Insights into the Use of Point-of-Care Ultrasound for Diagnosing Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Ultrasound of the Upper Airway
3.1.1. Tongue Parameters (Figure 1 and Figure 2)
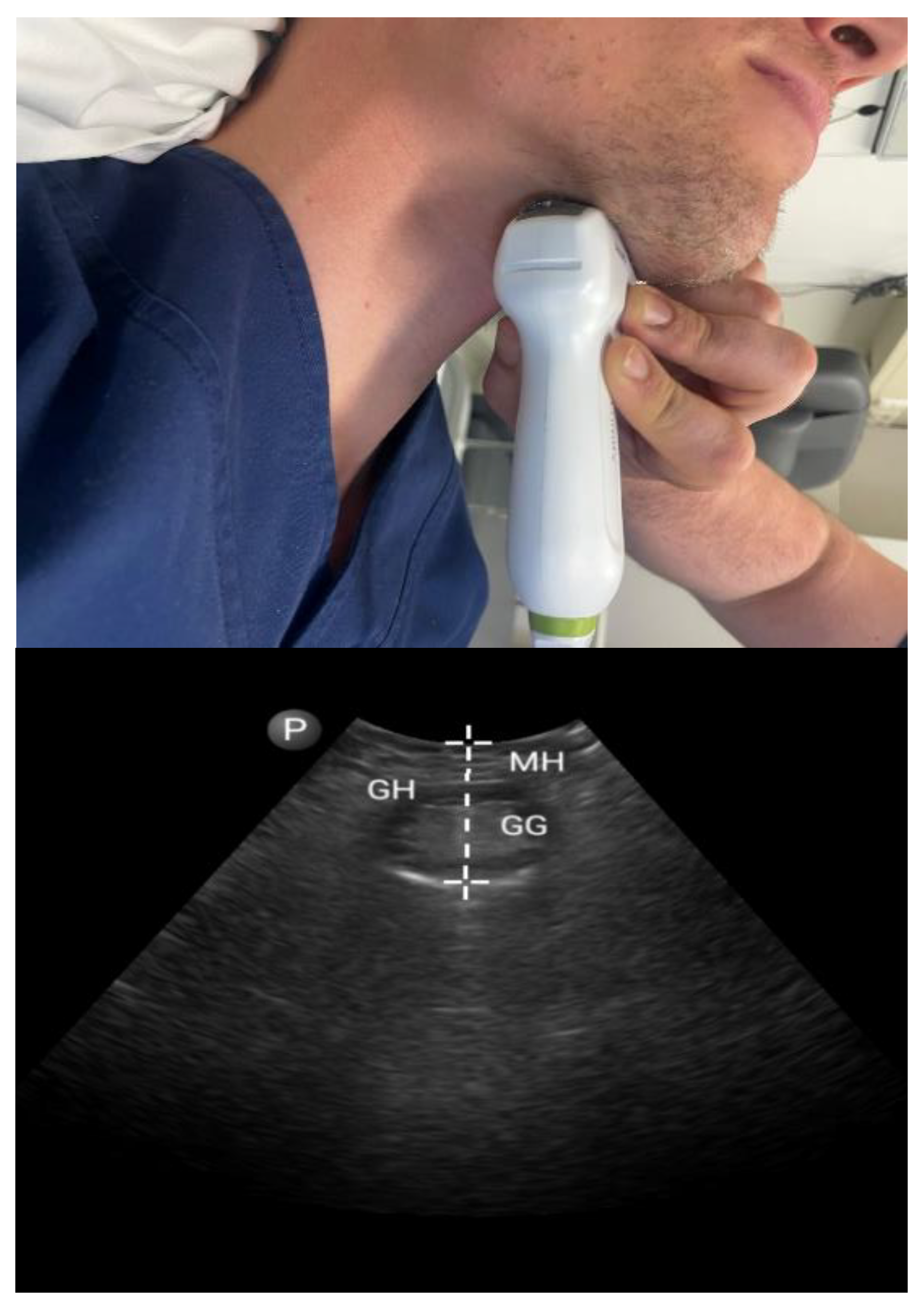
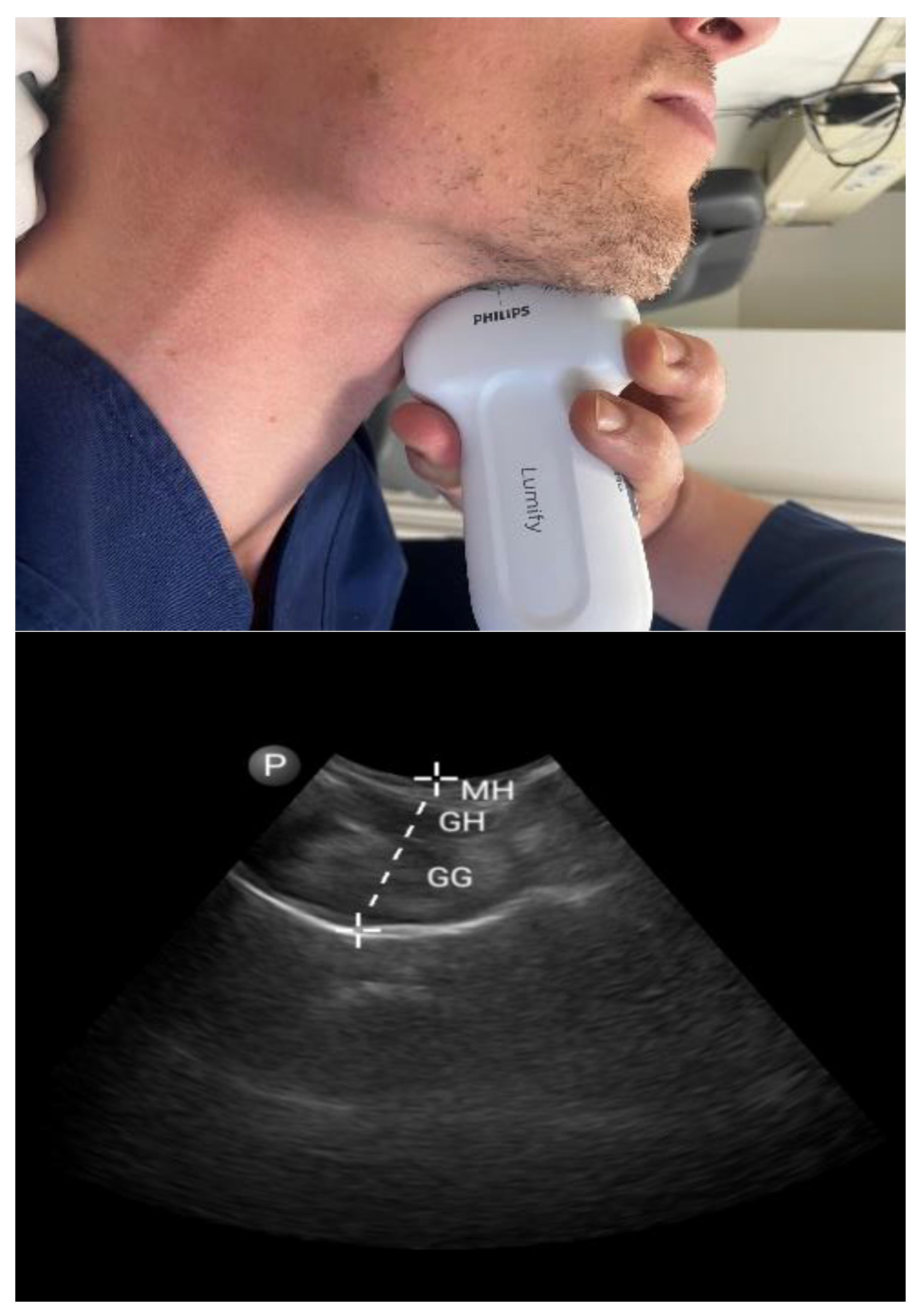
3.1.2. Tonsils
3.1.3. Pharyngeal Parameters (Figure 3)
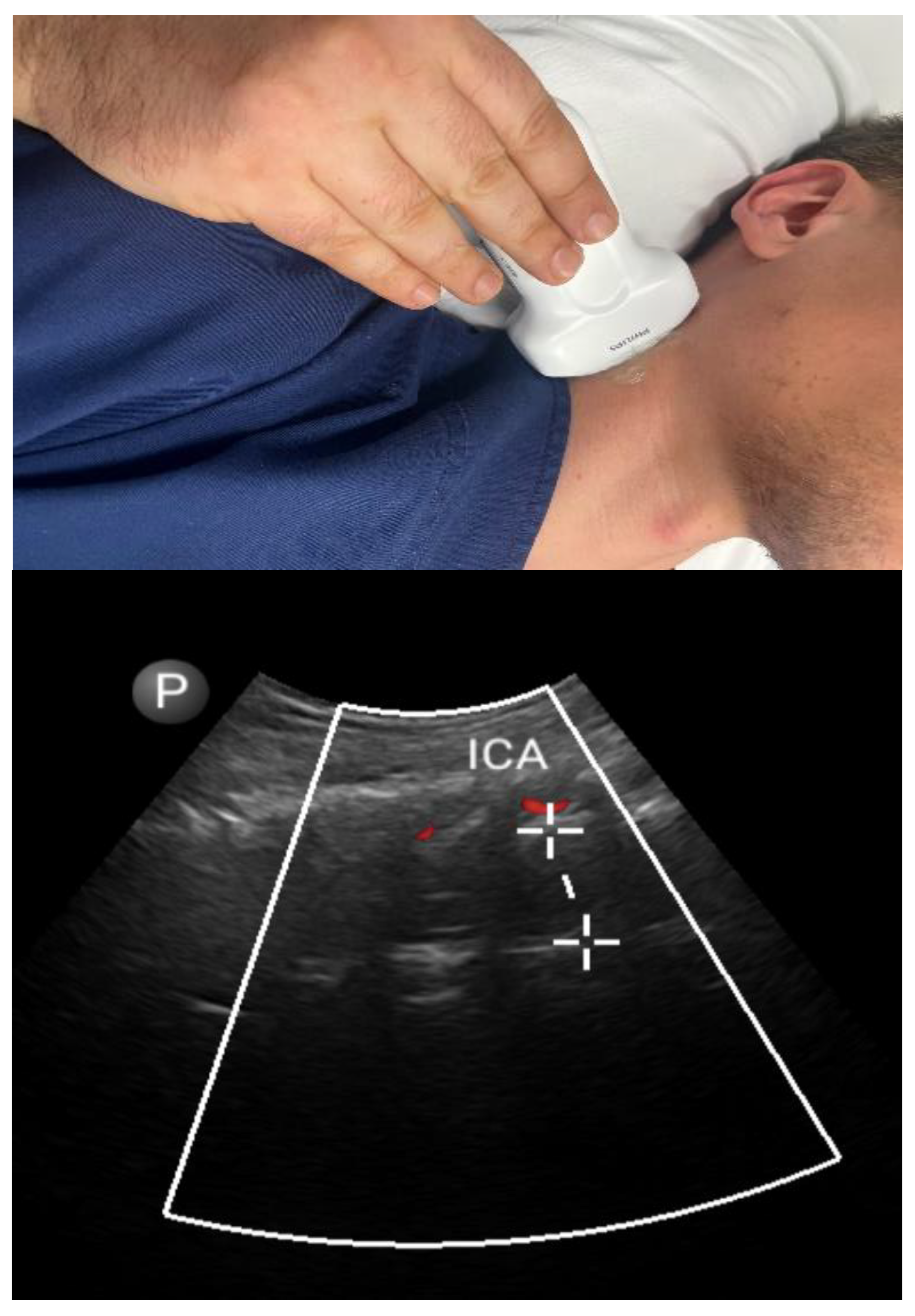
3.2. Non-Airway Parameters
3.2.1. Carotid Intima–Media Thickness
3.2.2. Adipose Tissue
3.2.3. Diaphragmatic Parameters
| Ref | Individuals | State of Consciousness | Ultrasound Parameters | OSA Outcomes | Main Findings |
|---|---|---|---|---|---|
| Airway Parameters | |||||
| [34] | 83 adults | Awake | Tongue echo intensity | AHI | Tongue echo intensity was significantly associated with higher AHI (adjusted rho = 0.27). |
| [35] | 26 adults | Awake and drug-induced sleep | Tongue parameters | AHI | During drug-induced sleep, the tongue muscles become thinner and the space between the two lingual arteries is significantly widened. The latter had a significant positive correlation with AHI (r = 0.51). |
| [36] | 100 adults | Awake | Tongue parameters | OSA (AHI ≥ 5/h) | In the prognosis of OSA, a US sensitivity of 94% and a specificity of 91% were detected. |
| [37] | 40 adults | Awake | Tongue base thickness | OSA (AHI ≥ 5/h) | Tongue base thickness during Müller’s maneuver was an independent predictor of OSA (OR = 2.11, 95% CI: 1.15, 3.87). |
| [38] | 41 male adults | Awake | Tongue parameters | Moderate-to-severe OSA (AHI > 15/h) | Distance between lingual arteries >30 mm had a sensitivity of 80% and a specificity of 67% for diagnosing moderate-to-severe OSA. |
| [39] | 90 adults | Awake | Tongue parameters | AHI | DLA and TBT were positively correlated with AHI. |
| [40] | 171 adults | Awake | Tongue parameters | OSA severity (mild, moderate, and severe) | Lingual height was an independent predictor of OSA severity (OR = 1.14, 95% CI: 1.04, 1.24). |
| [41] | 42 adults | Awake | Tongue movement | - | Agreement between MRI and ultrasound of posterior tongue displacement during inspiration. |
| [42] | 56 adults | Awake | Tongue parameters and tongue movement | OSA (AHI ≥ 5/h) | OSA patients had a larger midsagittal tongue area and restricted movement of the tongue muscles. |
| [43] | 18 adults | Awake | Ultrasound shear-wave elastography | - | Median shear-wave velocity increased during selective hypoglossal stimulation therapy. |
| [44] | 17 adults | Awake | Tongue movement (hyoid bone excursion) | Response after HNS (reduction in AHI > 50% and AHI < 20/h) | HBE > 0.85 cm had a sensitivity of 83.3% and a specificity of 80.0% in predicting response after HNS. |
| [45] | 12 adults | Awake | Tongue parameters and tongue movement | Change in airflow during sleep | Tongue protrusion with preservation of tongue shape predicted increases in patency during selective hypoglossal stimulation therapy. |
| [54] | 50 children and 35 adults | Awake | Tonsil’s size | - | A high correlation was observed between ultrasound measurements and Friedman’s parameters. |
| [57] | 76 adults | Awake | LPW thickness | AHI | LPWT correlated fairly to moderately with OSA severity (r = 0.37) but could lead to overestimation. |
| [58] | 100 adults | Awake | LPW thickness | OSA | A combination of US measurements of LPW and anthropometric parameters had a sensitivity of 93% and a specificity of 94% for the detection of OSA. |
| [59] | 34 children | Awake | LPW thickness | - | Ultrasound-based estimations were similar to MRI. |
| [60] | 43 adults | Awake | LPW thickness and other upper airway parameters | OSA (AHI ≥ 5/h) | OSA patients had increased LPWT (sensitivity of 100%, specificity of 60%) and DLA (sensitivity of 90.9%, specificity of 60%), with a decreased RPD (sensitivity of 54.5%, specificity of 100%). |
| [61] | 105 adults | Awake | Upper airway parameters | Severe OSA (AHI ≥ 30/h) | Change in retropalatal diameter during Müller’s maneuver and neck circumference had a sensitivity of 100% and a specificity of 65% for predicting severe OSA. |
| Non-airway parameters | |||||
| [16] | 87 adults | Awake | Carotid arteries | OSA (AHI ≥ 5/h) | Significant association between OSA and carotid intima–media thickness. |
| [62] | 156 adults | Awake | Carotid arteries | AHI and years from symptom onset | Association between OSA severity (r = 0.51) and duration (r = 0.34) and carotid intima–media thickness. |
| [63] | 30 adults | Awake | Carotid arteries | OSA (AHI ≥ 5/h) | Association between OSA severity and carotid intima–media thickness. |
| [64] | 985 adults | Awake | Carotid arteries | RDI | Weak correlation between respiratory disturbance index and carotid intima–media thickness. |
| [65] | 682 adults | Awake | Branchial artery | AHI and % TST with spO2 < 90% | Baseline brachial artery diameter was significantly associated with both the apnea–hypopnea index and the hypoxemia index. |
| [69] | 149 adults | Awake | Adipose tissue | AHI | Positive correlation of AHI with mesenteric (r = 0.43) and preperitoneal (r = 0.3) fat thickness. |
| [70] | 104 adults | Awake | Adipose tissue | AHI | Statistically significant correlation between AFI and AHI (r = 0.23). |
| [72] | 100 adults | Awake | Subcutaneous adipose tissue | OSA (AHI ≥ 5/h) | Anthropometric data, blood test parameters, and US SAT measures had a sensitivity of 100% and a specificity of 91.7% for predicting OSA. |
| [74] | 108 adults | Awake | Diaphragm | AHI | Diaphragm thickness was higher in OSA patients and positively correlated with disease severity (r = 0.41 for end-expiratory, r = 0.45 for end-inspiratory). |
| [75] | 100 adults | Awake | Diaphragm | OSA (AHI ≥ 5/h) | A combination of diaphragmatic dimensions, diaphragm dilation, age, sex, and BMI predicted the presence of OSA with 91% sensitivity and 81% specificity. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and management of obstructive sleep apnea: A review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Gali, B.; Whalen, F.X.; Schroeder, D.R.; Gay, P.C.; Plevak, D.J. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. J. Am. Soc. Anesthesiol. 2009, 110, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Schoch, O.D.; Rickli, H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc. Health Risk Manag. 2016, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Bartels, K.; Clavijo, C.; Scott, B.K.; Kacmar, R.; Bullard, K.; Moss, A.F.; Henderson, W.; Juarez-Colunga, E.; Jameson, L. Preoperatively screened obstructive sleep apnea is associated with worse postoperative outcomes than previously diagnosed obstructive sleep apnea. Anesth. Analg. 2017, 125, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A. Risk factors for obstructive sleep apnea syndrome in children: State of the art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.; Horner, R.L.; Kimoff, R.J.; Kozar, L.F.; Render-Teixeira, C.L.; Phillipson, E.A. Effect of obstructive sleep apnea versus sleep fragmentation on responses to airway occlusion. Am. J. Respir. Crit. Care Med. 1997, 155, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, W.S.; Tangel, D.J.; White, D.P. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 1996, 153, 1880–1887. [Google Scholar] [CrossRef]
- Flemons, W.W. Obstructive sleep apnea. N. Engl. J. Med. 2002, 347, 498–504. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events: Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Parrino, L.; Ferri, R.; Zucconi, M.; Fanfulla, F. Commentary from the Italian Association of Sleep Medicine on the AASM manual for the scoring of sleep and associated events: For debate and discussion. Sleep Med. 2009, 10, 799–808. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.-M.; Garcia III, A.J.; Anderson, T.M.; Koschnitzky, J.E.; Peng, Y.-J.; Kumar, G.K.; Prabhakar, N.R. Central and peripheral factors contributing to obstructive sleep apneas. Respir. Physiol. Neurobiol. 2013, 189, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.A.; Eckert, D.J.; Jordan, A.S. Obstructive sleep apnoea pathogenesis from mild to severe: Is it all the same? Respirology 2017, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.; Launois, S.; Isono, S.; Feroah, T.; Whitelaw, W.; Remmers, J. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 1993, 148, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Kubin, L. Neural control of the upper airway: Respiratory and state-dependent mechanisms. Compr. Physiol. 2016, 6, 1801. [Google Scholar]
- Apaydin, M.; Ayik, S.O.; Akhan, G.; Peker, S.; Uluc, E. Carotid intima-media thickness increase in patients with habitual simple snoring and obstructive sleep apnea syndrome is associated with metabolic syndrome. J. Clin. Ultrasound 2013, 41, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kaw, R.; Chung, F.; Pasupuleti, V.; Mehta, J.; Gay, P.; Hernández, A.V. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br. J. Anaesth. 2012, 109, 897–906. [Google Scholar] [CrossRef]
- Roure, N.; Gomez, S.; Mediano, O.; Duran, J.; de la Peña, M.; Capote, F.; Teran, J.; Masa, J.F.; Alonso, M.L.; Corral, J. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008, 9, 727–731. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Kirsch, D.B.; Kristo, D.A.; Olson, E.J.; Aurora, R.N.; Carden, K.A.; Chervin, R.D.; Martin, J.L.; Ramar, K.; Rosen, C.L. Polysomnography for obstructive sleep apnea should include arousal-based scoring: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2018, 14, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Khajehdehi, A.; Chung, F. A systematic review of screening questionnaires for obstructive sleep apnea. Can. J. Anesth./J. Can. Danesthésie 2010, 57, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Zhang, K.; Nagappa, M.; Saripella, A.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnoea in patients with cardiovascular risk factors: A systematic review and meta-analysis. BMJ Open Respir. Res. 2021, 8, e000848. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Yuan, H.; Chung, F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth. Analg. 2008, 107, 1543–1563. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.; Hillman, D.R.; Cooper, M.N.; Ward, K.L.; Hunter, M.; Cullen, S.; James, A.; Palmer, L.J.; Mukherjee, S.; Eastwood, P. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013, 17, 967–973. [Google Scholar] [CrossRef] [PubMed]
- You-Ten, K.E.; Siddiqui, N.; Teoh, W.H.; Kristensen, M.S. Point-of-care ultrasound (POCUS) of the upper airway. Can. J. Anesth./J. Can. Danesthésie 2018, 65, 473–484. [Google Scholar] [CrossRef]
- Naji, A.; Chappidi, M.; Ahmed, A.; Monga, A.; Sanders, J. Perioperative point-of-care ultrasound use by anesthesiologists. Cureus 2021, 13, e15217. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.M. The Great Leap Forward: The anatomic basis for the acquisition of speech and obstructive sleep apnea. Sleep Med. 2003, 4, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.G.; Vig, P.S.; Weyant, R.J.; Forrest, T.D.; Rockette, H.E., Jr. Craniofacial structure and obstructive sleep apnea syndrome—A qualitative analysis and meta-analysis of the literature. Am. J. Orthod. Dentofac. Orthop. 1996, 109, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, P.J.; Janczy, J.; Sharma, A.; Newsome, H.A.; Sparapani, R.A.; Rhee, J.S.; Woodson, B.T.; Garcia, G.J. Anatomical determinants of upper airway collapsibility in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 68, 101741. [Google Scholar] [CrossRef]
- Chi, L.; Comyn, F.-L.; Mitra, N.; Reilly, M.P.; Wan, F.; Maislin, G.; Chmiewski, L.; Thorne-FitzGerald, M.D.; Victor, U.N.; Pack, A.I. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur. Respir. J. 2011, 38, 348–358. [Google Scholar] [CrossRef]
- Osman, A.; Sum, K.M. Role of upper airway ultrasound in airway management. J. Intensive Care 2016, 4, 52. [Google Scholar] [CrossRef]
- Lee, E.J.; Cho, J.H. Meta-analysis of obstruction site observed with drug-induced sleep endoscopy in patients with obstructive sleep apnea. Laryngoscope 2019, 129, 1235–1243. [Google Scholar] [CrossRef]
- Friedman, M.; Tanyeri, H.; La Rosa, M.; Landsberg, R.; Vaidyanathan, K.; Pieri, S.; Caldarelli, D. Clinical predictors of obstructive sleep apnea. Laryngoscope 1999, 109, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.-G.; Gislason, T.; Lindholm, C. Computed tomography of the oropharynx in obstructive sleep apnea. Acta Radiol. 1988, 29, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Wiemken, A.; Schultz, S.M.; Keenan, B.T.; Sehgal, C.M.; Schwab, R.J. A comparison of ultrasound echo intensity to magnetic resonance imaging as a metric for tongue fat evaluation. Sleep 2022, 45, zsab295. [Google Scholar] [CrossRef] [PubMed]
- Abuan, M.R.A.; Lin, W.-N.; Hsin, L.-J.; Lee, L.-A.; Fang, T.-J.; Chen, N.-H.; Lo, Y.-L.; Li, H.-Y. Tongue imaging during drug-induced sleep ultrasound in obstructive sleep apnea patients. Auris Nasus Larynx 2020, 47, 828–836. [Google Scholar] [CrossRef]
- Molnár, V.; Lakner, Z.; Molnár, A.; Tárnoki, D.L.; Tárnoki, Á.D.; Kunos, L.; Jokkel, Z.; Tamás, L. Ultrasound and Magnetic Resonance Imaging of the Tongue in Obstructive Sleep Apnoea. Appl. Sci. 2022, 12, 9583. [Google Scholar] [CrossRef]
- Chen, J.-W.; Chang, C.-H.; Wang, S.-J.; Chang, Y.-T.; Huang, C.-C. Submental ultrasound measurement of dynamic tongue base thickness in patients with obstructive sleep apnea. Ultrasound Med. Biol. 2014, 40, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Lahav, Y.; Rosenzweig, E.; Heyman, Z.; Doljansky, J.; Green, A.; Dagan, Y. Tongue base ultrasound: A diagnostic tool for predicting obstructive sleep apnea. Ann. Otol. Rhinol. Laryngol. 2009, 118, 179–184. [Google Scholar] [CrossRef]
- Hussein, A.; Sedeek, R.; Higazi, M.; Abdelaziz, A. Neck Ultrasound Can Be Helpful in Diagnosing and Assessing Severity of OSA. In B66. An All-Inclusive Srn Experience: Diagnosis, Consequences, and Management of Sleep Disorders; American Thoracic Society: New York, NY, USA, 2022; p. A3337. [Google Scholar]
- Lun, H.M.; Liu, R.C.; Hu, Q.; Liu, Y.L.; Wei, L.S.; Wu, D.; Wang, F.; Zhu, S.Y. Potential ultrasonic anatomical markers of obstructive sleep apnoea-hypopnoea syndrome. Clin. Radiol. 2022, 78, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Kwan, B.C.; Jugé, L.; Gandevia, S.C.; Bilston, L.E. Sagittal measurement of tongue movement during respiration: Comparison between ultrasonography and magnetic resonance imaging. Ultrasound Med. Biol. 2019, 45, 921–934. [Google Scholar] [CrossRef]
- Manlises, C.O.; Chen, J.W.; Huang, C.C. Dynamic tongue area measurements in ultrasound images for adults with obstructive sleep apnea. J. Sleep Res. 2020, 29, e13032. [Google Scholar] [CrossRef] [PubMed]
- Arens, P.; Fischer, T.; Dommerich, S.; Olze, H.; Lerchbaumer, M.H. Ultrasound Shear Wave Elastography of the Tongue during Selective Hypoglossal Nerve Stimulation in Patients with Obstructive Sleep Apnea Syndrome. Ultrasound Med. Biol. 2021, 47, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Korotun, M.; Quintero, L.; Shikowitz, M.; Mayo, P.; Greenberg, H. Optimization of Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea with Ultrasound Assessment of Tongue Movement. Am. J. Ther. 2022, 29, e205–e211. [Google Scholar] [CrossRef] [PubMed]
- Fleury Curado, T.; Pham, L.; Otvos, T.; Klopfer, T.; Freire, C.; Amorim, M.R.; Nishimura, Y.; Sennes, L.U.; Psoter, K.J.; Abdelwahab, M.; et al. Changes in tongue morphology predict responses in pharyngeal patency to selective hypoglossal nerve stimulation. J. Clin. Sleep Med. 2023, 19, 947–955. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical Practice Guideline: Tonsillectomy in Children (Update)-Executive Summary. Otolaryngol. Head Neck Surg. 2019, 160, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Jara, S.M.; Weaver, E.M. Association of palatine tonsil size and obstructive sleep apnea in adults. Laryngoscope 2018, 128, 1002–1006. [Google Scholar] [CrossRef]
- Bozzini, M.F.; Di Francesco, R.C.; Soster, L.A. Clinical and anatomical characteristics associated with obstructive sleep apnea severity in children. Clinics 2022, 77, 100131. [Google Scholar] [CrossRef]
- Hsieh, H.S.; Kang, C.J.; Chuang, H.H.; Zhuo, M.Y.; Lee, G.S.; Huang, Y.S.; Chuang, L.P.; Kuo, T.B.; Yang, C.C.; Lee, L.A.; et al. Screening Severe Obstructive Sleep Apnea in Children with Snoring. Diagnostics 2021, 11, 1168. [Google Scholar] [CrossRef]
- Matarredona-Quiles, S.; Carrasco-Llatas, M.; Apodaca, P.M.; Ortega-Beltrá, N.; Dalmau-Galofre, J. Is there a relationship between tonsil volume and the success of pharyngeal surgery among adult patients with obstructive sleep apnea? Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S5), S156–S161. [Google Scholar] [CrossRef]
- Cahali, M.B.; Soares, C.F.; Dantas, D.A.; Formigoni, G.G. Tonsil volume, tonsil grade and obstructive sleep apnea: Is there any meaningful correlation? Clinics 2011, 66, 1347–1352. [Google Scholar] [CrossRef]
- Mehwish, A.; Usmani, A. Role of Ultrasound in Estimation of Palatine Tonsil Volume in Obstructive Sleep Apnea Patients. J. Bahria Univ. Med. Dent. Coll. 2020, 10, 228–233. [Google Scholar] [CrossRef]
- Mengi, E.; Sağtaş, E.; Kara, C.O. Assessment of tonsil volume with transcervical ultrasonography in both children and adults. J. Ultrasound Med. 2020, 39, 529–534. [Google Scholar] [CrossRef]
- Burns, D.W.; Chan, V.W.S.; Trivedi, A.; Englesakis, M.; Munshey, F.; Singh, M. Ready to scan? A systematic review of point of care ultrasound (PoCUS) for screening of obstructive sleep apnea (OSA) in the pediatric population. J. Clin. Anesth. 2022, 83, 110973. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Gupta, K.B.; Gefter, W.B.; Metzger, L.J.; Hoffman, E.A.; Pack, A.I. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am. J. Respir. Crit. Care Med. 1995, 152, 1673–1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-H.; Chu, W.C.; To, K.-W.; Ko, F.W.; Tong, M.W.; Chan, J.W.; Hui, D.S. Sonographic measurement of lateral parapharyngeal wall thickness in patients with obstructive sleep apnea. Sleep 2007, 30, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Molnár, V.; Molnár, A.; Lakner, Z.; Tárnoki, D.L.; Tárnoki, Á.D.; Jokkel, Z.; Kunos, L.; Tamás, L. The prognostic role of ultrasound and magnetic resonance imaging in obstructive sleep apnoea based on lateral oropharyngeal wall obstruction. Sleep Breath. 2023, 27, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Yuen, H.M.; Lai, A.C.; Liu, E.K.; Lee, M.C.; Chu, W.C.; Chan, J.W.; Chan, N.Y.; Wing, Y.K.; Li, A.M.; Chan, K.C. Validation of the Sonographic Measurement of Lateral Parapharyngeal Wall Thickness in Childhood Obstructive Sleep Apnea. Nat. Sci. Sleep 2022, 14, 2013–2021. [Google Scholar] [CrossRef]
- Hussein, S.A.; Kamel, K.M.; Kaddah, S.Z.; El-Hamid, E.E.A.; Shaban, M.M. Role of ultrasonography in assessment of anatomic upper airway changes in patients with obstructive sleep apnea. Adv. Respir. Med. 2020, 88, 548–557. [Google Scholar] [CrossRef]
- Shu, C.-C.; Lee, P.; Lin, J.-W.; Huang, C.-T.; Chang, Y.-C.; Yu, C.-J.; Wang, H.-C. The use of sub-mental ultrasonography for identifying patients with severe obstructive sleep apnea. PLoS ONE 2013, 8, e62848. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Scicchitano, P.; Mitacchione, G.; Zito, A.; Gesualdo, M.; Caputo, P.; Damiani, M.F.; Sassara, M.; Favale, S.; Resta, O. Is there a correlation between OSAS duration/severity and carotid intima-media thickness? Respir. Med. 2012, 106, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Altın, R.; Özdemir, H.; Mahmutyazıcıoğlu, K.; Kart, L.; Uzun, L.; Özer, T.; Savranlar, A.; Aydın, M. Evaluation of carotid artery wall thickness with high-resolution sonography in obstructive sleep apnea syndrome. J. Clin. Ultrasound 2005, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wattanakit, K.; Boland, L.; Punjabi, N.M.; Shahar, E. Relation of sleep-disordered breathing to carotid plaque and intima-media thickness. Atherosclerosis 2008, 197, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chami, H.A.; Keyes, M.J.; Vita, J.A.; Mitchell, G.F.; Larson, M.G.; Fan, S.; Vasan, R.S.; O’Connor, G.T.; Benjamin, E.J.; Gottlieb, D.J. Brachial artery diameter, blood flow and flow-mediated dilation in sleep-disordered breathing. Vasc. Med. 2009, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Pillar, G.; Shehadeh, N. Abdominal fat and sleep apnea: The chicken or the egg? Diabetes Care 2008, 31 (Suppl. S2), S303–S309. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.A.; Veasey, S.C. Daytime sleepiness in obesity: Mechanisms beyond obstructive sleep apnea—A review. Sleep 2012, 35, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Chan, Y.L.; Chan, W.B.; Chan, J.C.; Chu, C.W. Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diabetes Care 2006, 29, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Chu, W.C.; To, K.W.; Ko, F.W.; Ng, S.S.; Ngai, J.C.; Chan, J.W.; Ahuja, A.T.; Hui, D.S. Mesenteric fat thickness is associated with increased risk of obstructive sleep apnoea. Respirology 2014, 19, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Çetin, N.; Tatar, İ.G.; Yüceege, M.; Ergun, O.; Hekimoğlu, B. Ultrasonographic evaluation of abdominal wall fat index, carotid intima-media thickness and plaque score in obstructive sleep apnea syndrome. Med. Ultrason. 2019, 21, 422–426. [Google Scholar] [CrossRef]
- Tokunaga, K.; Matsuzawa, Y.; Ishikawa, K.; Tarui, S. A novel technique for the determination of body fat by computed tomography. Int. J. Obes. 1983, 7, 437–445. [Google Scholar]
- Molnár, V.; Lakner, Z.; Molnár, A.; Tárnoki, D.L.; Tárnoki, Á.D.; Kunos, L.; Tamás, L. The Predictive Role of Subcutaneous Adipose Tissue in the Pathogenesis of Obstructive Sleep Apnoea. Life 2022, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Pauleit, D.; Sudhop, T.; Gouni-Berthold, I.; Ewig, S.; Berthold, H.K. Body fat distribution, serum leptin, and cardio-vascular risk factors in men with obstructive sleep apnea. Chest 2002, 122, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Pazarlı, A.C.; Özmen, Z.; İnönü Köseoğlu, H.; Ekiz, T. Ultrasonographic measurement of the diaphragm thickness in patients with obstructive sleep apnea syndrome. Sleep Breath. 2020, 24, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Molnár, V.; Molnár, A.; Lakner, Z.; Tárnoki, D.L.; Tárnoki, Á.D.; Jokkel, Z.; Szabó, H.; Dienes, A.; Angyal, E.; Németh, F. Examination of the diaphragm in obstructive sleep apnea using ultrasound imaging. Sleep Breath. 2022, 26, 1333–1339. [Google Scholar] [CrossRef]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Peppard, P.E.; Nieto, F.J.; Hla, K.M. Burden of sleep apnea: Rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ 2009, 108, 246–249. [Google Scholar] [PubMed]
- US Preventive Services Task Force. Screening for Obstructive Sleep Apnea in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 407–414. [Google Scholar] [CrossRef]
- Schiza, S.E.; Bouloukaki, I. Screening for obstructive sleep apnoea in professional drivers. Breathe 2020, 16, 29364. [Google Scholar] [CrossRef]
- Wilcox, C.; Yang, J.; Filler, R.; Chen, P.H.; Hyun, Y.J.; Rielly, A.; Kales, S.N. Sleep Apnea Screening for Commercial Drivers: A Retrospective Comparison of the 2016 FMCSA MRB Recommendations and the 2006 Joint Task Force Consensus Guidelines. J. Occup. Environ. Med. 2020, 62, e442–e448. [Google Scholar] [CrossRef]
- Cheng, M.C.F.; Steier, J. Pre-operative screening for sleep disordered breathing: Obstructive sleep apnoea and beyond. Breathe 2022, 18, 220072. [Google Scholar] [CrossRef]
- Hashim, A.; Tahir, M.J.; Ullah, I.; Asghar, M.S.; Siddiqi, H.; Yousaf, Z. The utility of point of care ultrasonography (POCUS). Ann. Med. Surg. 2021, 71, 102982. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tuteja, A.; Wong, D.T.; Goel, A.; Trivedi, A.; Tomlinson, G.; Chan, V. Point-of-Care Ultrasound for Obstructive Sleep Apnea Screening: Are We There Yet? A Systematic Review and Meta-analysis. Anesth. Analg. 2019, 129, 1673–1691. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalkanis, A.; Testelmans, D.; Papadopoulos, D.; Van den Driessche, A.; Buyse, B. Insights into the Use of Point-of-Care Ultrasound for Diagnosing Obstructive Sleep Apnea. Diagnostics 2023, 13, 2262. https://doi.org/10.3390/diagnostics13132262
Kalkanis A, Testelmans D, Papadopoulos D, Van den Driessche A, Buyse B. Insights into the Use of Point-of-Care Ultrasound for Diagnosing Obstructive Sleep Apnea. Diagnostics. 2023; 13(13):2262. https://doi.org/10.3390/diagnostics13132262
Chicago/Turabian StyleKalkanis, Alexandros, Dries Testelmans, Dimitrios Papadopoulos, Annelies Van den Driessche, and Bertien Buyse. 2023. "Insights into the Use of Point-of-Care Ultrasound for Diagnosing Obstructive Sleep Apnea" Diagnostics 13, no. 13: 2262. https://doi.org/10.3390/diagnostics13132262
APA StyleKalkanis, A., Testelmans, D., Papadopoulos, D., Van den Driessche, A., & Buyse, B. (2023). Insights into the Use of Point-of-Care Ultrasound for Diagnosing Obstructive Sleep Apnea. Diagnostics, 13(13), 2262. https://doi.org/10.3390/diagnostics13132262






