Nuclear Polarization. Chem. Soc. Rev. 2014, 43, 1627–1659. [Google Scholar] [CrossRef] [Green Version]
- Woitek, R.; Gallagher, F.A. The Use of Hyperpolarised 13C MRI in Clinical Body Imaging to Probe Cancer Metabolism. Br. J. Cancer 2021, 124, 1187–1198. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Cheng, T.; Boero, G.; Roussel, C.; Comment, A. Thermal Annihilation of Photo-Induced Radicals Following Dynamic Nuclear Polarization to Produce Transportable Frozen Hyperpolarized 13 C-Substrates. Nat. Commun. 2017, 8, 15757. [Google Scholar] [CrossRef] [Green Version]
- Duckett, S.B.; Mewis, R.E. Application of Parahydrogen Induced Polarization Techniques in NMR Spectroscopy and Imaging. Acc. Chem. Res. 2012, 45, 1247–1257. [Google Scholar] [CrossRef]
- Reineri, F.; Boi, T.; Aime, S. ParaHydrogen Induced Polarization of 13C Carboxylate Resonance in Acetate and Pyruvate. Nat. Commun. 2015, 6, 5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; De Graaf, R.A. Deuterium Metabolic Imaging (DMI) for MRI-Based 3D Mapping of Metabolism in Vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Feyter, H.M.; de Graaf, R.A. Deuterium Metabolic Imaging—Back to the Future. J. Magn. Reson. 2021, 326, 106932. [Google Scholar] [CrossRef] [PubMed]
- Chen Ming Low, J.; Wright, A.J.; Hesse, F.; Cao, J.; Brindle, K.M. Metabolic Imaging with Deuterium Labeled Substrates. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 134–135, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, R.L.; Wang, J.; Wright, A.J.; Lewis, D.Y.; Denton, A.E.; Grenfell, R.; Miller, J.L.; Bielik, R.; Gehrung, M.; Fala, M.; et al. Magnetic Resonance Imaging Is More Sensitive than PET for Detecting Treatment-Induced Cell Death-Dependent Changes in Glycolysis. Cancer Res. 2019, 79, 3557–3569. [Google Scholar] [CrossRef]
- Witney, T.H.; Kettunen, M.I.; Brindle, K.M. Kinetic Modeling of Hyperpolarized 13C Label Exchange between Pyruvate and Lactate in Tumor Cells. J. Biol. Chem. 2011, 286, 24572–24580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, F.A.; Woitek, R.; McLean, M.A.; Gill, A.B.; Manzano Garcia, R.; Provenzano, E.; Riemer, F.; Kaggie, J.; Chhabra, A.; Ursprung, S.; et al. Imaging Breast Cancer Using Hyperpolarized Carbon-13 MRI. Proc. Natl. Acad. Sci. USA 2020, 117, 2092–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, J.; Gebhart, G.; Ruiz Borrego, M.; Stradella, A.; Bermejo, B.; Escrivá, S.; Calvo Martínez, L.; Ribelles, N.; Martinez, N.; Albacar, C.; et al. Chemotherapy (CT) de-Escalation Using an FDG-PET/CT (F-PET) and Pathological Response-Adapted Strategy in HER2[+] Early Breast Cancer (EBC): PHERGain Trial. J. Clin. Oncol. 2020, 38, 503. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Ros, S.; Wright, A.J.; D’Santos, P.; Hu, D.; Hesketh, R.L.; Lubling, Y.; Georgopoulou, D.; Lerda, G.; Couturier, D.L.; Razavi, P.; et al. Metabolic Imaging Detects Resistance to PI3Kα Inhibition Mediated by Persistent FOXM1 Expression in ER+ Breast Cancer. Cancer Cell 2020, 38, 516–533.e9. [Google Scholar] [CrossRef]
- Fala, M.; Somai, V.; Dannhorn, A.; Hamm, G.; Gibson, K.; Couturier, D.L.; Hesketh, R.; Wright, A.J.; Takats, Z.; Bunch, J.; et al. Comparison of 13C MRI of Hyperpolarized [1-13C]Pyruvate and Lactate with the Corresponding Mass Spectrometry Images in a Murine Lymphoma Model. Magn. Reson. Med. 2021, 85, 3027–3035. [Google Scholar] [CrossRef]
- Harris, T.; Eliyahu, G.; Frydman, L.; Degani, H. Kinetics of Hyperpolarized 13C1-Pyruvate Transport and Metabolism in Living Human Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18131–18136. [Google Scholar] [CrossRef]
- Macdonald, E.B.; Begovatz, P.; Barton, G.P.; Erickson-Bhatt, S.; Inman, D.R.; Cox, B.L.; Eliceiri, K.W.; Strigel, R.M.; Ponik, S.M.; Fain, S.B. Hyperpolarized 13C Magnetic Resonance Spectroscopic Imaging of Pyruvate Metabolism in Murine Breast Cancer Models of Different Metastatic Potential. Metabolites 2021, 11, 274. [Google Scholar] [CrossRef]
- Grashei, M.; Biechl, P.; Schilling, F.; Otto, A.M. Conversion of Hyperpolarized [1-13 C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment. Cancers 2022, 14, 1845. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, V.; Bok, R.A.; Santos, R.D.; Cunha, J.A.; Hsu, I.C.; Santos BS, J.D.; Lee, J.E.; Sukumar, S.; Larson, P.E.Z.; et al. Simultaneous Metabolic and Perfusion Imaging Using Hyperpolarized 13C MRI Can Evaluate Early and Dose-Dependent Responses to Radiotherapy in a Prostate Cancer Mouse Model. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 887. [Google Scholar] [CrossRef]
- Von Morze, C.; Larson, P.E.Z.; Hu, S.; Keshari, K.; Wilson, D.M.; Ardenkjaer-Larsen, J.H.; Goga, A.; Bok, R.; Kurhanewicz, J.; Vigneron, D.B. Imaging of Blood Flow Using Hyperpolarized [13C]Urea in Preclinical Cancer Models. J. Magn. Reson. Imaging 2011, 33, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Tang, S.; Riselli, A.M.; Bok, R.A.; Delos Santos, R.; van Criekinge, M.; Gordon, J.W.; Aggarwal, R.; Chen, R.; Goddard, G.; et al. Clinical Translation of Hyperpolarized 13C Pyruvate and Urea MRI for Simultaneous Metabolic and Perfusion Imaging. Magn. Reson. Med. 2022, 87, 138–149. [Google Scholar] [CrossRef]
- Gallagher, F.A.; Kettunen, M.I.; Hu, D.-E.; Jensen, P.R.; Zandt, R.I.’t.; Karlsson, M.; Gisselsson, A.; Nelson, S.K.; Witney, T.H.; Bohndiek, S.E.; et al. Production of Hyperpolarized [1,4-13C2]Malate from [1,4-13C2]Fumarate Is a Marker of Cell Necrosis and Treatment Response in Tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 19801–19806. [Google Scholar] [CrossRef]
- Feuerecker, B.; Durst, M.; Michalik, M.; Schneider, G.; Saur, D.; Menzel, M.; Schwaiger, M.; Schilling, F. Hyperpolarized 13C Diffusion MRS of Co-Polarized Pyruvate and Fumarate to Measure Lactate Export and Necrosis. J. Cancer 2017, 8, 3078–3085. [Google Scholar] [CrossRef] [Green Version]
- Hesse, F.; Somai, V.; Kreis, F.; Bulat, F.; Wright, A.J.; Brindle, K.M. Monitoring Tumor Cell Death in Murine Tumor Models Using Deuterium Magnetic Resonance Spectroscopy and Spectroscopic Imaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2014631118. [Google Scholar] [CrossRef] [PubMed]
- Kaggie, J.D.; Khan, A.S.; Matys, T.; Schulte, R.F.; Locke, M.J.; Grimmer, A.; Frary, A.; Menih, I.H.; Latimer, E.; Graves, M.J.; et al. Deuterium Metabolic Imaging and Hyperpolarized 13C-MRI of the Normal Human Brain at Clinical Field Strength Reveals Differential Cerebral Metabolism. Neuroimage 2022, 257, 119284. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Neoadjuvant Chemotherapy Use in Breast Cancer Is Greatest in Excellent Responders: Triple-Negative and HER2+ Subtypes. Ann. Surg. Oncol. 2018, 25, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.M.; Den Dekker, B.M.; Gilhuijs, K.G.A.; Van Diest, P.J.; Van Der Wall, E.; Elias, S.G. MRI to Assess Response after Neoadjuvant Chemotherapy in Breast Cancer Subtypes: A Systematic Review and Meta-Analysis. Npj Breast Cancer 2022, 8, 1–7. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Abeyakoon, O.; Latifoltojar, A.; Gong, F.; Papoutsaki, M.-V.; Chowdhury, R.; Glaser, M.; Jeraj, H.; Awais, R.; Holt, C.; Twyman, F.; et al. Hyperpolarised 13C MRI: A New Horizon for Non-Invasive Diagnosis of Aggressive Breast Cancer. BJR Case Rep. 2019, 5, 20190026. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Rueda, O.M.; Sammut, S.J.; Seoane, J.A.; Chin, S.F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of Breast-Cancer Relapse Reveal Late-Recurring ER-Positive Genomic Subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Woitek, R.; McLean, M.A.; Ursprung, S.; Rueda, O.M.; Garcia, R.M.; Locke, M.J.; Beer, L.; Baxter, G.; Rundo, L.; Provenzano, E.; et al. Hyperpolarized Carbon-13 MRI for Early Response Assessment of Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. 2021, 81, 6004–6017. [Google Scholar] [CrossRef]
- Woitek, R.; McLean, M.; Gill, A.B.; Grist, J.T.; Provenzano, E.; Patterson, A.J.; Ursprung, S.; Torheim, T.; Zaccagna, F.; Locke, M.; et al. Hyperpolarized 13C-MRI of Tumor Metabolism Demonstrates Early Metabolic Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiol. Imaging Cancer 2020, 2, e200017. [Google Scholar] [CrossRef]
- Ravoori, M.K.; Singh, S.P.; Lee, J.; Bankson, J.A.; Kundra, V. In Vivo Assessment of Ovarian Tumor Response to Tyrosine Kinase Inhibitor Pazopanib by Using Hyperpolarized 13C-Pyruvate MR Spectroscopy and 18F-FDG PET/CT Imaging in a Mouse Model. Radiology 2017, 285, 830–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodi, A.; Woods, S.M.; Ronen, S.M. Treatment with the MEK Inhibitor U0126 Induces Decreased Hyperpolarized Pyruvate to Lactate Conversion in Breast, but Not Prostate, Cancer Cells. NMR Biomed. 2013, 26, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodi, A.; Woods, S.M.; Ronen, S.M. MR-Detectable Metabolic Consequences of Mitogen-Activated Protein Kinase Kinase (MEK) Inhibition. NMR Biomed. 2014, 27, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Bohndiek, S.E.; Kettunen, M.I.; Hu, D.; Brindle, K.M. Hyperpolarized 13C Spectroscopy Detects Early Changes in Tumor Vasculature and Metabolism after VEGF Neutralization. Cancer Res. 2012, 72, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Granlund, K.L.; Tee, S.S.; Vargas, H.A.; Lyashchenko, S.K.; Reznik, E.; Fine, S.; Laudone, V.; Eastham, J.A.; Touijer, K.A.; Reuter, V.E.; et al. Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab. 2020, 31, 105–114.e3. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Serrao, E.M.; Kennedy, B.W.C.; Hu, D.-E.; Kettunen, M.I.; Brindle, K.M. Magnetic Resonance Imaging of Tumor Glycolysis Using Hyperpolarized 13C-Labeled Glucose. Nat. Med. 2014, 20, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Livraghi, L.; Garber, J.E. PARP Inhibitors in the Management of Breast Cancer: Current Data and Future Prospects. BMC Med. 2015, 13, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, C.; Zhang, C.; Luo, T.; Vyas, A.; Chen, S.H.; Liu, C.; Kassab, M.A.; Yang, Y.; Kong, M.; Yu, X. NADP+ Is an Endogenous PARP Inhibitor in DNA Damage Response and Tumor Suppression. Nat. Commun. 2019, 10, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP Inhibition: PARP1 and Beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Vyas, A.; Kassab, M.A.; Singh, A.K.; Yu, X. The Role of Poly ADP-Ribosylation in the First Wave of DNA Damage Response. Nucleic Acids Res. 2017, 45, 8129–8141. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Soliman, H.; Bragagnolo, N.D.; Sahgal, A.; Geraghty, B.J.; Chen, A.P.; Endre, R.; Perks, W.J.; Detsky, J.S.; Leung, E.; et al. Predicting Response to Radiotherapy of Intracranial Metastases with Hyperpolarized 13C MRI. J. Neurooncol. 2021, 152, 551–557. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, F.; Bradbury, C.M.; Kaushal, A.; Li, L.; Spitz, D.R.; Aft, R.L.; Gius, D. 2-Deoxy-D-Glucose-Induced Cytotoxicity and Radiosensitization in Tumor Cells Is Mediated via Disruptions in Thiol Metabolism. Cancer Res. 2003, 63, 3413–3417. [Google Scholar]
- Milshteyn, E.; von Morze, C.; Reed, G.D.; Shang, H.; Shin, P.J.; Zhu, Z.; Chen, H.Y.; Bok, R.; Goga, A.; Kurhanewicz, J.; et al. Development of High Resolution 3D Hyperpolarized Carbon-13 MR Molecular Imaging Techniques. Magn. Reson. Imaging 2017, 38, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Shang, H.; Sukumar, S.; von Morze, C.; Bok, R.A.; Marco-Rius, I.; Kerr, A.; Reed, G.D.; Milshteyn, E.; Ohliger, M.A.; Kurhanewicz, J.; et al. Spectrally Selective Three-Dimensional Dynamic Balanced Steady-State Free Precession for Hyperpolarized C-13 Metabolic Imaging with Spectrally Selective Radiofrequency Pulses. Magn. Reson. Med. 2017, 78, 963–975. [Google Scholar] [CrossRef]
- Milshteyn, E.; von Morze, C.; Gordon, J.W.; Zhu, Z.; Larson, P.E.Z.; Vigneron, D.B. High Spatiotemporal Resolution BSSFP Imaging of Hyperpolarized [1-13C]Pyruvate and [1-13C]Lactate with Spectral Suppression of Alanine and Pyruvate-Hydrate. Magn. Reson. Med. 2018, 80, 1048–1060. [Google Scholar] [CrossRef]
- Chowdhury, R.; Mueller, C.A.; Smith, L.; Gong, F.; Papoutsaki, M.V.; Rogers, H.; Syer, T.; Singh, S.; Brembilla, G.; Retter, A.; et al. Quantification of Prostate Cancer Metabolism Using 3D Multiecho BSSFP and Hyperpolarized [1-13C] Pyruvate: Metabolism Differs Between Tumors of the Same Gleason Grade. J. Magn. Reson. Imaging 2022, 57, 1865–1875. [Google Scholar] [CrossRef]
- Stødkilde-Jørgensen, H.; Laustsen, C.; Hansen, E.S.S.; Schulte, R.; Ardenkjaer-Larsen, J.H.; Comment, A.; Frøkiær, J.; Ringgaard, S.; Bertelsen, L.B.; Ladekarl, M.; et al. Pilot Study Experiences With Hyperpolarized [1-13C]Pyruvate MRI in Pancreatic Cancer Patients. J. Magn. Reson. Imaging 2019, 51, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Ursprung, S.; Woitek, R.; McLean, M.A.; Priest, A.N.; Crispin-Ortuzar, M.; Brodie, C.R.; Gill, A.B.; Gehrung, M.; Beer, L.; Riddick, A.C.P.; et al. Hyperpolarized13C-Pyruvate Metabolism as a Surrogate for Tumor Grade and Poor Outcome in Renal Cell Carcinoma—A Proof of Principle Study. Cancers 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Latifoltojar, A.; Neves, J.B.; Papoutsaki, M.-V.; Gong, F.; Comment, A.; Costa, A.S.H.; Glaser, M.; Tran-Dang, M.-A.; El Sheikh, S.; et al. First-in-Human in Vivo Non-Invasive Assessment of Intra-Tumoral Metabolic Heterogeneity in Renal Cell Carcinoma. BJR Case Rep. 2019, 5, 20190003. [Google Scholar] [CrossRef] [PubMed]
- Zaccagna, F.; McLean, M.A.; Grist, J.T.; Kaggie, J.; Mair, R.; Riemer, F.; Woitek, R.; Gill, A.B.; Deen, S.; Daniels, C.J.; et al. Imaging Glioblastoma Metabolism by Using Hyperpolarized [1-13C]Pyruvate Demonstrates Heterogeneity in Lactate Labeling: A Proof of Principle Study. Radiol. Imaging Cancer 2022, 4, 210076. [Google Scholar] [CrossRef]
- Grist, J.T.; McLean, M.A.; Riemer, F.; Schulte, R.F.; Deen, S.S.; Zaccagna, F.; Woitek, R.; Daniels, C.J.; Kaggie, J.D.; Matyz, T.; et al. Quantifying Normal Human Brain Metabolism Using Hyperpolarized [1-13C]Pyruvate and Magnetic Resonance Imaging. Neuroimage 2019, 189, 171–179. [Google Scholar] [CrossRef]
- European Medicines Agency information on Keytruda. Available online: https://www.Ema.Europa.Eu/En/Medicines/Human/EPAR/Keytrud (accessed on 7 June 2023).
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. IRECIST: Guidelines for Response Criteria for Use in Trials Testing Immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Kaira, K.; Higuchi, T.; Naruse, I.; Arisaka, Y.; Tokue, A.; Altan, B.; Suda, S.; Mogi, A.; Shimizu, K.; Sunaga, N.; et al. Metabolic Activity by 18F–FDG-PET/CT Is Predictive of Early Response after Nivolumab in Previously Treated NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bier, G.; Hoffmann, V.; Kloth, C.; Othman, A.E.; Eigentler, T.; Garbe, C.; La Fougère, C.; Pfannenberg, C.; Nikolaou, K.; Klumpp, B. CT Imaging of Bone and Bone Marrow Infiltration in Malignant Melanoma-Challenges and Limitations for Clinical Staging in Comparison to 18FDG-PET/CT. Eur. J. Radiol. 2016, 85, 732–738. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lipson, E.J.; Im, H.J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 2017, 58, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Saunders, C.; Liniker, E.; Ramanujam, S.; Guminski, A.; Scolyer, R.; Kefford, R.; Menzies, A.; Long, G.; Carlino, M. Metabolic Activity in Metastatic Melanoma after Long-Term Treatment with Anti-PD-1 Antibodies. Eur. J. Cancer 2015, 51, S665. [Google Scholar] [CrossRef]
- de Kouchkovsky, I.; Chen, H.Y.; Ohliger, M.A.; Wang, Z.J.; Bok, R.A.; Gordon, J.W.; Larson, P.E.Z.; Frost, M.; Okamoto, K.; Cooperberg, M.R.; et al. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Immune Checkpoint Inhibitor Therapy in Prostate Cancer. Eur. Urol. 2022, 81, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Neveu, M.A.; Bouzin, C.; Knezevic, Z.; Gallez, B.; Leucci, E.; Baurain, J.F.; Mignion, L.; Jordan, B.F. Hyperpolarized 13C-Pyruvate to Assess Response to Anti-PD1 Immune Checkpoint Inhibition in YUMMER 1.7 Melanoma Xenografts. Int. J. Mol. Sci. 2023, 24, 2499. [Google Scholar] [CrossRef] [PubMed]
- Saida, Y.; Brender, J.R.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C.; Kishimoto, S. Multimodal Molecular Imaging Detects Early Responses to Immune Checkpoint Blockade. Cancer Res. 2021, 81, 3693–3705. [Google Scholar] [CrossRef] [PubMed]

| Reference | Tumor | Imaging | Results |
|---|---|---|---|
| [25] | Human colorectal (Colo205) and breast adenocarcinoma (MDA-MB-231) xenografts in mice treated with an apoptosis-inducing agent (TRAIL agonist) | 13C-MRI with hyperpolarized [1-13C]pyruvate [18F]FDG-PET | At only 24 h after treatment, there was a decrease in lactate labeling, whereas [18F]FDG uptake remained unchanged. |
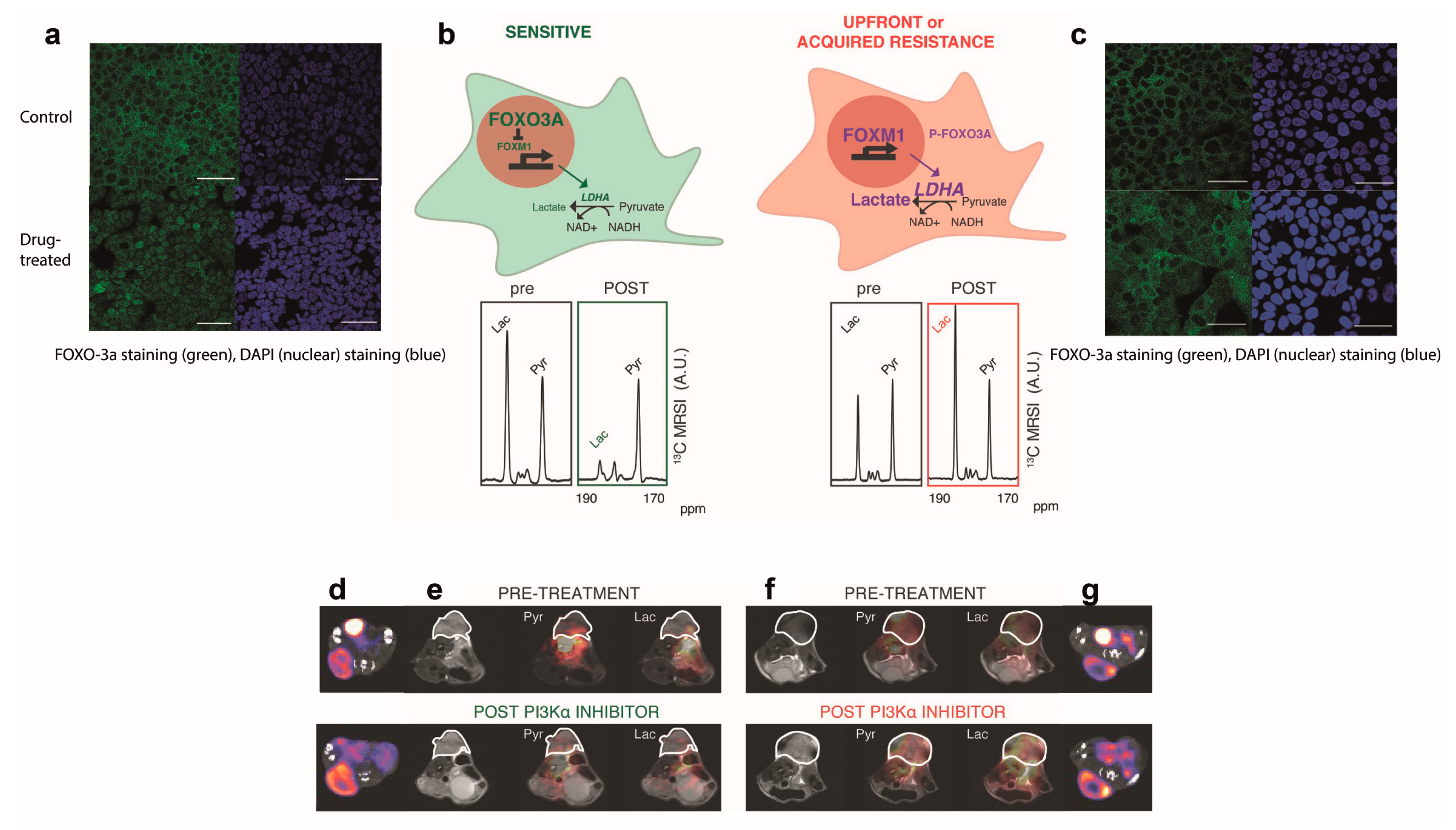
| [26] | Patient-derived ER+ breast cancer xenografts treated with a PI3Kα inhibitor | 13C-MRI with hyperpolarized [1-13C]pyruvate [18F]FDG-PET | 13C-label exchange between pyruvate and lactate was decreased in drug-sensitive but not in drug-resistant tumors, whereas [18F]FDG uptake was unaffected in both. |
| [32] | TRAMP (Transgenic Adenocarcinoma of Mouse Prostate) mice undergoing radiotherapy | 13C-MRI with co-polarized [1-13C]pyruvate and [13C]urea | After 1, 4, and 7 days a decrease in lactate-to-pyruvate conversion was found with a concomitant increase in the hyperpolarized urea signal, indicating increased perfusion. Similar results indicated increased perfusion were obtained with DCE MRI. |
| [15] | Suspensions of MDA-MB-231 breast cancer cells and mice with subcutaneous MDA-MB-231 tumors following their treatment with doxorubicin | Co-polarized [1-13C]pyruvate and [1,4-13C2]fumarate | Treatment-induced cell death was accompanied by a decrease in 13C-label exchange between [1-13C]pyruvate and lactate and a concomitant increase in flux between fumarate and malate, which occurred before changes in tumor size. |
| [33] | Human breast cancer (MDA-MB-231) xenografts | 2H-MRSI at 7T after injection of [2,3-2H2]fumarate | At only 48 h after treatment with a TRAIL agonist, the malate-to-fumarate ratio increased significantly. |
| [34] | One patient with early triple-negative breast cancer undergoing platinum-based neoadjuvant chemotherapy | 13C-MRI with hyperpolarized [1-13C]pyruvate 1H-MRI including DCE MRI | In a patient with eventual pathologic complete response (pCR), after the first cycle of neoadjuvant chemotherapy, the lactate-to-pyruvate ratio and the apparent first-order rate constant describing label flux from pyruvate to lactate (kPL) had decreased while Ktrans on DCE MRI had increased. |
| [35] | Seven patients with early triple negative or HER2+ breast cancer undergoing neoadjuvant treatment; a subgroup received Olaparib (PARP inhibitor) treatment. | 13C-MRI with hyperpolarized [1-13C]pyruvate 1H-MRI including DCE MRI and DWI | At 7–11 days into treatment, an early increase in the lactate-to-pyruvate ratio of ≥20% was observed among patients with pCR, but not those without eventual pCR; neither DCE MRI with pharmacokinetic modeling nor DWI allowed a distinction between these two outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woitek, R.; Brindle, K.M. Hyperpolarized Carbon-13 MRI in Breast Cancer. Diagnostics 2023, 13, 2311. https://doi.org/10.3390/diagnostics13132311
Woitek R, Brindle KM. Hyperpolarized Carbon-13 MRI in Breast Cancer. Diagnostics. 2023; 13(13):2311. https://doi.org/10.3390/diagnostics13132311
Chicago/Turabian StyleWoitek, Ramona, and Kevin M. Brindle. 2023. "Hyperpolarized Carbon-13 MRI in Breast Cancer" Diagnostics 13, no. 13: 2311. https://doi.org/10.3390/diagnostics13132311
APA StyleWoitek, R., & Brindle, K. M. (2023). Hyperpolarized Carbon-13 MRI in Breast Cancer. Diagnostics, 13(13), 2311. https://doi.org/10.3390/diagnostics13132311







