Optic Nerve Sheath Meningiomas: Solving Diagnostic Challenges with 68Ga-DOTATOC PET/CT
Abstract
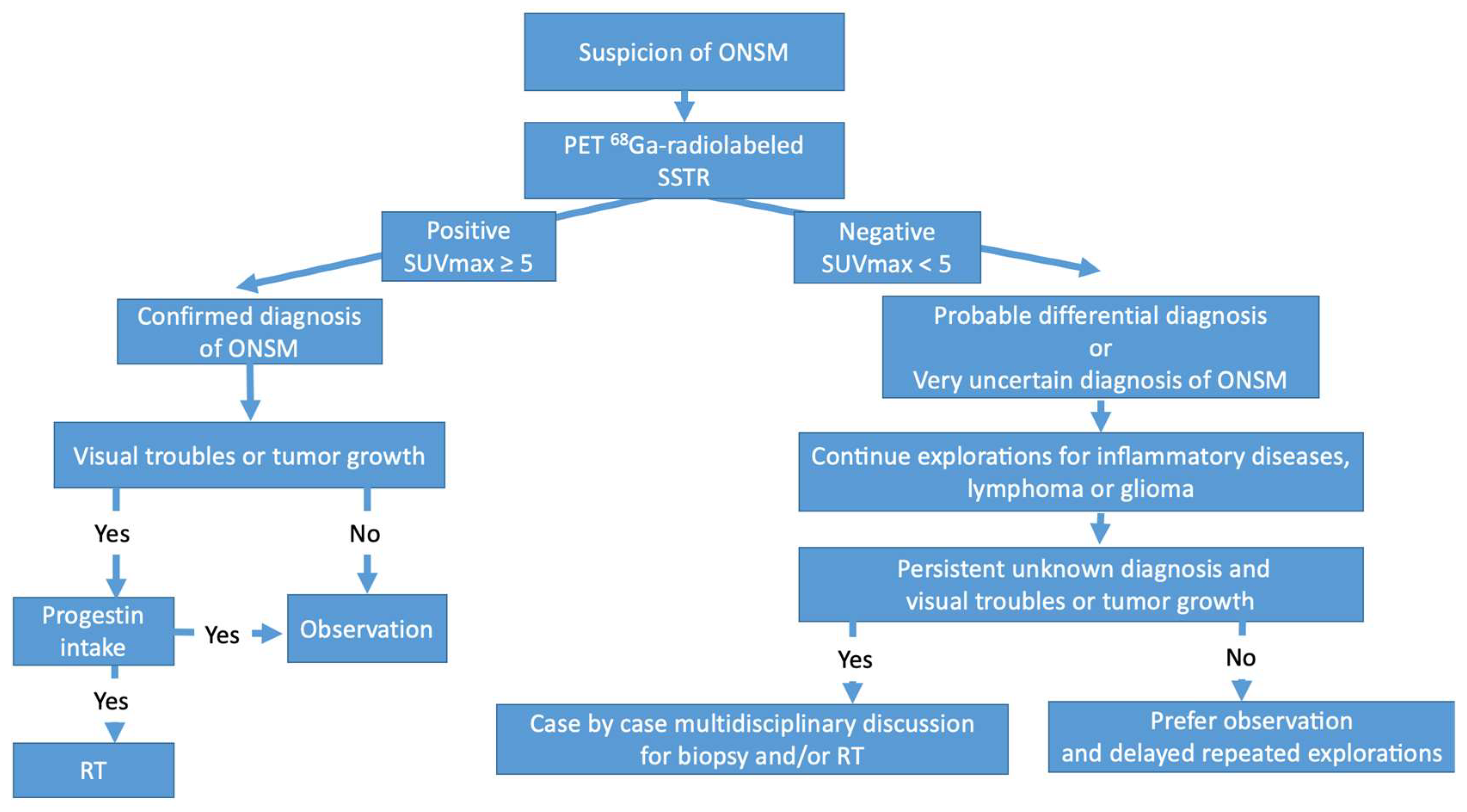
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
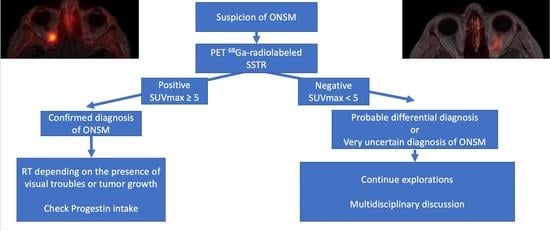
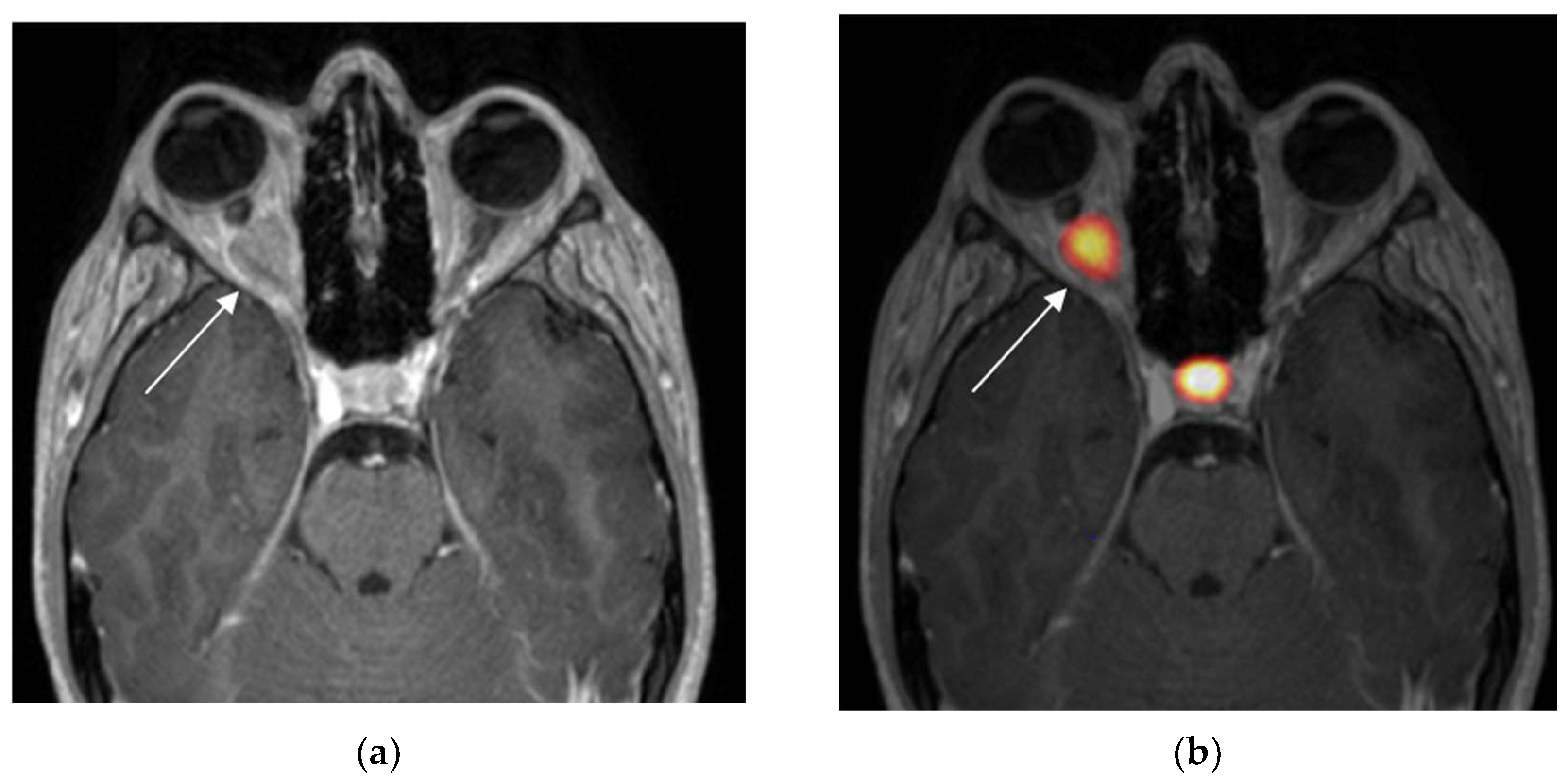
3.1. Positive 68Ga-DOTATOC PET
3.2. Negative 68Ga-DOTATOC PET
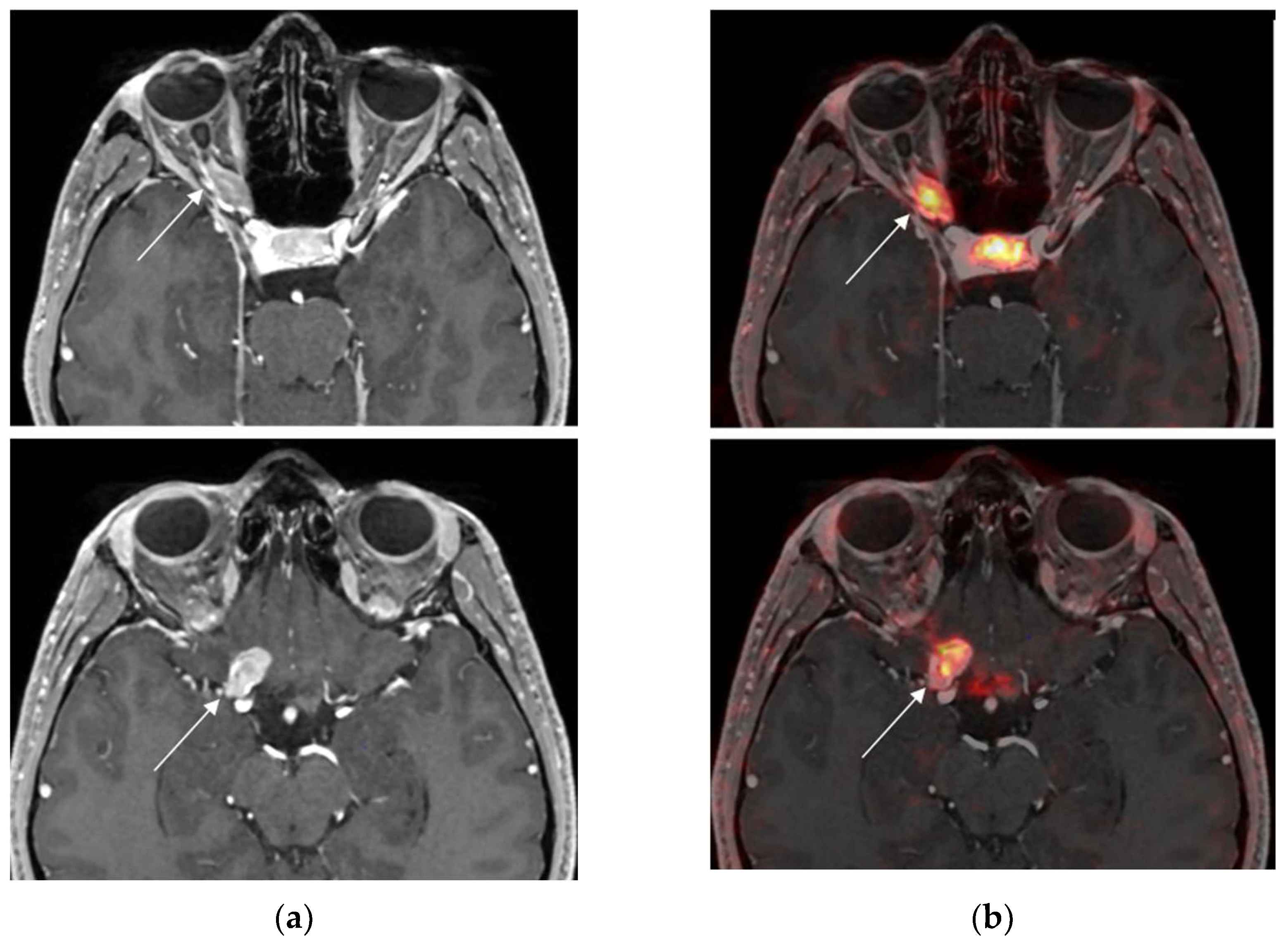
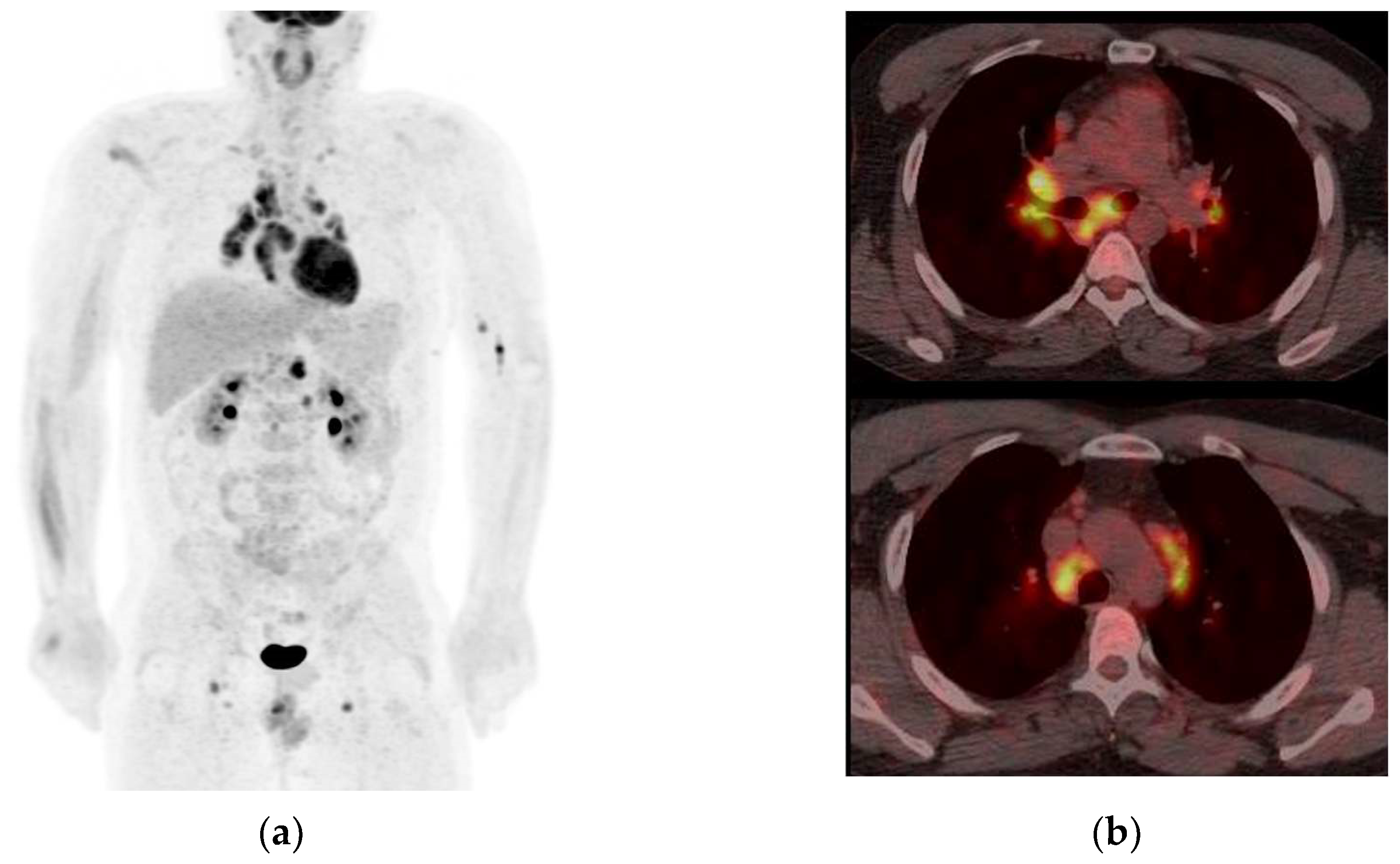
3.3. Added Value of 68Ga-DOTATOC PET before RT in Two Positive Cases
4. Discussion
4.1. Is Positive Radiolabelled SSTR2A-PET True Positive?
4.2. True Negative Radiolabelled SSTR2A-PET
4.3. False Negative 68Ga-DOTATOC PET
4.4. What Is the Optimal Threshold to Differentiate ONSM from Non-Meningioma Optic Nerve Lesions?
4.5. What Is the Place for Radiolabelled SSTR2A-PET in ONSM?
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapey, J.; Sabin, H.I.; Danesh-Meyer, H.V.; Kaye, A.H. Diagnosis and management of optic nerve sheath meningiomas. J. Clin. Neurosci. 2013, 20, 1045–1056. [Google Scholar] [CrossRef]
- Dutton, J.J. Optic nerve sheath meningiomas. Surv. Ophthalmol. 1992, 37, 167–183. [Google Scholar] [CrossRef]
- Patel, B.C.; De Jesus, O.; Margolin, E. Optic Nerve Sheath Meningioma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430868/ (accessed on 5 June 2023).
- Kanamalla, U.S. The Optic Nerve Tram-Track Sign. Radiology 2003, 227, 718–719. [Google Scholar] [CrossRef]
- Galldiks, N.; Albert, N.L.; Sommerauer, M.; Grosu, A.L.; Ganswindt, U.; Law, I.; Preusser, M.; Le Rhun, E.; Vogelbaum, M.A.; Zadeh, G.; et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro Oncol. 2017, 19, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Salgues, B.; Graillon, T.; Guedj, E. [68Ga]Ga-DOTA-TOC PET/CT fused to MRI in post-operative evaluation of olfactory groove meningioma: A case on millimetric remnants. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.; Schuhmacher, J.; Hipp, P.; Kowalski, J.; Becker, D.W.; Doll, J.; Mäcke, H.R.; Hofmann, M.; Debus, J.; Haberkorn, U. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: First results in patients with meningiomas. J. Nucl. Med. 2001, 42, 1053–1056. [Google Scholar] [PubMed]
- Klingenstein, A.; Haug, A.R.; Miller, C.; Hintschich, C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit 2015, 34, 16–22. [Google Scholar] [CrossRef]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Pöschl, J.; Schüller, U.; Schichor, C.; Thon, N.; Tonn, J.-C. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Al Feghali, K.A.; Yeboa, D.N.; Chasen, B.; Gule, M.K.; Johnson, J.M.; Chung, C. The Use of 68Ga-DOTATATE PET/CT in the Non-invasive Diagnosis of Optic Nerve Sheath Meningioma: A Case Report. Front. Oncol. 2018, 8, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolar Bilge, A.; Yazici, B.; Güngör, A.F.; Yazici, Z. An Atypical Paediatric Optic Nerve Sheath Meningioma. Neuroophthalmology 2020, 44, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Graef, J.; Furth, C.; Kluge, A.K.; Acker, G.; Kord, M.; Zimmermann, Z.; Amthauer, H.; Makowski, M.; Loebel, F.; Vajkoczy, P.; et al. 68Ga-DOTATOC-PET/MRI—A Secure One-Stop Shop Imaging Tool for Robotic Radiosurgery Treatment Planning in Patients with Optic Nerve Sheath Meningioma. Cancers 2021, 13, 3305. [Google Scholar] [CrossRef]
- Kiviniemi, A.; Gardberg, M.; Frantzén, J.; Pesola, M.; Vuorinen, V.; Parkkola, R.; Tolvanen, T.; Suilamo, S.; Johansson, J.; Luoto, P.; et al. Somatostatin receptor subtype 2 in high-grade gliomas: PET/CT with (68)Ga-DOTA-peptides, correlation to prognostic markers, and implications for targeted radiotherapy. EJNMMI Res. 2015, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Roytman, M.; Pisapia, D.J.; Liechty, B.; Lin, E.; Skafida, M.; Magge, R.S.; Osborne, J.R.; Pannullo, S.C.; Knisely, J.P.; Ramakrishna, R.; et al. Somatostatin receptor-2 negative meningioma: Pathologic correlation and imaging implications. Clin. Imaging 2020, 66, 18–22. [Google Scholar] [CrossRef]
- Graillon, T.; Romano, D.; Defilles, C.; Saveanu, A.; Mohamed, A.; Figarella-Branger, D.; Roche, P.-H.; Fuentes, S.; Chinot, O.; Dufour, H.; et al. Octreotide therapy in meningiomas: In Vitro study, clinical correlation, and literature review. J. Neurosurg. 2017, 127, 660–669. [Google Scholar] [CrossRef]
- Behling, F.; Fodi, C.; Hoffmann, E.; Renovanz, M.; Skardelly, M.; Tabatabai, G.; Schittenhelm, J.; Honegger, J.; Tatagiba, M. The role of Simpson grading in meningiomas after integration of the updated WHO classification and adjuvant radiotherapy. Neurosurg. Rev. 2021, 44, 2329–2336. [Google Scholar] [CrossRef]
- Behling, F.; Fodi, C.; Skardelly, M.; Renovanz, M.; Castaneda, S.; Tabatabai, G.; Honegger, J.; Tatagiba, M.; Schittenhelm, J. Differences in the expression of SSTR1-5 in meningiomas and its therapeutic potential. Neurosurg. Rev. 2022, 45, 467–478. [Google Scholar] [CrossRef]
- Dijkstra, B.M.; Motekallemi, A.; den Dunnen, W.F.A.; Jeltema, J.R.; van Dam, G.M.; Kruyt, F.A.E.; Groen, R.J.M. SSTR-2 as a potential tumour-specific marker for fluorescence-guided meningioma surgery. Acta Neurochir. (Wien) 2018, 160, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Boulagnon-Rombi, C.; Fleury, C.; Fichel, C.; Lefour, S.; Marchal Bressenot, A.; Gauchotte, G. Immunohistochemical Approach to the Differential Diagnosis of Meningiomas and Their Mimics. J. Neuropathol. Exp. Neurol. 2017, 76, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Menke, J.R.; Raleigh, D.R.; Gown, A.M.; Thomas, S.; Perry, A.; Tihan, T. Somatostatin receptor 2a is a more sensitive diagnostic marker of meningioma than epithelial membrane antigen. Acta Neuropathol. 2015, 130, 441–443. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Silva, C.B.; Ongaratti, B.R.; Trott, G.; Haag, T.; Ferreira, N.P.; Leães, C.G.S.; Pereira-Lima, J.F.S.; da Costa Oliveira, M. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int. J. Clin. Exp. Pathol. 2015, 8, 13185–13192. [Google Scholar]
- Agaimy, A.; Buslei, R.; Coras, R.; Rubin, B.P.; Mentzel, T. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology 2014, 65, 60–70. [Google Scholar] [CrossRef]
- Barresi, V.; Alafaci, C.; Salpietro, F.; Tuccari, G. Sstr2A immunohistochemical expression in human meningiomas: Is there a correlation with the histological grade, proliferation or microvessel density? Oncol. Rep. 2008, 20, 485–492. [Google Scholar] [CrossRef]
- Durand, A.; Champier, J.; Jouvet, A.; Labrousse, F.; Honnorat, J.; Guyotat, J.; Fèvre-Montange, M. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: Relation to grades or histotypes. Clin. Neuropathol. 2008, 27, 334–345. [Google Scholar] [CrossRef]
- Arena, S.; Barbieri, F.; Thellung, S.; Pirani, P.; Corsaro, A.; Villa, V.; Dadati, P.; Dorcaratto, A.; Lapertosa, G.; Ravetti, J.L.; et al. Expression of Somatostatin Receptor mRNA in Human Meningiomas and their Implication in in vitro Antiproliferative Activity. J. Neurooncol. 2004, 66, 155–166. [Google Scholar] [CrossRef]
- Schulz, S.; Pauli, S.U.; Schulz, S.; Händel, M.; Dietzmann, K.; Firsching, R.; Höllt, V. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin. Cancer Res. 2000, 6, 1865–1874. [Google Scholar]
- Dutour, A.; Kumar, U.; Panetta, R.; Ouafik, L.H.; Fina, F.; Sasi, R.; Patel, Y.C. Expression of somatostatin receptor subtypes in human brain tumors. Int. J. Cancer 1998, 76, 620–627. [Google Scholar] [CrossRef]
- Graillon, T.; Boissonneau, S.; Appay, R.; Boucekine, M.; Peyrière, H.; Meyer, M.; Farah, K.; Albarel, F.; Morange, I.; Castinetti, F.; et al. Meningiomas in patients with long-term exposition to progestins: Characteristics and outcome. Neurochirurgie 2021, 67, 556–563. [Google Scholar] [CrossRef]
- Stade, F.; Dittmar, J.O.; Jäkel, O.; Kratochwil, C.; Haberkorn, U.; Debus, J.; Combs, S.E. Influence of 68Ga-DOTATOC on sparing of normal tissue for radiation therapy of skull base meningioma: Differential impact of photon and proton radiotherapy. Radiat. Oncol. 2018, 13, 58. [Google Scholar] [CrossRef] [Green Version]



| Case | Age (Years), Sex | Main Symptoms | 68Ga- DOTATOC PET | SUVmax | Lesion/Pituitary Ratio | Obtained Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 17, F | Progressive vision loss and exophthalmos | Positive | 11.9 | 0.7 | ONSM | Radiotherapy |
| 2 | 38, F | Progressive loss of vision | Positive | 6.9 | 0.8 | ONSM | Radiotherapy |
| 3 | 82, F | Rapid vision loss | Positive | 11.0 | 0.9 | ONSM | Radiotherapy |
| 4 | 41, F | Retro ocular pain and no vision loss | Positive | 19.6 | 1.1 | ONSM | Observation and hormonal treatment withdrawal |
| 5 | 74, F | Blurred vision and rapid vision loss | Positive | 6.3 | 0.4 | ONSM | Radiotherapy proposed |
| 6 | 59, F | Recent vision loss after tomotherapy 6 years ago | Positive | 9.1 | 0.8 | ONSM | Radiotherapy |
| 7 | 35, M | Blurred vision and progressive vision loss | Negative | 3.6 | 0.3 | Sarcoidosis | Corticosteroid therapy and then infliximab |
| 8 | 22, F | Progressive vision loss and history of cranial nerve palsy (III and VI) | Negative | 2.2 | 0.2 | Unknown | Follow-up |
| 9 | 38, F | Visual discomfort and narrowing of the visual field without vision loss | Negative | 2.1 | 0.3 | Unknown | Follow-up |
| 10 | 25, M | No vision loss and fortuitous discovery of papilledema | Negative | 3.3 | 0.1 | Compressive neuropathy of unknown origin | Follow-up |
| 11 | 57, F | Progression vision loss | Negative | 1.2 | 0.1 | Uncertain, possible glioma | Follow-up |
| 12 | 67, M | Brutal retro-ocular pain and diplopia | Negative | 1.5 | 0.1 | Cavernous malformation | Follow-up |
| Study | Age (Years), Sex | Main Symptoms | Radiolabelled SSTR2A-PET | SUVmax | Obtained Diagnosis | Treatment |
|---|---|---|---|---|---|---|
| Current Study Horowitz et al. | 17, F | Progressive vision loss and exophthalmos | Positive | 11.9 | ONSM | Radiotherapy |
| 38, F | Progressive loss of vision | Positive | 6.9 | ONSM | Radiotherapy | |
| 82, F | Rapid vision loss | Positive | 11.0 | ONSM | Radiotherapy | |
| 41, F | Retro-ocular pain and no vision loss | Positive | 19.6 | ONSM | Observation and hormonal treatment withdrawal | |
| 74, F | Blurred vision and rapid vision loss | Positive | 6.3 | ONSM | Radiotherapy proposed | |
| 59, F | Vision loss after tomotherapy 6 years ago | Positive | 9.1 | ONSM | Radiotherapy | |
| Dolar Bilge et al., 2020 [11] | 16, M | Three-year history of proptosis | Positive | Visually high but NA | ONSM | Radiotherapy |
| Al Feghali et al., 2018 [10] | 28, F | Progressive temporal vision loss over a year | Positive | 10.8 | ONSM | Radiotherapy |
| Klingenstein et al. [8] | 29, F | Photopsia | Positive | 6 | ONSM | Radiotherapy |
| 50, F | Lower vision | Positive | 12.6 | Optic nerve meningioma | Radiotherapy and surgery | |
| 57, M | Lower vision | Positive | 17.6 | Optic nerve meningioma | Cyberknife |
| Study | Age (Years), Sex | Main Symptoms | Radiolabelled SSTR2A-PET | SUVmax | Obtained Diagnosis | Treatment |
|---|---|---|---|---|---|---|
| Current study by Horowitz et al. | 35, M | Blurred vision and a progressive vision loss | True negative | 3.6 | Sarcoidosis | Corticosteroid therapy and then infliximab |
| 67, M | Brutal retro-ocular pain and diplopia | True negative | 1.5 | Cavernous malformation | Follow-up | |
| Klingenstein et al., 2015 [8] | 61, M | Lower vision | True negative | 3.2 | Gastric carcinoma metastasis | Cyberknife and chemotherapy |
| 66, F | Lower vision | True negative | 1.7 | Leukaemic infiltration | Chemotherapy | |
| 48, F | Double vision | True negative | 1.4 | Histologically proved inflammatory collagenous connective tissue | Corticosteroid therapy | |
| Graef et al., 2021 [12] | NA | NA | False negative | 1.7 | Histologically confirmed ONSM (4 mm lesion) | Radiotherapy |
| Klingenstein et al., 2015 [8] | 33, F | Lower vision | Non-histologically confirmed false negative | 4.1 | ONSM | Radiotherapy |
| 44, F | Lower vision and headache | Non histologically confirmed false negative | 3 | ONSM | Radiotherapy | |
| Current study by Horowitz et al. | 22, F | Progressive vision loss and history of cranial nerve palsy (III and VI) | Unknown | 2.2 | Unknown | Follow-up |
| 38, F | Visual discomfort and narrowing of the visual field without vision loss | Unknown | 2.1 | Unknown | Follow-up | |
| 25, M | No vision loss and fortuitous discovery of papilledema | Unknown | 3.3 | Compressive neuropathy of unknown origin | Follow-up | |
| 57, F | Progression vision loss | Unknown | 1.2 | Uncertain, possible glioma | Follow-up |
| Study | Behling et al., 2022 [17] | Dijkstra et al., 2018 [18] | Boulagnon-Rombi et al., 2017 [19] | Graillon et al., 2017 [15] | Menke et al., 2015 [20] | Silva et al., 2015 [21] | Agaimy et al., 2014 [22] | Barresi et al., 2008 [23] | Durand et al., 2008 [24] | Arena et al., 2004 [25] | Schulz et al., 2000 [26] | Dutour et al., 1998 [27] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of tumours | 726 | 148 | 127 | 50 | 176 | 60 | 68 | 35 | 22 | 26 | 42 | 40 | 20 | |
| Method | IHC | IHC | IHC | PCR | IHC | IHC | IHC | IHC | IHC | PCR | PCR | IHC | PCR | |
| SSTR2A expression | Negative | 0.6% | 0% | 5% | 0% | 0% | 0% | 13% | 26% | 32% | 0% | 21% | 28% | 0% |
| Low | 8% | 18.2% | NA | <25% | NA | 73% | 13% | 23% | 41% | NA | NA | 23% | NA | |
| Used scale for low expression definition | 1–4 * | Weak/focal staining 1/3 | − | +/+++ ** | − | +/+++ | +/+++ | 1–4 * | +/− vs. + | − | − | +/+++ | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horowitz, T.; Salgues, B.; Padovani, L.; Farah, K.; Dufour, H.; Chinot, O.; Guedj, E.; Graillon, T. Optic Nerve Sheath Meningiomas: Solving Diagnostic Challenges with 68Ga-DOTATOC PET/CT. Diagnostics 2023, 13, 2307. https://doi.org/10.3390/diagnostics13132307
Horowitz T, Salgues B, Padovani L, Farah K, Dufour H, Chinot O, Guedj E, Graillon T. Optic Nerve Sheath Meningiomas: Solving Diagnostic Challenges with 68Ga-DOTATOC PET/CT. Diagnostics. 2023; 13(13):2307. https://doi.org/10.3390/diagnostics13132307
Chicago/Turabian StyleHorowitz, Tatiana, Betty Salgues, Laetitia Padovani, Kaissar Farah, Henry Dufour, Olivier Chinot, Eric Guedj, and Thomas Graillon. 2023. "Optic Nerve Sheath Meningiomas: Solving Diagnostic Challenges with 68Ga-DOTATOC PET/CT" Diagnostics 13, no. 13: 2307. https://doi.org/10.3390/diagnostics13132307
APA StyleHorowitz, T., Salgues, B., Padovani, L., Farah, K., Dufour, H., Chinot, O., Guedj, E., & Graillon, T. (2023). Optic Nerve Sheath Meningiomas: Solving Diagnostic Challenges with 68Ga-DOTATOC PET/CT. Diagnostics, 13(13), 2307. https://doi.org/10.3390/diagnostics13132307