Pretreatment CT Texture Parameters as Predictive Biomarkers of Progression-Free Survival in Follicular Lymphoma Treated with Immunochemotherapy and Rituximab Maintenance
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Follow-Up and Endpoints
2.3. 18F-FDG PET/CT Acquisition and Analysis
2.4. CT Texture Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
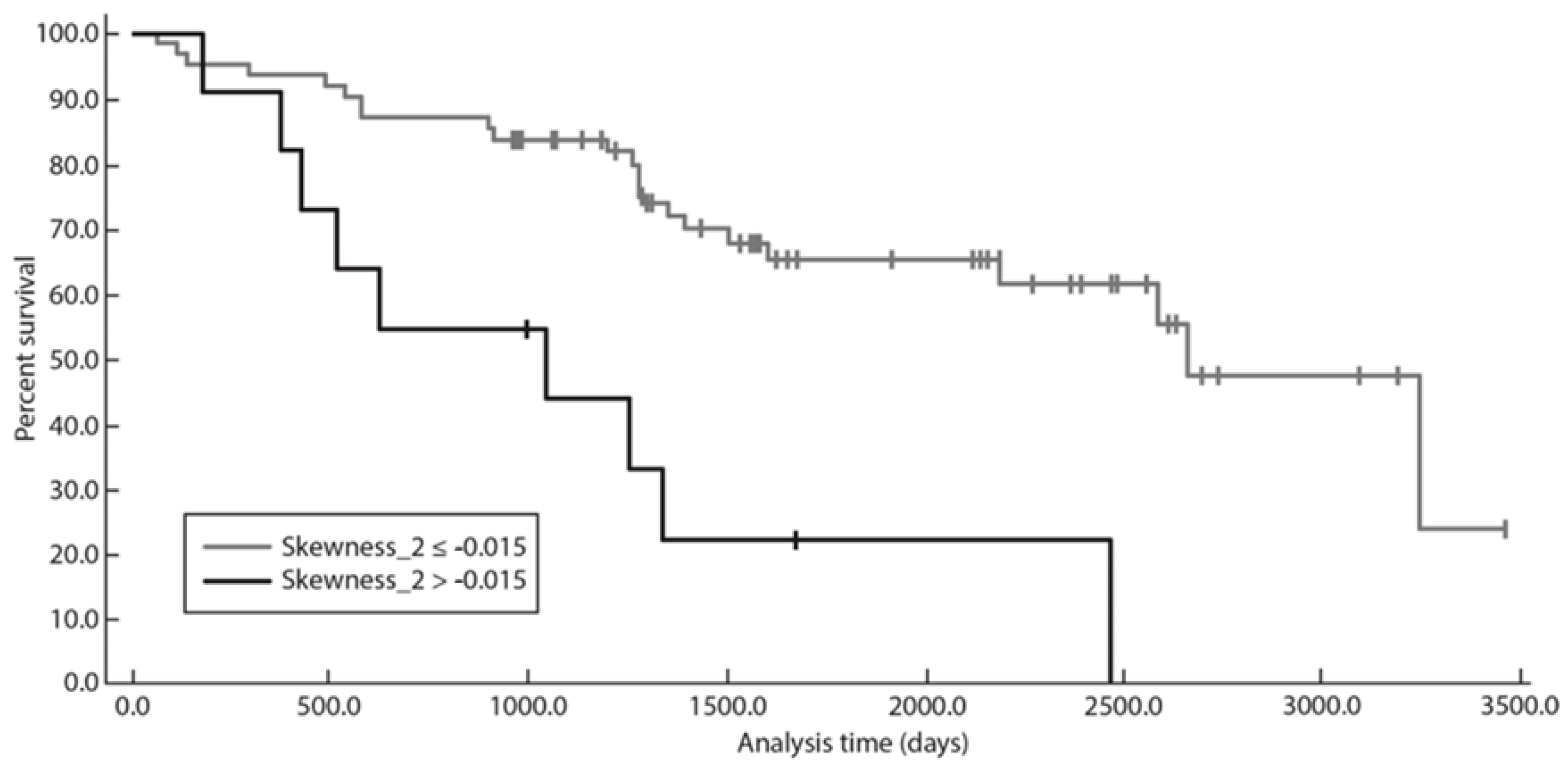
3.2. Progression-Free Survival Analysis
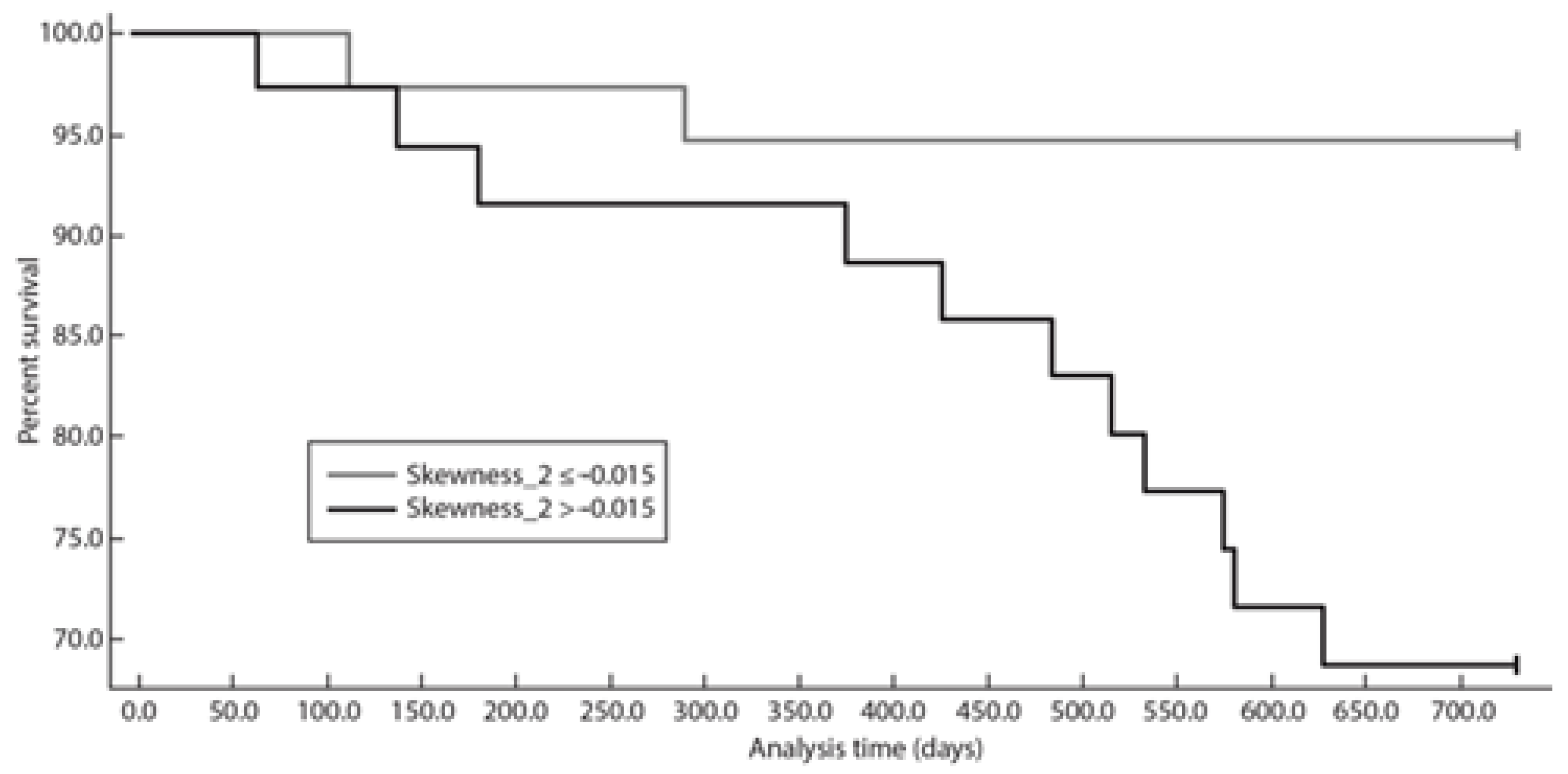
3.3. Progression-Free Survival at 24 Months Analysis
3.4. Time to Next Treatment Analysis
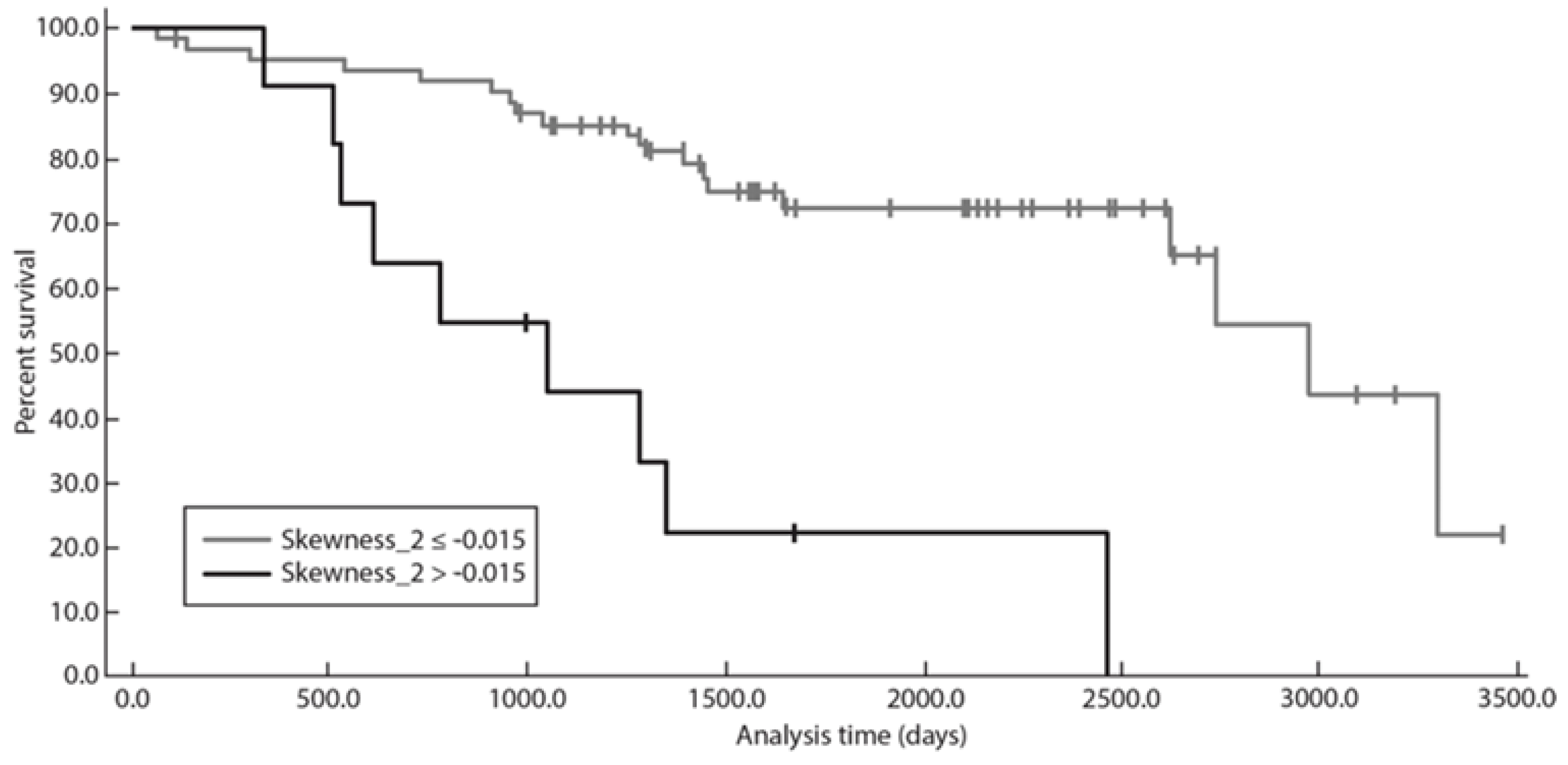
3.5. Overall Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teras, L.R.; DeSantis, C.E.; Cerhan, J.R.; Morton, L.M.; Jemal, A.; Flowers, C.R. US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016, 66, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, B.E.; Yri, O.E.; Kimby, E.; Holte, H.; Delabie, J.; Smeland, E.B.; Sundström, C.; Christensson, B.; Sander, B. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br. J. Haematol. 2012, 156, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar]
- Salles, G.; Mounier, N.; de Guibert, S.; Morschhauser, F.; Doyen, C.; Rossi, J.F.; Haioun, C.; Brice, P.; Mahé, B.; Bouabdallah, R.; et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA-GOELAMS FL2000 study. Blood 2008, 112, 4824–4831. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Ghesquières, H. Current and future management of follicular lymphoma. Int. J. Hematol. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.S.; Yang, D.T. Follicular lymphoma: Evolving therapeutic strategies. Blood 2016, 127, 2055–2063. [Google Scholar] [CrossRef]
- Salles, G.; Seymour, J.F.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011, 377, 42–51. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Tan, D.; Horning, S.J.; Hoppe, R.T.; Levy, R.; Rosenberg, S.A.; Sigal, B.M.; Warnke, R.A.; Natkunam, Y.; Han, S.S.; Yuen, A.; et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood 2013, 122, 981–987. [Google Scholar] [CrossRef]
- Sorigue, M.; Sancho, J.-M. Current prognostic and predictive factors in follicular lymphoma. Ann. Hematol. 2018, 97, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Seymour, J.F.; Feugier, P.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Catalano, J.V.; Brice, P.; Lemonnier, F.; et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients with Follicular Lymphoma: Long-Term Results of the PRIMA Study. J. Clin. Oncol. 2019, 37, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early Relapse of Follicular Lymphoma after Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis from the National LymphoCare Study. J. Clin. Oncol. 2015, 34, 1430. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.J.; Bachy, E.; Ghesquières, H.; Ansell, S.M.; Nowakowski, G.S.; Thompson, C.A.; Inwards, D.J.; Allmer, C.; Chassagne-Clément, C.; Nicolas-Virelizier, E.; et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am. J. Hematol. 2016, 91, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Solal-Céligny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D.; et al. Follicular lymphoma international prognostic index. Blood 2004, 104, 1258–1265. [Google Scholar] [CrossRef]
- Federico, M.; Bellei, M.; Marcheselli, L.; Luminari, S.; Lopez-Guillermo, A.; Vitolo, U.; Pro, B.; Pileri, S.; Pulsoni, A.; Soubeyran, P.; et al. Follicular lymphoma international prognostic index 2: A new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J. Clin. Oncol. 2009, 27, 4555–4562. [Google Scholar] [CrossRef]
- Meignan, M.; Cottereau, A.S.; Versari, A.; Chartier, L.; Dupuis, J.; Boussetta, S.; Grassi, I.; Casasnovas, R.O.; Haioun, C.; Tilly, H.; et al. Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J. Clin. Oncol. 2016, 34, 3618–3626. [Google Scholar] [CrossRef]
- Schöder, H.; Moskowitz, C. Metabolic Tumor Volume in Lymphoma: Hype or Hope? J. Clin. Oncol. 2016, 34, 3591–3594. [Google Scholar] [CrossRef]
- Adams, H.J.A.; Kwee, T.C. Overestimated Value of Baseline Total Metabolic Tumor Volume at 18F-Labeled Fluorodeoxyglucose Positron Emission Tomography in Follicular Lymphoma. J. Clin. Oncol. 2017, 35, 918–919. [Google Scholar] [CrossRef]
- Rai, A.; Nastoupil, L.J.; Williams, J.N.; Lipscomb, J.; Ward, K.C.; Howard, D.H.; Lee, D.; Flowers, C.R. Patterns of use and survival outcomes of positron emission tomography for initial staging in elderly follicular lymphoma patients. Leuk. Lymphoma 2017, 58, 1570–1580. [Google Scholar] [CrossRef]
- Ganeshan, B.; Miles, K.A. Quantifying tumour heterogeneity with CT. Cancer Imaging 2013, 13, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Boeken, T.; Feydy, J.; Lecler, A.; Soyer, P.; Feydy, A.; Barat, M.; Duron, L. Artificial intelligence in diagnostic and interventional radiology: Where are we now? Diagn. Interv. Imaging 2023, 104, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhou, Q.; Xi, H.; Zhou, J. Radiomics: A review of current applications and possibilities in the assessment of tumor microenvironment. Diagn. Interv. Imaging 2023, 104, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Durot, C.; Barat, M.; Djelouah, M.; Grange, F.; Mulé, S.; Soyer, P.; Hoeffel, C. CT texture analysis as a predictor of favorable response to anti-PD1 monoclonal antibodies in metastatic skin melanoma. Diagn. Interv. Imaging 2022, 103, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Durot, C.; Mulé, S.; Soyer, P.; Marchal, A.; Grange, F.; Hoeffel, C. Metastatic melanoma: Pretreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with pembrolizumab. Eur. Radiol. 2019, 29, 3183–3191. [Google Scholar] [CrossRef]
- Yip, C.; Landau, D.; Kozarski, R.; Ganeshan, B.; Thomas, R.; Michaelidou, A.; Goh, V. Primary esophageal cancer: Heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 2014, 270, 141–148. [Google Scholar] [CrossRef]
- Zhang, H.; Graham, C.M.; Elci, O.; Griswold, M.E.; Zhang, X.; Khan, M.A.; Pitman, K.; Caudell, J.J.; Hamilton, R.D.; Ganeshan, B.; et al. Locally advanced squamous cell carcinoma of the head and neck: CT texture and histogram analysis allow independent prediction of overall survival in patients treated with induction chemotherapy. Radiology 2013, 269, 801–809. [Google Scholar] [CrossRef]
- Ganeshan, B.; Panayiotou, E.; Burnand, K.; Dizdarevic, S.; Miles, K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: A potential marker of survival. Eur. Radiol. 2012, 22, 796–802. [Google Scholar] [CrossRef]
- Miles, K.A. How to use CT texture analysis for prognostication of non-small cell lung cancer. Cancer Imaging 2016, 16, 10. [Google Scholar] [CrossRef]
- Soliman, R.K.; Essa, A.A.; Elhakeem, A.A.S.; Gamal, S.A.; Zaitoun, M.M.A. Texture analysis of apparent diffusion coefficient (ADC) map for glioma grading: Analysis of whole tumoral and peri-tumoral tissue. Diagn. Interv. Imaging 2021, 102, 287–295. [Google Scholar] [CrossRef]
- Mulé, S.; Thiefin, G.; Costentin, C.; Durot, C.; Rahmouni, A.; Luciani, A.; Hoeffel, C. Advanced Hepatocellular Carcinoma: Pretreatment Contrast-enhanced CT Texture Parameters as Predictive Biomarkers of Survival in Patients Treated with Sorafenib. Radiology 2018, 288, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Brenet Defour, L.; Mulé, S.; Tenenhaus, A.; Piardi, T.; Sommacale, D.; Hoeffel, C.; Thiéfin, G. Hepatocellular carcinoma: CT texture analysis as a predictor of survival after surgical resection. Eur. Radiol. 2019, 29, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, B.; Miles, K.A.; Babikir, S.; Shortman, R.; Afaq, A.; Ardeshna, K.M.; Groves, A.M.; Kayani, I. CT-based texture analysis potentially provides prognostic information complementary to interim FDG-PET for patients with hodgkin’s and aggressive non-hodgkin’s lymphomas. Eur. Radiol. 2017, 27, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Barat, M.; Jannot, A.S.; Dohan, A.; Soyer, P. How to report and compare quantitative variables in a radiology article. Diagn. Interv. Imaging 2022, 103, 571–573. [Google Scholar] [CrossRef]
- Yun, Z.; Lin, Q. Hypoxia and regulation of cancer cell stemness. Adv. Exp. Med. Biol. 2014, 772, 41–53. [Google Scholar]
- Miles, K.A.; Ganeshan, B.; Hayball, M.P. CT texture analysis using the filtration-histogram method: What do the measurements mean? Cancer Imaging 2013, 13, 400–406. [Google Scholar] [CrossRef]
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503. [Google Scholar] [CrossRef]
- Ng, F.; Ganeshan, B.; Kozarski, R.; Miles, K.A.; Goh, V. Assessment of Primary Colorectal Cancer Heterogeneity by Using Whole-Tumor Texture Analysis: Contrast-enhanced CT Texture as a Biomarker of 5-year Survival. Radiology 2013, 266, 177–184. [Google Scholar] [CrossRef]
- Pellat, A.; Barat, M.; Coriat, R.; Soyer, P.; Dohan, A. Artificial intelligence: A review of current applications in hepatocellular carcinoma imaging. Diagn. Interv. Imaging 2023, 104, 24–36. [Google Scholar] [CrossRef]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.; Wood, P.; Hawkins, T.; MacDonald, D.; Simpson, D.; Kolibaba, K.; Issa, S.; Chang, J.; et al. First-Line Treatment of Patients with Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma with Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J. Clin. Oncol. 2019, 37, 984–991. [Google Scholar] [CrossRef]
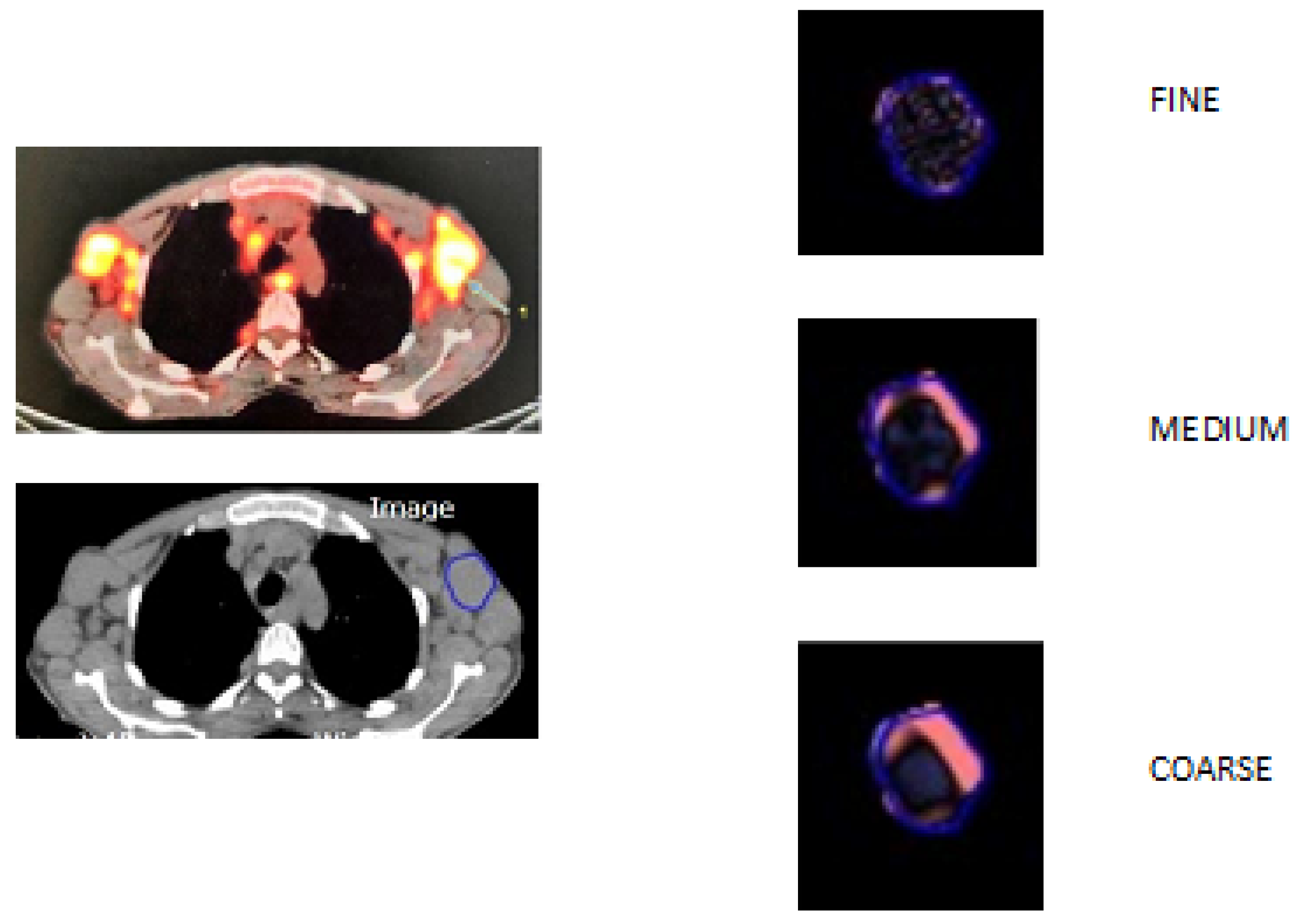
| Variable | Values |
|---|---|
| Age (years) | 61 (24–85; 52–68) |
| Age > 60 years | 38 (52) |
| Male sex | 43 (60) |
| Histologic grade | |
| 1–2 | 54 (90) |
| 3a | 6 (10) |
| ECOG > 1 | 5 (7) |
| Clinical symptoms | 14 (19) |
| LDH > upper limit of normal | 26 (36) |
| B2 microglobulin > 3 mg/L | 26 (38) |
| Hemoglobin < 12 g/dL | 10 (15) |
| Platelets < 150 × 109/L | 11 (17) |
| Albumin < 40 g/L | 28 (51) |
| Stage III–IV | 65 (89) |
| Nodal sites involvement > 4 | 46 (63) |
| Bone marrow involvement | 25 (58) |
| Extranodal sites involvement (other than bone marrow) | 38 (53) |
| LoDLIN > 6 cm | 35 (49) |
| Effusion syndrome | 5 (7) |
| Compression syndrome | 13 (18) |
| Circulating malignant cells | 8 (11) |
| Treatment | |
| R-CHOP | 66 (92) |
| R-CVP | 2 (3) |
| R-bendamustine | 4 (5) |
| Rituximab maintenance | 68 (94) |
| T-MTV (cm3) | 381 (12–3329; 155–807) |
| T-MTV > 510 cm3 | 29 (40) |
| SUVmax | 10.5 (2.7–22.2; 6.1–14.1) |
| Fails to achieve PFS 24 | 13 (18) |
| Death | 10 (14) |
| Parameters | HR (95% CI) | p Value |
|---|---|---|
| Patient sex | 4.81 (1.84, 12.55) | 0.001 * |
| ECOG > 1 | 5. 5.53 (1.55, 19.79) | 0.008 * |
| FLIPI RC | 1.73 (0.95, 3.14) | 0.071 |
| TMTV > 510 cm3 | 1.41 (0.64, 3.15) | 0.395 |
| Kurtosis_SSF0 | 1.22 (1.04, 1.44) | 0.013 * |
| Skewness_SSF2 | 3.72 (1.15, 12.11) | 0.028 * |
| Parameters | HR (95% CI) | p Value |
|---|---|---|
| ECOG > 1 | 3.31 (0.88, 12.33) | 0.075 |
| FLIPI RC | 2.28 (1.29, 6.11) | 0.101 |
| Skewness_SSF2 | 13.38 (1.29, 138.13) | 0.029 * |
| Parameters | HR (95% CI) | p Value |
|---|---|---|
| Patient sex | 3.72 (1.45, 9.53) | 0.006 * |
| ECOG > 1 | 5.90 (1.58, 21.98) | 0.008 * |
| TMTV > 510 cm3 | 2.07 (0.88, 4.87) | 0.093 |
| Kurtosis_SSF0 | 1.23 (1.04, 1.46) | 0.013 * |
| Skewness_SSF2 | 5.11 (1.18, 22.13) | 0.029 * |
| Parameters | HR (95% CI) | p Value |
|---|---|---|
| Patient sex | 19 (1.66, 217.82) | 0.018 * |
| ECOG > 1 | 3.47 (0.61, 19.86) | 0.162 |
| B2-microglobuline | 0.87 (0.18, 4.25) | 0.868 |
| FLIPI RC | 6.43 (1.35, 30.66) | 0.019 * |
| Mean_SSF2 | 0.92 (0.83, 1.01) | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durot, C.; Durot, E.; Mulé, S.; Morland, D.; Godard, F.; Quinquenel, A.; Delmer, A.; Soyer, P.; Hoeffel, C. Pretreatment CT Texture Parameters as Predictive Biomarkers of Progression-Free Survival in Follicular Lymphoma Treated with Immunochemotherapy and Rituximab Maintenance. Diagnostics 2023, 13, 2237. https://doi.org/10.3390/diagnostics13132237
Durot C, Durot E, Mulé S, Morland D, Godard F, Quinquenel A, Delmer A, Soyer P, Hoeffel C. Pretreatment CT Texture Parameters as Predictive Biomarkers of Progression-Free Survival in Follicular Lymphoma Treated with Immunochemotherapy and Rituximab Maintenance. Diagnostics. 2023; 13(13):2237. https://doi.org/10.3390/diagnostics13132237
Chicago/Turabian StyleDurot, Carole, Eric Durot, Sébastien Mulé, David Morland, François Godard, Anne Quinquenel, Alain Delmer, Philippe Soyer, and Christine Hoeffel. 2023. "Pretreatment CT Texture Parameters as Predictive Biomarkers of Progression-Free Survival in Follicular Lymphoma Treated with Immunochemotherapy and Rituximab Maintenance" Diagnostics 13, no. 13: 2237. https://doi.org/10.3390/diagnostics13132237
APA StyleDurot, C., Durot, E., Mulé, S., Morland, D., Godard, F., Quinquenel, A., Delmer, A., Soyer, P., & Hoeffel, C. (2023). Pretreatment CT Texture Parameters as Predictive Biomarkers of Progression-Free Survival in Follicular Lymphoma Treated with Immunochemotherapy and Rituximab Maintenance. Diagnostics, 13(13), 2237. https://doi.org/10.3390/diagnostics13132237







