Hyperoxic BOLD-MRI-Based Characterization of Breast Cancer Molecular Subtypes Is Independent of the Supplied Amount of Oxygen: A Preclinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Mouse Model
2.2. MRI
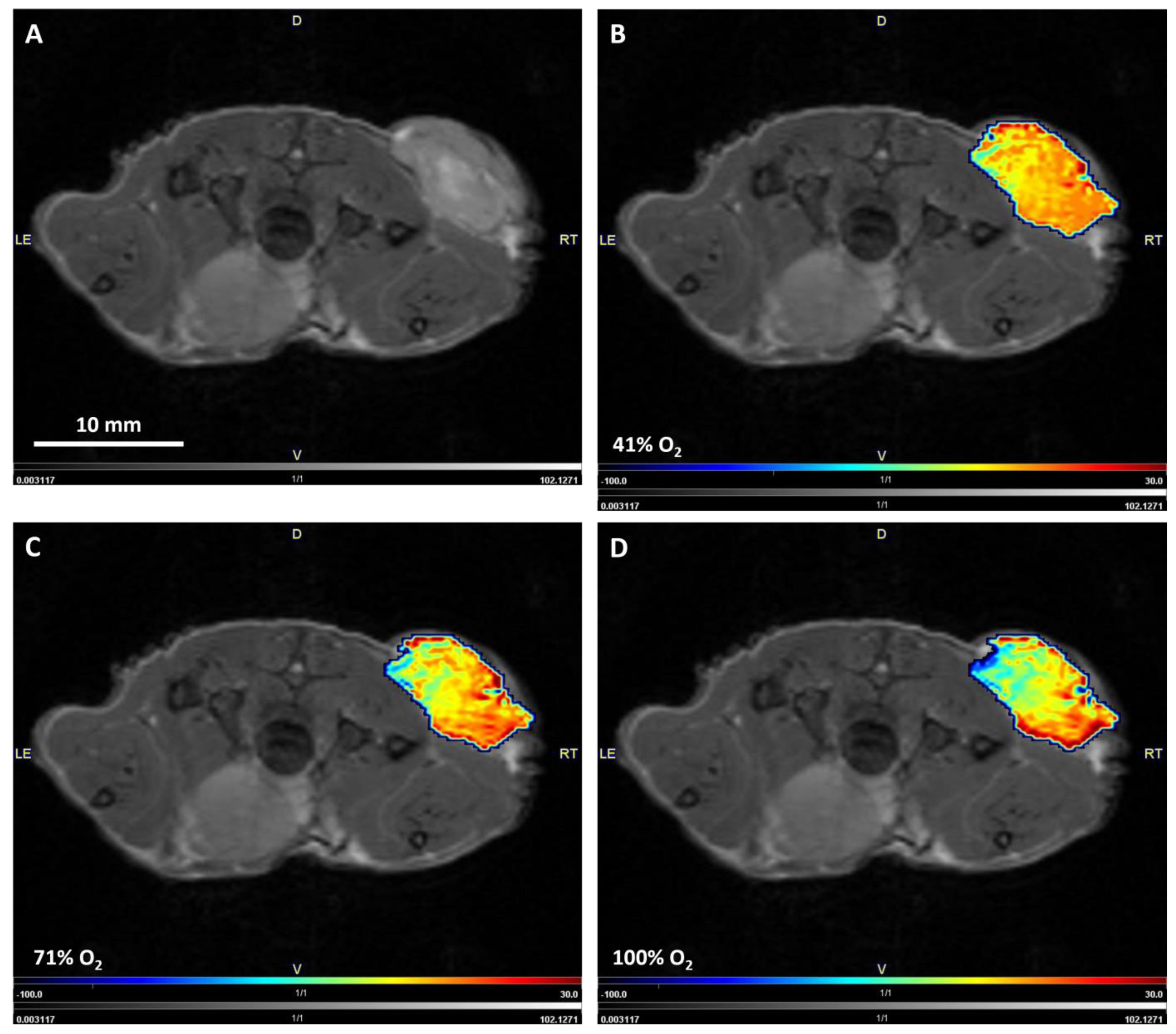
2.3. Image Postprocessing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol. Med. 2001, 8, S62–S67. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Tatum, J.L.; Kelloff, G.J.; Gillies, R.J.; Arbeit, J.M.; Brown, J.M.; Chao, K.S.; Chapman, J.D.; Eckelman, W.C.; Fyles, A.W.; Giaccia, A.J.; et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006, 82, 699–757. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Song, G.; Ouyang, G. Role of hypoxia in the hallmarks of human cancer. J. Cell Biochem. 2009, 107, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Hypoxia and aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist 2008, 13 (Suppl. S3), 21–26. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Ajduković, J. HIF-1—A big chapter in the cancer tale. Exp. Oncol. 2016, 38, 9–12. [Google Scholar] [CrossRef]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef]
- Haynes, B.; Sarma, A.; Nangia-Makker, P.; Shekhar, M.P. Breast cancer complexity: Implications of intratumoral heterogeneity in clinical management. Cancer Metastasis Rev. 2017, 36, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. (Lausanne) 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. In Breast Cancer Chemosensitivity; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–22. [Google Scholar]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumor hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef]
- Pauling, L.; Coryell, C.D. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. USA 1936, 22, 210–216. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Waterton, J.C.; Matthews, P.M.; Radda, G.K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1982, 714, 265–270. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Raichle, M.E. Behind the scenes of functional brain imaging: A historical and physiological perspective. Proc. Natl. Acad. Sci. USA 1998, 95, 765–772. [Google Scholar] [CrossRef]
- Pinker, K.; Helbich, T.H.; Morris, E.A. The potential of multiparametric MRI of the breast. Br. J. Radiol. 2017, 90, 20160715. [Google Scholar] [CrossRef]
- Panek, R.; Welsh, L.; Baker, L.C.J.; Schmidt, M.A.; Wong, K.H.; Riddell, A.M.; Koh, D.M.; Dunlop, A.; McQuaid, D.; d’Arcy, J.A.; et al. Noninvasive Imaging of Cycling Hypoxia in Head and Neck Cancer Using Intrinsic Susceptibility MRI. Clin. Cancer Res. 2017, 23, 4233–4241. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Naish, J.H.; Jackson, A.; Waterton, J.C.; Watson, Y.; Cheung, S.; Buckley, D.L.; McGrath, D.M.; Buonaccorsi, G.A.; Mills, S.J.; et al. Comparison of normal tissue R1 and R*2 modulation by oxygen and carbogen. Magn. Reson. Med. 2009, 61, 75–83. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed]
- Severinghaus, J.W. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J. Appl. Physiol. 1958, 12, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.M.; Arai, T.J.; Campbell, J.W., 3rd; Gerberich, J.L.; Zhou, H.; Mason, R.P. Oxygen-sensitive MRI assessment of tumor response to hypoxic gas breathing challenge. NMR Biomed. 2019, 32, e4101. [Google Scholar] [CrossRef]
- Parkins, K.M.; Krishnamachary, B.; Jacob, D.; Kakkad, S.M.; Solaiyappan, M.; Mishra, A.; Mironchik, Y.; Penet, M.F.; McMahon, M.T.; Knopf, P.; et al. PET/MRI and Bioluminescent Imaging Identify Hypoxia as a Cause of Programmed Cell Death Ligand 1 Image Heterogeneity. Radiol. Imaging Cancer 2023, 5, e220138. [Google Scholar] [CrossRef]
- Padhani, A.R.; Krohn, K.A.; Lewis, J.S.; Alber, M. Imaging oxygenation of human tumours. Eur. Radiol. 2007, 17, 861–872. [Google Scholar] [CrossRef]
- Rakow-Penner, R.; Daniel, B.; Glover, G.H. Detecting blood oxygen level-dependent (BOLD) contrast in the breast. J. Magn. Reson. Imaging 2010, 32, 120–129. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, J.Y.; Suh, H.B.; Hwangbo, L.; Lee, N.K.; Kim, S.; Lee, J.W.; Choo, K.S.; Nam, K.J.; Kang, T.; et al. Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Eur. Radiol. 2022, 32, 822–833. [Google Scholar] [CrossRef]
- Koo, H.R.; Cho, N.; Song, I.C.; Kim, H.; Chang, J.M.; Yi, A.; Yun, B.L.; Moon, W.K. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J. Magn. Reson. Imaging 2012, 36, 145–151. [Google Scholar] [CrossRef]
- Youk, J.H.; Son, E.J.; Chung, J.; Kim, J.A.; Kim, E.K. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: Comparison with other breast cancer subtypes. Eur. Radiol. 2012, 22, 1724–1734. [Google Scholar] [CrossRef]
- Robinson, S.P.; Rijken, P.F.; Howe, F.A.; McSheehy, P.M.; van der Sanden, B.P.; Heerschap, A.; Stubbs, M.; van der Kogel, A.J.; Griffiths, J.R. Tumor vascular architecture and function evaluated by non-invasive susceptibility MRI methods and immunohistochemistry. J. Magn. Reson. Imaging 2003, 17, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Howe, F.A.; Robinson, S.P.; McIntyre, D.J.; Stubbs, M.; Griffiths, J.R. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001, 14, 497–506. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Zimmermann, M.; Bennani-Baiti, B.; Helbich, T.H.; Baltzer, P.; Clauser, P.; Kapetas, P.; Bago-Horvath, Z.; Pinker, K. Development of a Non-invasive Assessment of Hypoxia and Neovascularization with Magnetic Resonance Imaging in Benign and Malignant Breast Tumors: Initial Results. Mol. Imaging Biol. 2019, 21, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Virani, N.; Kwon, J.; Zhou, H.; Mason, R.; Berbeco, R.; Protti, A. In vivo hypoxia characterization using blood oxygen level dependent magnetic resonance imaging in a preclinical glioblastoma mouse model. Magn. Reson. Imaging 2021, 76, 52–60. [Google Scholar] [CrossRef] [PubMed]
- McPhail, L.D.; Robinson, S.P. Intrinsic susceptibility MR imaging of chemically induced rat mammary tumors: Relationship to histologic assessment of hypoxia and fibrosis. Radiology 2010, 254, 110–118. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, L.; Hahn, E.W.; Mason, R.P. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn. Reson. Med. 2009, 62, 357–364. [Google Scholar] [CrossRef]
- Hallac, R.R.; Zhou, H.; Pidikiti, R.; Song, K.; Stojadinovic, S.; Zhao, D.; Solberg, T.; Peschke, P.; Mason, R.P. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn. Reson. Med. 2014, 71, 1863–1873. [Google Scholar] [CrossRef]
- Arai, T.J.; Yang, D.M.; Campbell, J.W., 3rd; Chiu, T.; Cheng, X.; Stojadinovic, S.; Peschke, P.; Mason, R.P. Oxygen-Sensitive MRI: A Predictive Imaging Biomarker for Tumor Radiation Response? Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1519–1529. [Google Scholar] [CrossRef]
- Liu, M.; Guo, X.; Wang, S.; Jin, M.; Wang, Y.; Li, J.; Liu, J. BOLD-MRI of breast invasive ductal carcinoma: Correlation of R2* value and the expression of HIF-1alpha. Eur. Radiol. 2013, 23, 3221–3227. [Google Scholar] [CrossRef]
- O’Flynn, E.A.; deSouza, N.M. Functional magnetic resonance: Biomarkers of response in breast cancer. Breast Cancer Res. 2011, 13, 204. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Mattace Raso, M.; Vallone, P.; De Rosa, A.P.; Siani, C.; Di Bonito, M.; Petrillo, A.; Sansone, M. Blood Oxygenation Level Dependent Magnetic Resonance Imaging (MRI), Dynamic Contrast Enhanced MRI, and Diffusion Weighted MRI for Benign and Malignant Breast Cancer Discrimination: A Preliminary Experience. Cancers 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Pariante, P.; Cerciello, V.; Siani, C.; Di Bonito, M.; Valentino, M.; Sansone, M.; Botti, G.; Petrillo, A. Blood oxygenation level dependent magnetic resonance imaging and diffusion weighted MRI imaging for benign and malignant breast cancer discrimination. Magn. Reson. Imaging 2021, 75, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Duyn, J.H. The future of ultra-high field MRI and fMRI for study of the human brain. Neuroimage 2012, 62, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Mittal, S.; Wu, Z.; Neelavalli, J.; Cheng, Y.C. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. AJNR Am. J. Neuroradiol. 2009, 30, 19–30. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
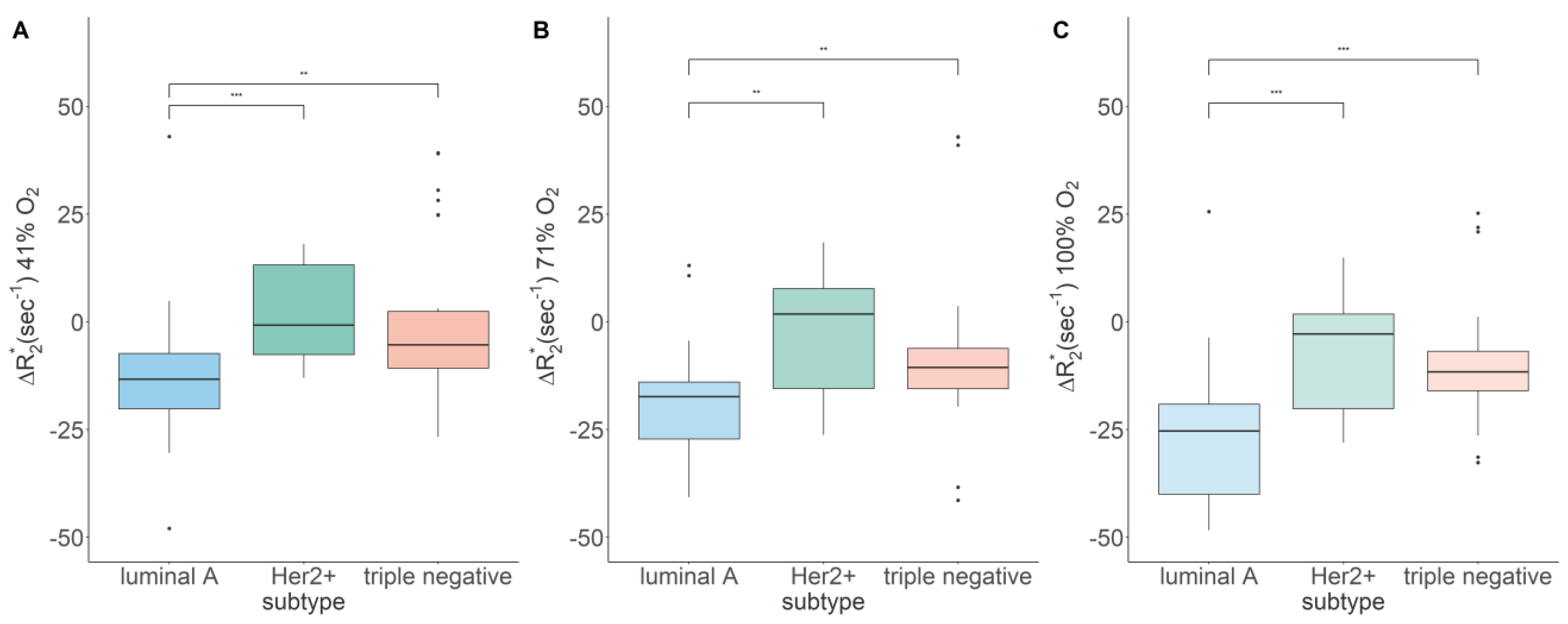
| 21% O2 [s−1] | 41% O2 [s−1] | 71% O2 [s−1] | 100% O2 [s−1] | |
|---|---|---|---|---|
| Luminal A | 145.19, 67.56 | −13.46, 14.20 a | −20.37, 17.06 | −28.42, 24.35 a |
| Her2+ | 140.60, 33.13 | −0.76, 20.80 *** | 1.80, 23.19 ** | −2.85, 21.95 *** |
| Triple negative | 110.06, 20.93 | −5.32, 13.15 ** | −10.58, 9.38 ** | −11.64, 9.15 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartsch, S.J.; Ehret, V.; Friske, J.; Fröhlich, V.; Laimer-Gruber, D.; Helbich, T.H.; Pinker, K. Hyperoxic BOLD-MRI-Based Characterization of Breast Cancer Molecular Subtypes Is Independent of the Supplied Amount of Oxygen: A Preclinical Study. Diagnostics 2023, 13, 2946. https://doi.org/10.3390/diagnostics13182946
Bartsch SJ, Ehret V, Friske J, Fröhlich V, Laimer-Gruber D, Helbich TH, Pinker K. Hyperoxic BOLD-MRI-Based Characterization of Breast Cancer Molecular Subtypes Is Independent of the Supplied Amount of Oxygen: A Preclinical Study. Diagnostics. 2023; 13(18):2946. https://doi.org/10.3390/diagnostics13182946
Chicago/Turabian StyleBartsch, Silvester J., Viktoria Ehret, Joachim Friske, Vanessa Fröhlich, Daniela Laimer-Gruber, Thomas H. Helbich, and Katja Pinker. 2023. "Hyperoxic BOLD-MRI-Based Characterization of Breast Cancer Molecular Subtypes Is Independent of the Supplied Amount of Oxygen: A Preclinical Study" Diagnostics 13, no. 18: 2946. https://doi.org/10.3390/diagnostics13182946
APA StyleBartsch, S. J., Ehret, V., Friske, J., Fröhlich, V., Laimer-Gruber, D., Helbich, T. H., & Pinker, K. (2023). Hyperoxic BOLD-MRI-Based Characterization of Breast Cancer Molecular Subtypes Is Independent of the Supplied Amount of Oxygen: A Preclinical Study. Diagnostics, 13(18), 2946. https://doi.org/10.3390/diagnostics13182946








