Nail Ultrasound in Psoriasis and Psoriatic Arthritis—A Narrative Review
Abstract
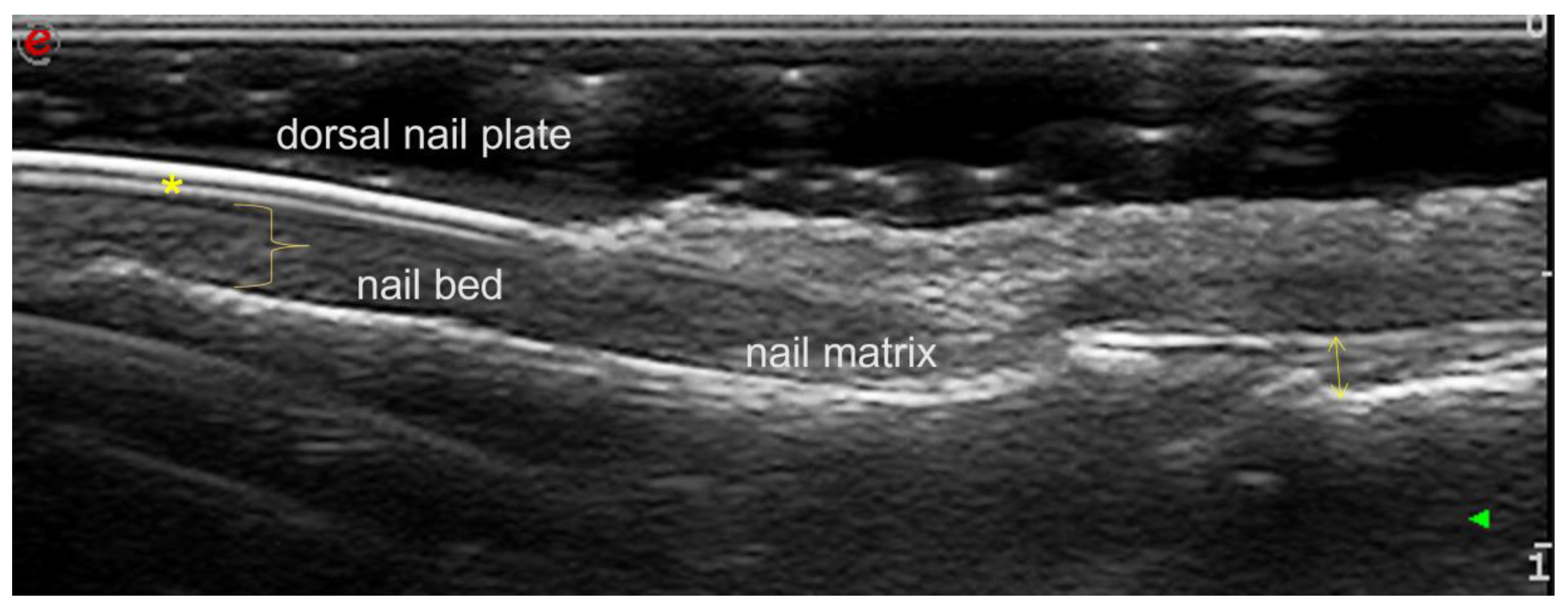
:1. Introduction
2. Assessing Nail Parameters
3. Evaluation of the Nail and Enthesis Treatment Response
4. The Connection between DIP Erosion and Nails
5. The Connection between Nails and Enthesitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gladman, D.D.; Brockbank, J. Psoriatic arthritis. Expert Opin. Investig. Drugs 2000, 9, 1511–1522. [Google Scholar] [PubMed]
- McGonagle, D. Enthesitis: An autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23 (Suppl. S1), 9–13. [Google Scholar] [CrossRef] [PubMed]
- Schons, K.R.; Knob, C.F.; Murussi, N.; Beber, A.A.; Neumaier, W.; Monticielo, O.A. Nail psoriasis: A review of the literature. An. Bras. Dermatol. 2014, 89, 312–317. [Google Scholar] [CrossRef]
- Haneke, E. Nail psoriasis: Clinical features, pathogenesis, differential diagnoses, and management. Psoriasis 2017, 7, 51–63. [Google Scholar] [CrossRef]
- Wilson, F.C.; Icen, M.; Crowson, C.S.; McEvoy, M.T.; Gabriel, S.E.; Kremers, H.M. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: A population-based study. Arthritis Rheumatol. 2009, 61, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Raposo, I.; Torres, T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermo-Sifiliográficas 2015, 106, 452–457. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.M.; Seegers, B.A.; Gulinck, M.K.; Boezeman, J.B.; van de Kerkhof, P.C. Psoriasis of the nails associated with disability in a large number of patients: Results of a recent interview with 1728 patients. Dermatology 1996, 193, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Guéro, S.; Guichard, S.; Fraitag, S.R. Ligamentary structure of the base of the nail. Surg. Radiol. Anat. 1994, 16, 47–52. [Google Scholar] [CrossRef]
- McGonagle, D.; Lories, R.J.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for under-standing joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheumatol. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Tan, A.L.; Benjamin, M.; Toumi, H.; Grainger, A.J.; Tanner, S.F.; Emery, P.; McGonagle, D. The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis—A high-resolution MRI and histological study. Rheumatology 2006, 46, 253–256. [Google Scholar] [CrossRef]
- Ortner, V.K.; Mandel, V.D.; Bertugno, S.; Philipsen, P.A.; Haedersdal, M. Imaging of the nail unit in psoriatic patients: A systematic scoping review of techniques and terminology. Exp. Dermatol. 2022, 31, 828–840. [Google Scholar] [CrossRef]
- Fassio, A.; Giovannini, I.; Idolazzi, L.; Zabotti, A.; Iagnocco, A.; Sakellariou, G. Nail ultrasonography for psoriatic arthritis and psoriasis patients: A systematic literature review. Clin. Rheumatol. 2020, 39, 1391–1404. [Google Scholar] [CrossRef]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Möller, I.; Naredo, E.; et al. OMERACT Ultrasound Task Force members. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef]
- Ximena Wortsman, C.; Elizabeth, A.H.; Gregor, B.E.J.; Monika, G. Ultrasonido de alta resolucion (15 MHz) en el estudio de la uña psoriatica. Rev. Chil. Radiol. 2004, 10, 6–11. [Google Scholar]
- Gutierrez, M.; Filippucci, E.; De Angelis, R.; Filosa, G.; Kane, D.; Grassi, W. A sonographic spectrum of psoriatic arthritis: “The five targets”. Clin. Rheumatol. 2010, 29, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Naredo, E.; Keen, H.I.; Bruyn, G.A.W.; Iagnocco, A.; Wakefield, R.J.; Conaghan, P.G.; Maxwell, L.J.; Beaton, D.E.; Boers, M.; et al. The OMERACT Stepwise Approach to Select and Develop Imaging Outcome Measurement Instruments: The Musculoskeletal Ultrasound Example. J. Rheumatol. 2019, 46, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Gisondi, P.; Fassio, A.; Viapiana, O.; Giollo, A.; Rossini, M.; Girolomoni, G.; Gatti, D. Ultrasonography of the nail unit reveals quantitative and qualitative alterations in patients with psoriasis and psoriatic arthritis. Med. Ultrason. 2018, 20, 177–184. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wiktorowicz, A.; Wojtkiewicz, J. Ultrasound Assessment of Changes in Nails in Psoriasis and Psoriatic Arthritis. Biomed. Res. Int. 2018, 2018, 8251097. [Google Scholar] [CrossRef]
- Mondal, S.; Dutta, S.; Lahiri, D.; Sinha, D.; Sircar, G.; Mandal, A.K.; Kejriwal, M.; Ghosh, A. Assessment of nail unit structures by ultrasound in patients with psoriatic arthritis and their correlations with disease activity indices: A case-control study. Rheumatol. Int. 2018, 38, 2087–2093. [Google Scholar] [CrossRef]
- Mahmoud, I.; Rahmouni, S.; Ben Tekaya, A.; Bouden, S.; Rouached, L.; Tekaya, R.; Saidane, O.; Abdelmoula, L. AB0956 To what extend is nail ultrasound discriminative between psoriatic arthritis and healthy subjects? Ann. Rheum. Dis. 2022, 81, 1606–1607. [Google Scholar] [CrossRef]
- Naredo, E.; Janta, I.; Baniandrés-Rodríguez, O.; Valor, L.; Hinojosa, M.; Bello, N.; Serrano, B.; Garrido, J. To what extend is nail ultrasound discriminative between psoriasis, psoriatic arthritis and healthy subjects? Rheumatol. Int. 2019, 39, 697–705. [Google Scholar] [CrossRef]
- Aydin, S.Z.; Mathew, A.J.; Koppikar, S.; Eder, L.; Østergaard, M. Imaging in the diagnosis and management of peripheral psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101594. [Google Scholar] [CrossRef]
- Aydin, S.Z.; Castillo-Gallego, C.; Ash, Z.R.; Marzo-Ortega, H.; Wakefield, R.; McGonagle, D. Vascularity of nail bed by ultrasound to discriminate psoriasis, psoriatic arthritis and healthy controls. Clin. Exp. Rheumatol. 2017, 35, 872. [Google Scholar] [PubMed]
- Kasman, S.A.; Gezer, H.H.; Baklacıoğlu, H.Ş.; Gürsoy, D.E.; Duruöz, M.T. A standardized sonographic analysis of nails in psoriatic arthritis and healthy controls: Feasibility, reliability, diagnostic performance, and demographic and clinical associations. Jt. Bone Spine 2021, 88, 105197. [Google Scholar] [CrossRef]
- Bellinato, F.; Gisondi, P.; Filippucci, E.; Tozzi, F.; Fassio, A.; Adami, G.; Idolazzi, L. Systematic study on nail plate assessment: Differences in nail plate shape, thickness, power Doppler signal and scanning approach. Arch. Dermatol. Res. 2023, 315, 593–600. [Google Scholar] [CrossRef]
- De Rossi, S.D.; Mendonça, J.A.; Palominos, P.E.; Kohem, C.L.; Cestari, T.F.; da Silva Chakr, R.M. Ultrasonographic and resistance index evaluation of nails in psoriatic arthritis, psoriasis, and control groups: A cross-sectional study. Adv. Rheumatol. 2021, 61, 48. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Zabotti, A.; Fassio, A.; Errichetti, E.; Benini, C.; Vantaggiato, E.; Rossini, M.; De Vita, S.; Viapiana, O. The ultrasonographic study of the nail reveals differences in patients affected by inflammatory and degenerative conditions. Clin. Rheumatol. 2019, 38, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Krajewska-Włodarczyk, M.; Żuber, Z.; Owczarczyk-Saczonek, A. Ultrasound Evaluation of the Effectiveness of the Use of Acitretin in the Treatment of Nail Psoriasis. J. Clin. Med. 2021, 10, 2122. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wojtkiewicz, J. Effect of Methotrexate in the Treatment of Distal Interphalangeal Joint Extensor Tendon Enthesopathy in Patients with Nail Psoriasis. J. Clin. Med. 2018, 7, 546. [Google Scholar] [CrossRef]
- Muñoz-Santos, C.; Sola-Ortigosa, J.; Vidal, D.; Guilabert, A. Apremilast improves quality of life and ultrasonography parameters in patients with nail psoriasis: A prospective cohort study. J. Dermatol. 2021, 48, 1593–1596. [Google Scholar] [CrossRef]
- Nash, P.; Mease, P.J.; Kirkham, B.; Singhal, A.; Quebe-Fehling, E.; Pricop, L.; Gaillez, C. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin. Exp. Rheumatol. 2022, 40, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.S.; Allard, A.; Rambojun, A.; Lovell, C.R.; Shaddick, G.; Robinson, G.; Jadon, D.R.; Holland, R.; Cavill, C.; Korendowych, E.; et al. Psoriatic Nail Dystrophy Is Associated with Erosive Disease in the Distal Interphalangeal Joints in Psoriatic Arthritis: A Retrospective Cohort Study. J. Rheumatol. 2019, 46, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.P.; Boutroy, S.; Coutisson, C.; Carlier, M.C.; Barets, L.; Marotte, H.; Richert, B.; Chapurlat, R.D.; Jullien, D.; Confavreux, C.B. Distal phalangeal bone erosions observed by HR-pQCT in patients with psoriatic onycholysis. Rheumatology 2021, 60, 1176–1184. [Google Scholar] [CrossRef]
- Moya Alvarado, P.; Roé Crespo, E.; Muñoz-Garza, F.Z.; López-Ferrer, A.; Laiz Alonso, A.; Vilarrassa Rull, E.; Casademont I Pou, J.; Puig Sanz, L. Subclinical enthesopathy of extensor digitorum tendon is highly prevalent and associated with clinical and ultrasound alterations of the adjacent fingernails in patients with psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1728–1736. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Wojtkiewicz, M.; Wiktorowicz, A.; Wojtkiewicz, J. Distal inter-phalangeal joint extensor tendon enthesopathy in patients with nail psoriasis. Sci. Rep. 2019, 9, 3628. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; McGonagle, D.; Rooney, M. Integrating imaging and biomarker assessment to better define psoriatic arthritis and predict response to biologic therapy. Rheumatology 2021, 60 (Suppl. S6), vi38–vi52. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, I.; Rahmouni, S.; Ben Tekaya, A.; Bouden, S.; Rouached, L.; Tekaya, R.; Saidane, O.; Abdelmoula, L. AB0958 The relationship between the extensor tendon enthesis and the nail in distal interphalangeal joint disease in psoriatic arthritis: Ultrasound study. Ann. Rheum. Dis. 2022, 81, 1607. [Google Scholar] [CrossRef]
- Huang, Y.S.; Huang, Y.H.; Lin, C.H.; Kuo, C.F.; Huang, Y.J. Ultrasound Can Be Usefully Integrated with the Clinical Assessment of Nail and Enthesis Involvement in Psoriasis and Psoriatic Arthritis. J. Clin. Med. 2022, 11, 6296. [Google Scholar] [CrossRef]
- Guldberg-Møller, J.; Mogensen, M.; Ellegaard, K.; Zavareh, A.; Wakefield, R.J.; Tan, A.L.; Boesen, M.; Dehmeshki, J.; Kubassova, O.; Dreyer, L.; et al. Multimodal imaging of the distal interphalangeal-joint synovio-entheseal complex in pso-riatic arthritis (MIDAS): A cross-sectional study on the diagnostic accuracy of different imaging modalities comparing psoriatic arthritis to psoriasis and osteoarthritis. RMD Open 2022, 8, e002109. [Google Scholar]
- Ruscitti, P.; Esposito, M.; Gianneramo, C.; Di Cola, I.; De Berardinis, A.; Martinese, A.; Nkamtse Tochap, G.; Conforti, A.; Masciocchi, C.; Cipriani, P.; et al. Nail and enthesis assessment in patients with psoriatic disease by high frequency ultra-sonography: Findings from a single-centre cross-sectional study. Radiol Med. 2022, 127, 1400–1406. [Google Scholar] [CrossRef]
- Perrin, C. Are There Signs of Enthesitis in Nail Psoriasis? An Immunohistological Study of Nail Psoriasis With and Without Psoriatic Arthritis. Am. J. Dermatopathol. 2023, 45, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Orbai, A.M.; de Wit, M.; Mease, P.J.; Callis Duffin, K.; Elmamoun, M.; Tillett, W.; Campbell, W.; FitzGerald, O.; Gladman, D.D.; Goel, N.; et al. Updating the Psoriatic Arthritis (PsA) Core Domain Set: A Report from the PsA Workshop at OMERACT 2016. J. Rheumatol. 2017, 44, 1522–1528. [Google Scholar] [CrossRef] [PubMed]

| type I | Intact and hyperechoic dorsal plate with focal hyperechoic areas on the ventral plate |
| type II | A normal hyperechoic dorsal plate with blurring and loss of ventral plate margins |
| type III | Wavy appearance of both nail plates |
| type IV | Loss of definition of both plates |
| Study | Subjects | Probe (MHz) | Correlation between Nail US Changes (Morphological Parameters) and DIP US (Thickness of Extensor Tendon) | Correlation between Nail US Changes (Morphological Parameters) and Peripheral Enthesitis Score |
|---|---|---|---|---|
| Moya Alvarado et al., 2018 [34] | 48 PsO 23 PsA | 18–22 | Not correlated significantly | - |
| Krajewska-Włodarczyk et al., 2019 [35] | 41 PsO 31 PsA 30 HC | 24 | PsO: extensor tendon thickness correlated with NBT (r = 0.316, p = 0.027) and NMT (r = 0.421, p = 0.012); PsA: extensor tendon thickness correlated with NBT (r = 0.402, p = 0.031) | - |
| Elliot et al., 2021 [36] | 46 PsA | 18 | Correlation between the nail US score and DIP US (r = 0.43, p = 0.003) | Correlation between nail US score and the active peripheral enthesitis score (MASEI-active; r = 0.35, p = 0.018) |
| Mahmoud et al., 2022 [37] | 31 PsA | NS | Finger extensor tendon thickness correlated with NBT (r = 0.412, p = 0.00), NPT (r = 0.310, p = 0.00) and the thickness of the adjacent skin (r = 0.509, p = 0.00) | - |
| Huang et al., 2022 [38] | 35 PsO 154 PsA | 18 | - | GUESS scores were significantly associated with nail thickness (p = 0.020) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agache, M.; Popescu, C.C.; Enache, L.; Dumitrescu, B.M.; Codreanu, C. Nail Ultrasound in Psoriasis and Psoriatic Arthritis—A Narrative Review. Diagnostics 2023, 13, 2236. https://doi.org/10.3390/diagnostics13132236
Agache M, Popescu CC, Enache L, Dumitrescu BM, Codreanu C. Nail Ultrasound in Psoriasis and Psoriatic Arthritis—A Narrative Review. Diagnostics. 2023; 13(13):2236. https://doi.org/10.3390/diagnostics13132236
Chicago/Turabian StyleAgache, Mihaela, Claudiu C. Popescu, Luminița Enache, Bianca M. Dumitrescu, and Cătălin Codreanu. 2023. "Nail Ultrasound in Psoriasis and Psoriatic Arthritis—A Narrative Review" Diagnostics 13, no. 13: 2236. https://doi.org/10.3390/diagnostics13132236
APA StyleAgache, M., Popescu, C. C., Enache, L., Dumitrescu, B. M., & Codreanu, C. (2023). Nail Ultrasound in Psoriasis and Psoriatic Arthritis—A Narrative Review. Diagnostics, 13(13), 2236. https://doi.org/10.3390/diagnostics13132236





