Image-Based Numerical Analysis for Isolated Type II SLAP Lesions in Shoulder Abduction and External Rotation
Abstract
1. Introduction
2. Methods
2.1. 3D Model
2.2. Surface Reconstruction
3. Materials
3.1. Yeoh Model
3.2. Finite Element Model and Boundary Conditions
3.3. A Joint Model with an Isolated Type II SLAP
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordin, M.; Frankel, H.V. Basic Biomechanics of the Musculoskeletal System, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Rudy, C.C.; Daya, M.R. Shoulder. In Rosen’s Emergency Medicine: Concepts and Clinical Practice, 10th ed.; Mosby/Elsevier: Philadelphia, PA, USA, 2022. [Google Scholar]
- Lodha, S.; Mazloom, S.; Resler, A.G.; Frank, R.M.; Ghodadra, N.S.; Romeo, A.A.; Kim, J.Y.; Jadgchew, R.J.; Provencher, M.T. Shoulder Instability Treatment and Rehabilitation, 4th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2018. [Google Scholar]
- de SA, D.; Arakgi, M.; Lian, J.; Crum, R.J.; Lin, A.; Lesniak, B.P. Labral Repair Versus Biceps Tenodesis for Primary Surgical Management of Type II Superior Labrum Anterior to Posterior Tears: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.J.; Karzel, R.P.; Del Pizzo, W.; Ferkel, R.D.; Friedman, M.J. SLAP lesions of the shoulder. Arthrosc. J. Arthrosc. Relat. Surg. 1990, 6, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Frantz, T.L.; Shacklett, A.G.; Martin, A.S.; Barlow, J.D.; Jones, G.L.; Neviaser, A.S.; Cvetanovich, G.L. Biceps Tenodesis for Superior Labrum Anterior-Posterior Tear in the Overhead Athlete: A Systematic Review. Am. J. Sports Med. 2021, 49, 522–528. [Google Scholar] [CrossRef]
- Nam, E.K.; Snyder, S.J. The Diagnosis and Treatment of Superior Labrum, Anterior and Posterior (SLAP) Lesions. Am. J. Sports Med. 2003, 31, 798–810. [Google Scholar] [CrossRef]
- Morgan, C.D.; McHale, K.J. Throwing Acquired Anterior Rotator Interval Pathology. In Elite Techniques in Shoulder Arthroscopy; Kelly, J.K., IV, Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–63. [Google Scholar] [CrossRef]
- Powell, S.E.; Nord, K.D.; Ryu, R.K.N. The Diagnosis, Classification, and Treatment of SLAP Lesions. Oper. Tech. Sports Med. 2012, 20, 46–56. [Google Scholar] [CrossRef]
- Snyder, S.J.; Banas, M.P.; Karzel, R.P. An analysis of 140 injuries to the superior glenoid labrum. J. Shoulder Elb. Surg. 1995, 4, 243–248. [Google Scholar] [CrossRef]
- Brockmeyer, M.; Tompkins, M.; Kohn, D.M.; Lorbach, O. SLAP lesions: A treatment algorithm. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Parratte, S.; Chuinard, C.; Roussanne, Y.; Shia, D.; Bicknell, R. Arthroscopic Treatment of Isolated Type II SLAP Lesions. Am. J. Sports Med. 2009, 37, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Hutchinson, I.D.; Curry, E.J.; Marinko, L.; Li, X. Surgical management of type II superior labrum anterior posterior (SLAP) lesions: A review of outcomes and prognostic indicators. Phys. Sportsmed. 2019, 47, 375–386. [Google Scholar] [CrossRef]
- Shin, M.H.; Baek, S.; Kim, T.M.; Kim, H.; Oh, K.-S.; Chung, S.W. Biceps Tenodesis Versus Superior Labral Anterior and Posterior (SLAP) Lesion Repair for the Treatment of SLAP Lesion in Overhead Athletes: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2022, 50, 3987–3997. [Google Scholar] [CrossRef]
- Li, M.; Shaikh, A.B.; Sun, J.; Shang, P.; Shang, X. Effectiveness of biceps tenodesis versus SLAP repair for surgical treatment of isolated SLAP lesions: A systemic review and meta-analysis. J. Orthop. Transl. 2019, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mathew, C.J.; Lintner, D.M. Superior Labral Anterior to Posterior Tear Management in Athletes. Open Orthop. J. 2018, 12, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Stathellis, A.; Brilakis, E.; Georgoulis, J.-D.; Antonogiannakis, E.; Georgoulis, A. Treatment of SLAP lesions. Open Orthop. J. 2018, 12, 288–294. [Google Scholar] [CrossRef]
- Schrøder, C.P. “SLAP lesions, an opinion piece. Open Orthop. J. 2018, 12, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Burkhart, S.; Palmeri, M.; Gillespie, M. Type II SLAP lesions: Three subtypes and their relationships to superior instability and rotator cuff tears. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 553–565. [Google Scholar] [CrossRef]
- Drury, N.J. Evaluating the Anterior Stability Provided by the Glenohumeral Capsule: A Finite Element Approach; University of Pittsburgh ETD: Pittsburgh, PA, USA, 2008. [Google Scholar]
- Drury, N.J.; Ellis, B.J.; Weiss, J.A.; McMahon, P.J.; Debski, R.E. The impact of glenoid labrum thickness and modulus on labrum and glenohumeral capsule function. J. Biomech. Eng. 2010, 132, 121003. [Google Scholar] [CrossRef]
- Drury, N.J.; Ellis, B.J.; Weiss, J.A.; McMahon, P.J.; Debski, R.E. Finding consistent strain distributions in the glenohumeral capsule between two subjects: Implications for development of physical examinations. J. Biomech. 2011, 44, 607–613. [Google Scholar] [CrossRef]
- Swanson, K. Finite Element Analysis of Glenohumeral Labral Tears in The Shoulder Joint; Worcester Polytechnic Institute: Worcester, MA, USA, 2017. [Google Scholar]
- Lemieux, P.O.; Tétreault, P.; Hagemeister, N.; Nuño, N. Influence of prosthetic humeral head size and medial offset on the mechanics of the shoulder with cuff tear arthropathy: A numerical study. J. Biomech. 2013, 46, 806–812. [Google Scholar] [CrossRef]
- Ratajczak, M.; Ptak, M.; Kwiatkowski, A.; Kubicki, K.; Fernandes, F.A.O.; Wilhelm, J.; Dymek, M.; Sawicki, M.; Żółkiewski, S. Symmetry of the Human Head—Are Symmetrical Models More Applicable in Numerical Analysis? Symmetry 2021, 13, 1252. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Sabesan, V.J.; Callanan, M.; Youderian, A.; Iannotti, J.P. 3D CT Assessment of the Relationship Between Humeral Head Alignment and Glenoid Retroversion in Glenohumeral Osteoarthritis. J. Bone Jt. Vol. 2014, 96, e64. [Google Scholar] [CrossRef] [PubMed]
- Itoi, E.; Lee, S.B.; Berglund, L.J.; Berge, L.L.; An, K.N. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: A cadaveric study. J. Bone Jt. Surg. Ser. A 2000, 82, 35–46. [Google Scholar] [CrossRef]
- Mercer, D.; Saltzman, M.D.; Neradilek, M.B.; Gilmer, B.B.; Warme, W.J.; Matsen, F.A. A reproducible and practical method for documenting the position of the humeral head center relative to the scapula on standardized plain radiographs. J. Shoulder Elb. Surg. 2011, 20, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Stehle, J.H.; Rainis, E.J.; McMahon, P.J.; Debski, R.E. The current anatomical description of the inferior glenohumeral ligament does not correlate with its functional role in positions of external rotation. J. Orthop. Res. 2008, 26, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Ellis, B.; Weiss, J.A.; McMahon, P.J.; Debski, R.E. The glenohumeral capsule should be evaluated as a sheet of fibrous tissue: A validated finite element model. Ann. Biomed. Eng. 2010, 38, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Kamran, A.; Siddiqui, M.Z.; Farhan, M. Mechanical Characterization and FE Modelling of a Hyperelastic Material. Mater. Res. 2015, 18, 918–924. [Google Scholar] [CrossRef]
- Safshekan, F.; Tafazzoli-Shadpour, M.; Abdouss, M.; Shadmehr, M.B. Mechanical characterization and constitutive modeling of human trachea: Age and gender dependency. Materials 2016, 9, 456. [Google Scholar] [CrossRef]
- ANSYS Inc. ANSYS® Academic Research Mechanical, Release 19.2, Help System; ANSYS, Inc.: Canonsburg, PA, USA, 2018. [Google Scholar]
- Debski, R.E.; Wong, E.K.; Woo, S.L.Y.; Sakane, M.; Fu, F.H.; Warner, J.J.P. In situ force distribution in the glenohumeral joint capsule during anterior-posterior loading. J. Orthop. Res. 1999, 17, 769–776. [Google Scholar] [CrossRef]
- Metan, S.S.; Krishna, P.; Mohankumar, G.C. FEM model an effective tool to evaluate von Mises stresses in shoulder joint and muscles for adduction and abduction. Procedia Mater. Sci. 2014, 5, 2090–2098. [Google Scholar] [CrossRef]
- Moore, S.M.; Musahl, V.; McMahon, P.J.; Debski, R.E. Multidirectional kinematics of the glenohumeral joint during simulated simple translation tests: Impact on clinical diagnoses. J. Orthop. Res. 2004, 22, 889–894. [Google Scholar] [CrossRef]
- Musahl, V.; Moore, S.M.; McMahon, P.J.; Debski, R.E. Orientation feedback during simulated simple translation tests has little clinical significance on the magnitude and precision of glenohumeral joint translations. Knee Surg. Sport Traumatol. Arthrosc. 2006, 14, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Tennent, T.D.; Beach, W.R.; Meyers, J.F. A review of the special tests associated with shoulder examination. Part II: Laxity, instability, and superior labral anterior and posterior (SLAP) lesions. Am. J. Sports Med. 2003, 31, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Popp, D. Superior labral anterior posterior lesions of the shoulder: Current diagnostic and therapeutic standards. World J. Orthop. 2015, 6, 660–671. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, M.R.; Mancini, M.R.; Hawthorne, B.C.; Romeo, A.A.; Calvo, E.; Mazzocca, A.D. SLAP tears and return to sport and work: Current concepts. J. ISAKOS 2021, 6, 204–211. [Google Scholar] [CrossRef]
- Wilk, K.E.; Macrina, L.C.; Cain, E.L.; Dugas, J.R.; Andrews, J.R. The recognition and treatment of superior labral (slap) lesions in the overhead athlete. Int. J. Sports Phys. Ther. 2013, 8, 579–600. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24175139 (accessed on 15 January 2020).
- Modarresi, S.; Motamedi, D.; Jude, C.M. Superior Labral Anteroposterior Lesions of the Shoulder: Part 1, Anatomy and Anatomic Variants. Am. J. Roentgenol. 2011, 197, 596–603. [Google Scholar] [CrossRef]
- Mohana-Borges, A.V.R.; Chung, C.B.; Resnick, D. Superior Labral Anteroposterior Tear: Classification and Diagnosis on MRI and MR Arthrography. Am. J. Roentgenol. 2003, 181, 1449–1462. [Google Scholar] [CrossRef]
- Allred, J.J.; Flores-Hernandez, C.; Hoenecke, H.R.; D’Lima, D.D. Posterior augmented glenoid implants require less bone removal and generate lower stresses: A finite element analysis. J. Shoulder Elb. Surg. 2016, 25, 823–830. [Google Scholar] [CrossRef]
- Bryce, C.D.; Davison, A.C.; Lewis, G.S.; Wang, L.; Flemming, D.J.; Armstrong, A.D. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J. Bone Jt. Surg. Ser. A 2010, 92, 692–699. [Google Scholar] [CrossRef]
- Meriç, G.; Zeybek, G.; Kiray, A.; Atik, A.; Budeyri, A.; Koşay, C. Utilization of the bicipital groove axis for confirming alignment of the humerus with transepicondylar and ulnar shaft axes during intramedullary nailing. Acta Orthop. Traumatol. Turc. 2015, 49, 184–189. [Google Scholar] [CrossRef]
- Yoshida, M.; Saho, Y.; Katayose, M. Reliability of Measuring Humeral Retroversion Using Ultrasound Imaging in a Healthy Nonthrowing Population. J. Sport Rehabil. 2016, 19, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Walz, D.M.; Burge, A.J.; Steinbach, L. Imaging of shoulder instability. Semin. Musculoskelet. Radiol. 2015, 19, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Cools, A.M.; Borms, D.; Cottens, S.; Himpe, M.; Meersdom, S.; Cagnie, B. Rehabilitation Exercises for Athletes With Biceps Disorders and SLAP Lesions. Am. J. Sports Med. 2014, 42, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Bey, M.J.; Elders, G.J.; Huston, L.J.; Kuhn, J.E.; Blasier, R.B.; Soslowsky, L.J. The mechanism of creation of superior labrum, anterior, and posterior lesions in a dynamic biomechanical model of the shoulder: The role of inferior subluxation. J. Shoulder Elb. Surg. 1998, 7, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Moore-Reed, S.D.; Seekins, K.A.; Kibler, W.B.; Sciascia, A.D.; Uhl, T.L. Conservative Treatment for Patients with Suspected SLAP Tears: A Case Series. Turk. Klin. J. Health Sci. 2017, 2, 121–128. [Google Scholar] [CrossRef]
- Hashiguchi, H.; Iwashita, S.; Yoneda, M.; Takai, S. Factors influencing outcomes of nonsurgical treatment for baseball players with SLAP lesion. Asia-Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2018, 14, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Nashikkar, P.S.; Rhee, S.-M.; Desai, C.V.; Oh, J.H. Is Anatomical Healing Essential for Better Clinical Outcome in Type II SLAP Repair? Clinico-Radiological Outcome after Type II SLAP Repair. Clin. Orthop. Surg. 2018, 10, 358. [Google Scholar] [CrossRef]
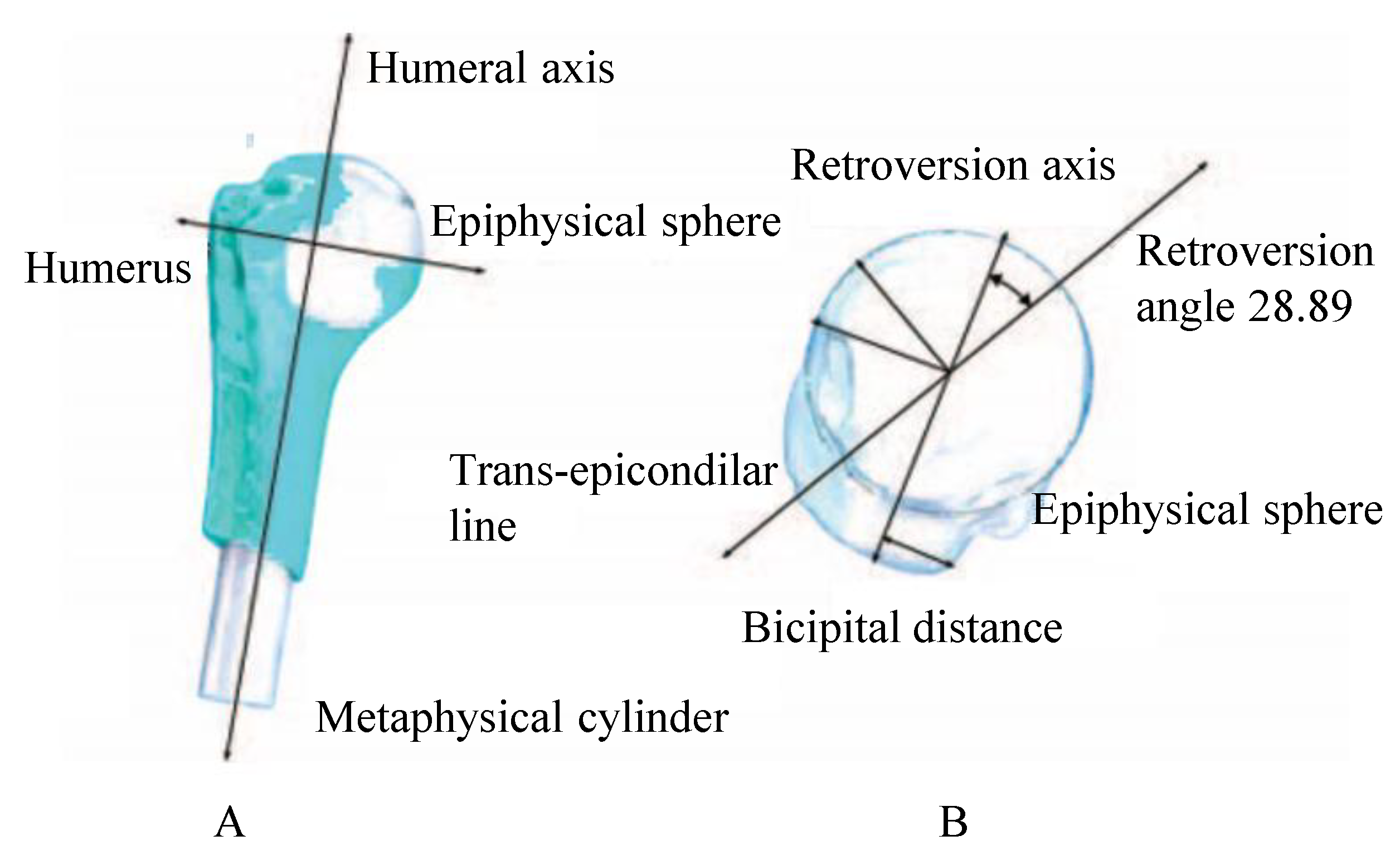
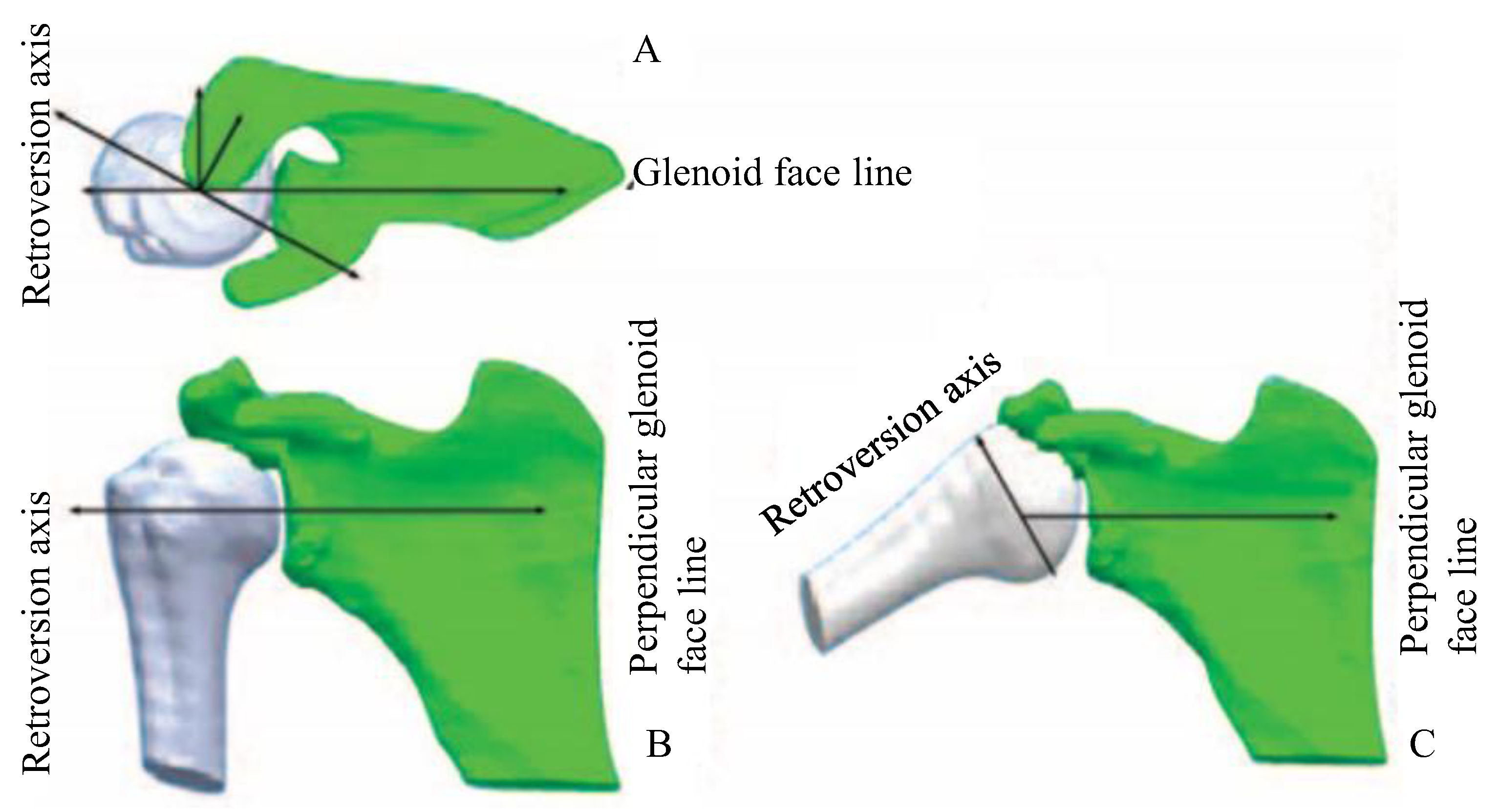
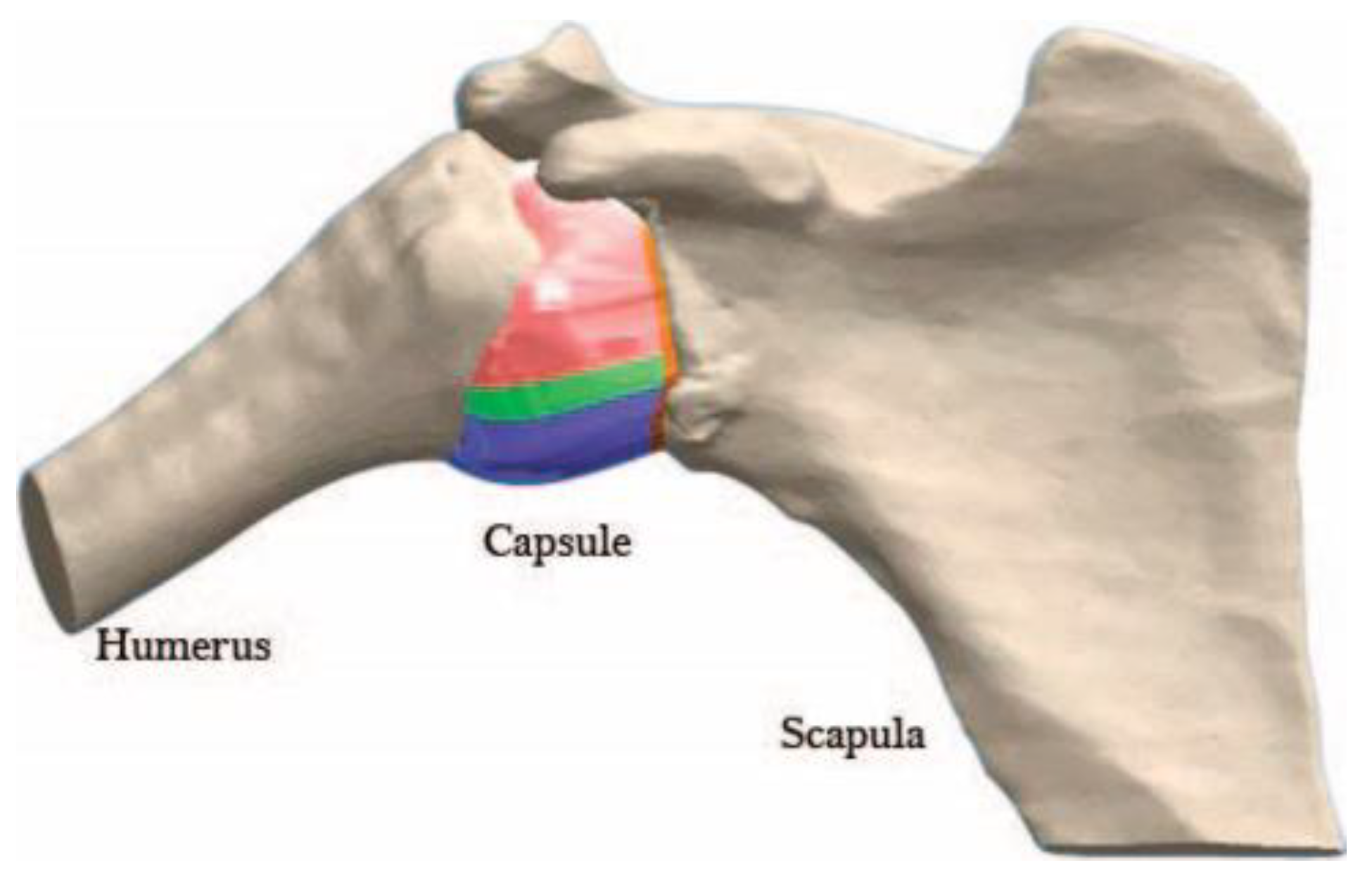
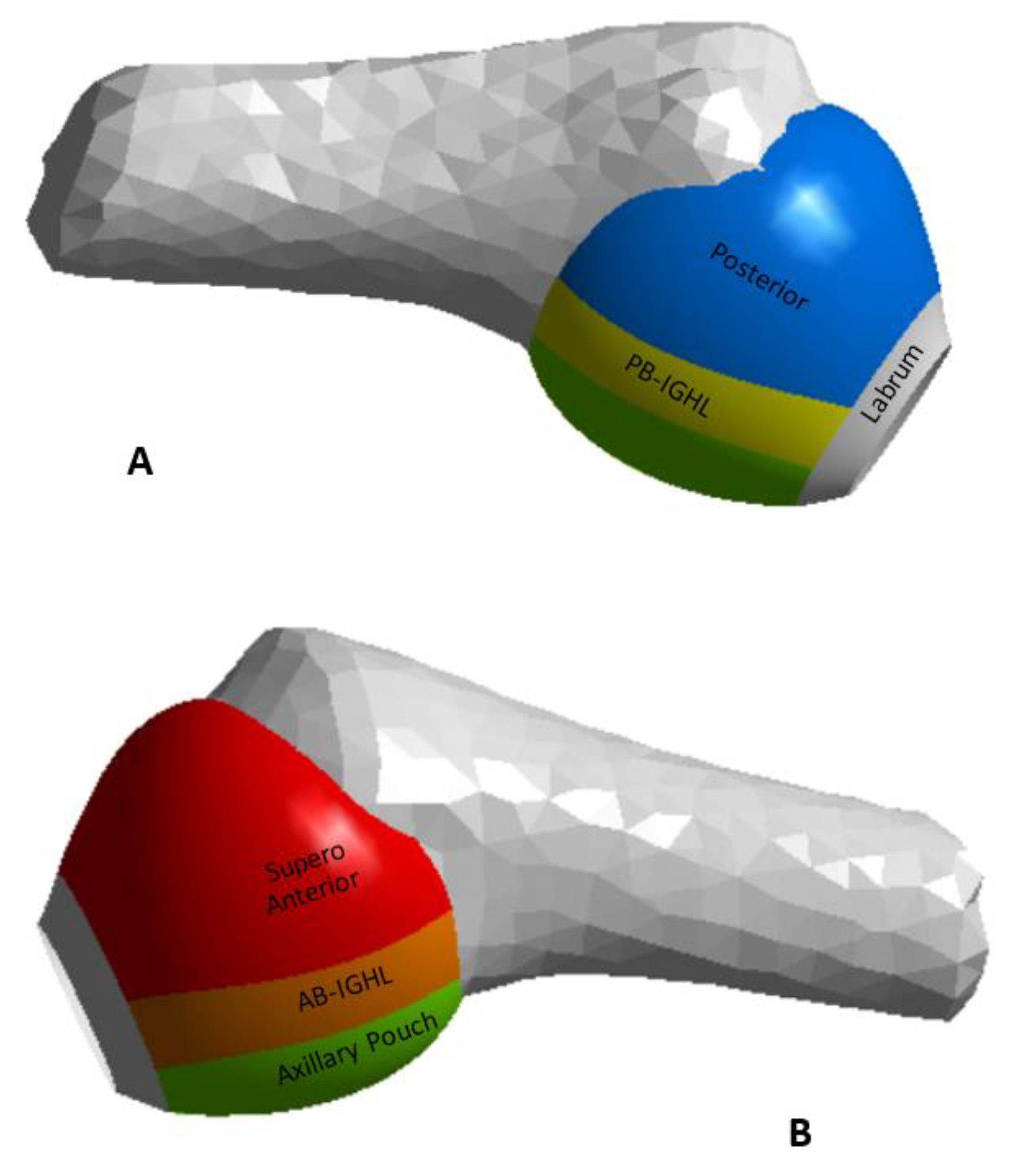

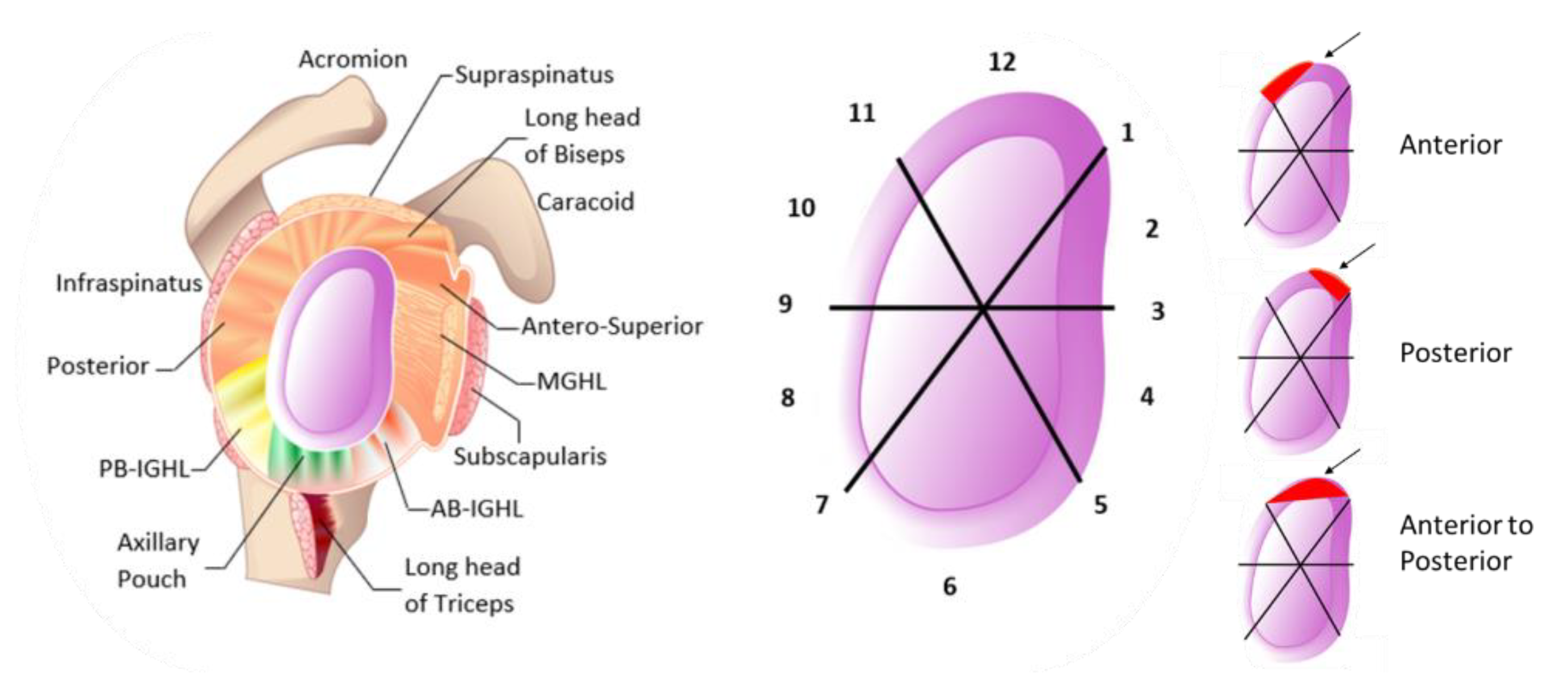
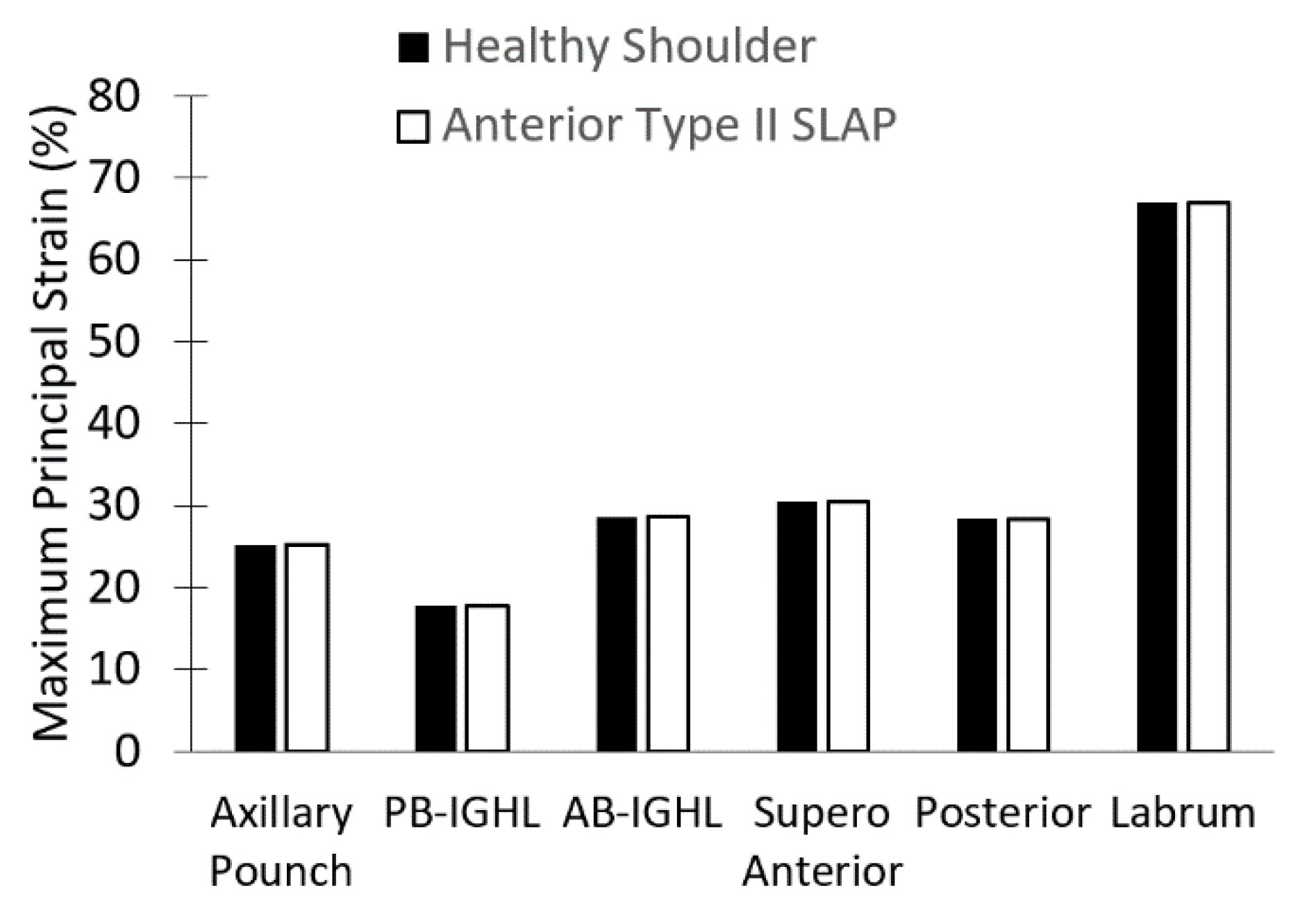

| Region | C1 [MPa] | C2 [MPa] | C3 [MPa] | R2 | Thickness [mm] |
|---|---|---|---|---|---|
| Antero Superior | 20.5 | −26.5 | 185.3 | 0.99 | 2.8 |
| Posterior | 13.2 | −19.6 | 125.9 | 0.99 | 1.5 |
| AB-IGHL | 11.9 | −11.1 | 95.8 | 0.99 | 2.8 |
| PB-IGHL | 29.1 | −18.4 | 206.5 | 0.99 | 1.3 |
| Axillary pouch | 0.22 | −17.4 | 111.9 | 0.99 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, J.A.; Puentes, D.A.; Quintero, I.D.; González-Estrada, O.A.; Villegas, D.F. Image-Based Numerical Analysis for Isolated Type II SLAP Lesions in Shoulder Abduction and External Rotation. Diagnostics 2023, 13, 1819. https://doi.org/10.3390/diagnostics13101819
Maldonado JA, Puentes DA, Quintero ID, González-Estrada OA, Villegas DF. Image-Based Numerical Analysis for Isolated Type II SLAP Lesions in Shoulder Abduction and External Rotation. Diagnostics. 2023; 13(10):1819. https://doi.org/10.3390/diagnostics13101819
Chicago/Turabian StyleMaldonado, Javier A., Duvert A. Puentes, Ivan D. Quintero, Octavio A. González-Estrada, and Diego F. Villegas. 2023. "Image-Based Numerical Analysis for Isolated Type II SLAP Lesions in Shoulder Abduction and External Rotation" Diagnostics 13, no. 10: 1819. https://doi.org/10.3390/diagnostics13101819
APA StyleMaldonado, J. A., Puentes, D. A., Quintero, I. D., González-Estrada, O. A., & Villegas, D. F. (2023). Image-Based Numerical Analysis for Isolated Type II SLAP Lesions in Shoulder Abduction and External Rotation. Diagnostics, 13(10), 1819. https://doi.org/10.3390/diagnostics13101819









