Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation
Abstract
1. Introduction
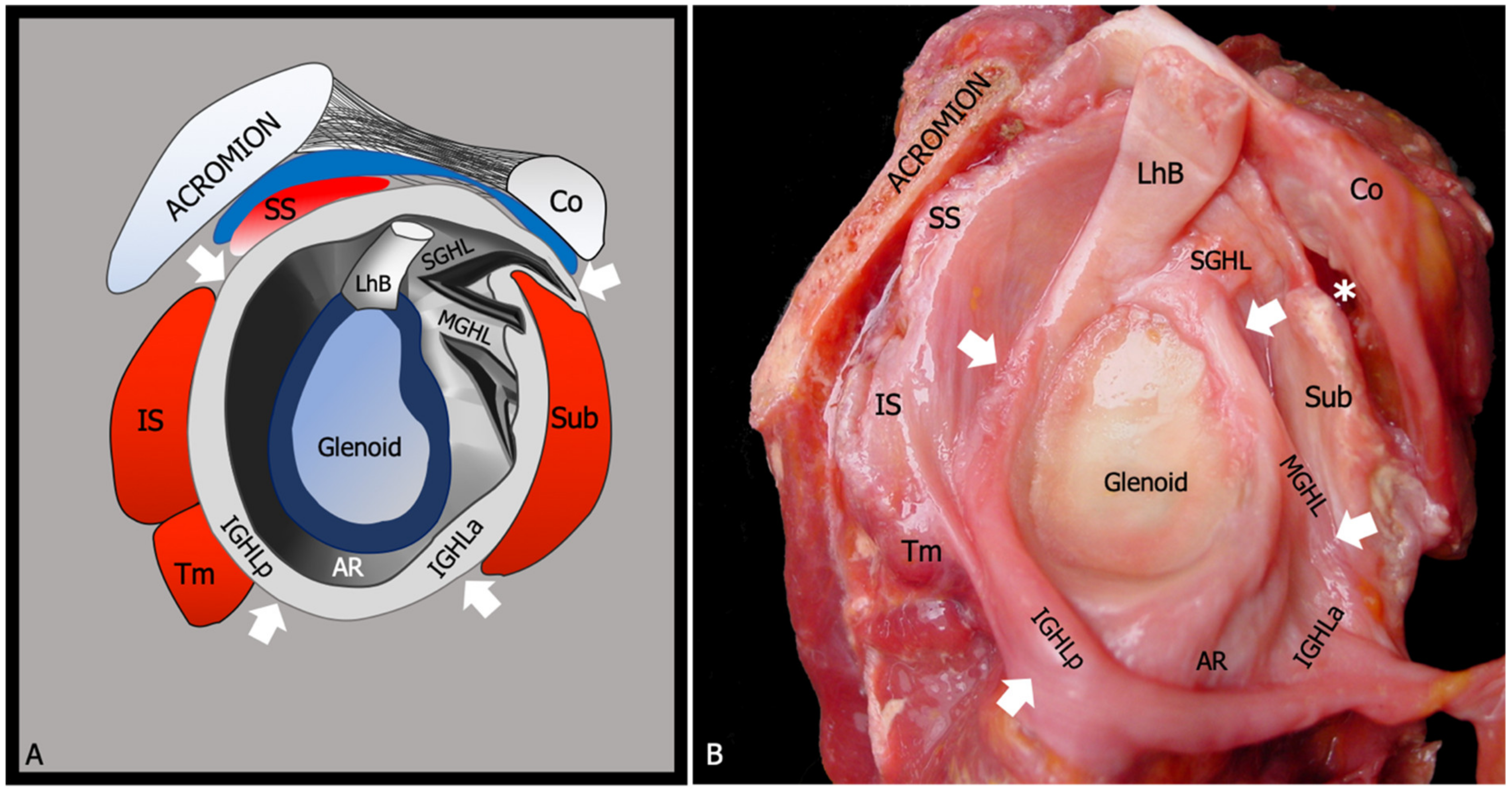
2. Relevant Anatomy
3. Epidemiology and Risk Factors
4. Pathogenesis
5. Natural History
- First stage (painful stage): It occurs in the first three months, and it is characterized by the insurgence of ill-defined ache around the deltoid area, which tends to worsen at night. Mild restriction of glenohumeral joint range of motion may also be observed. From a histological point of view, this phase is distinguished by the development of an inflammatory infiltrate, synovitis, and hypervascularity at the level of the glenohumeral joint capsule.
- Second stage (freezing stage): Between the third and the ninth month the patient experiences progressive joint stiffness and the increase of pain. Joint stiffness is particularly evident in forward flexion, abduction, and extra rotation. The capsule shows macroscopic alteration with thickening and hypervascularity of the synovial membrane, disorganized deposition of collagen, and adhesions. The first and second stages have been grouped together in recent descriptions.
- Third stage (frozen stage): This phase may last until the fourteenth month and is distinguished by severe restriction of joint movements and an initial decrease of pain, which in this phase is less evident at rest but remains intense during passive mobilization of the glenohumeral joint.
- The fourth stage (thawing stage): The last phase is distinguished by the spontaneous resolution of joint stiffness and pain, which may require up to two years. Histologically, mature and adhering hypercellular collagen is detected in the capsular tissue in the third and fourth stages whereas inflammatory signs are less evident.
6. Clinical Diagnostic Work-Up
7. Role of Imaging
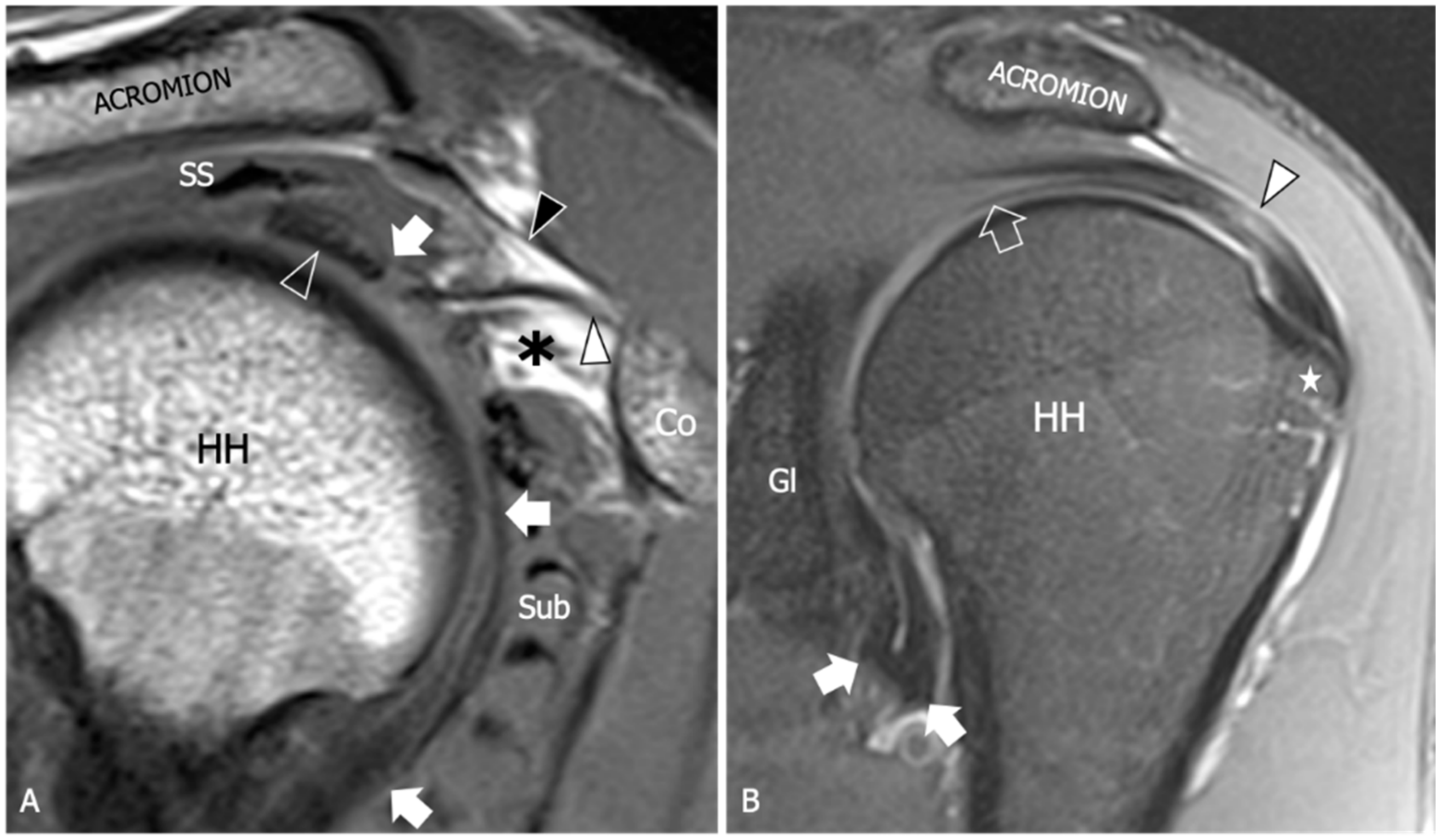
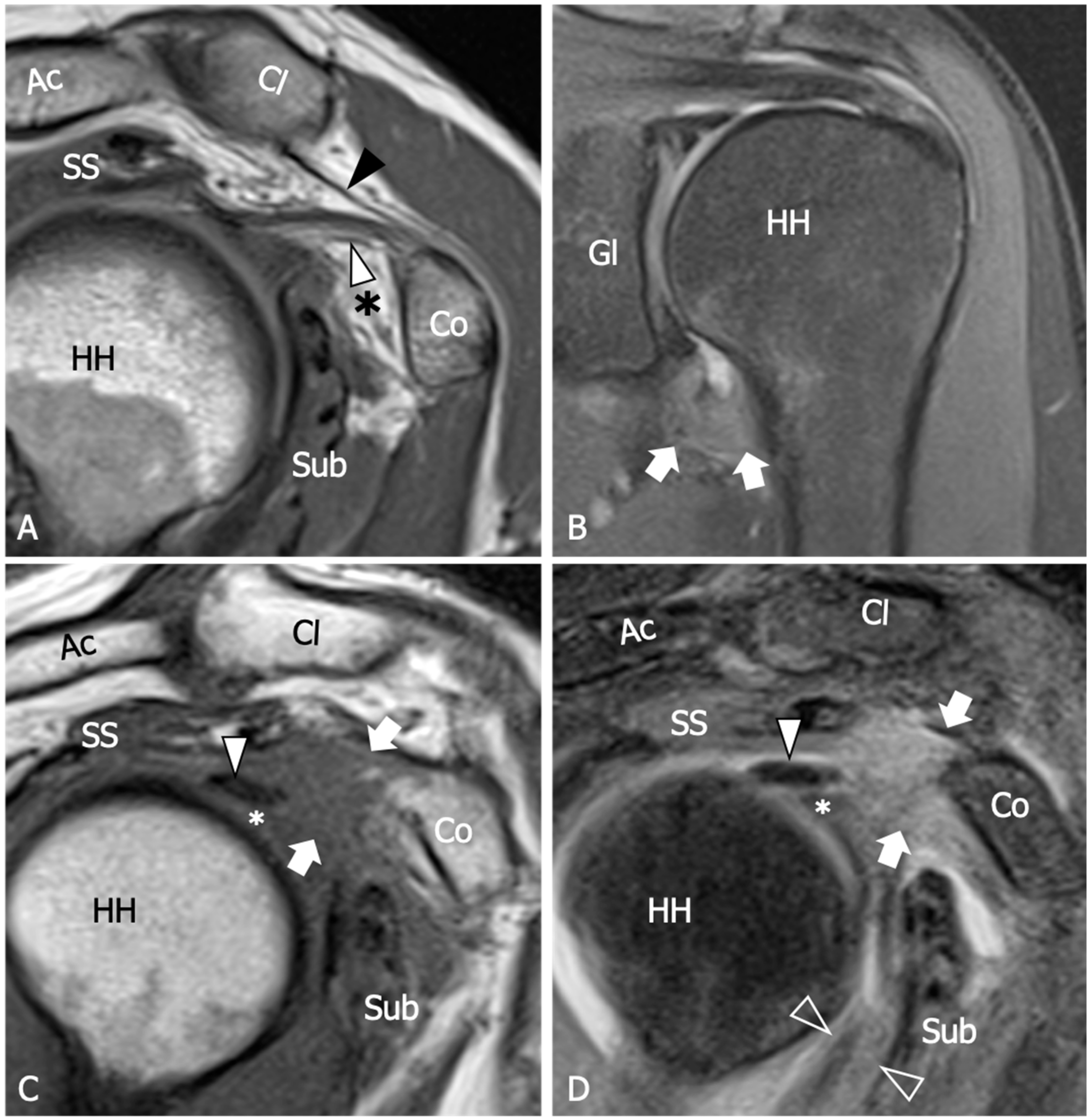
8. Magnetic Resonance Imaging
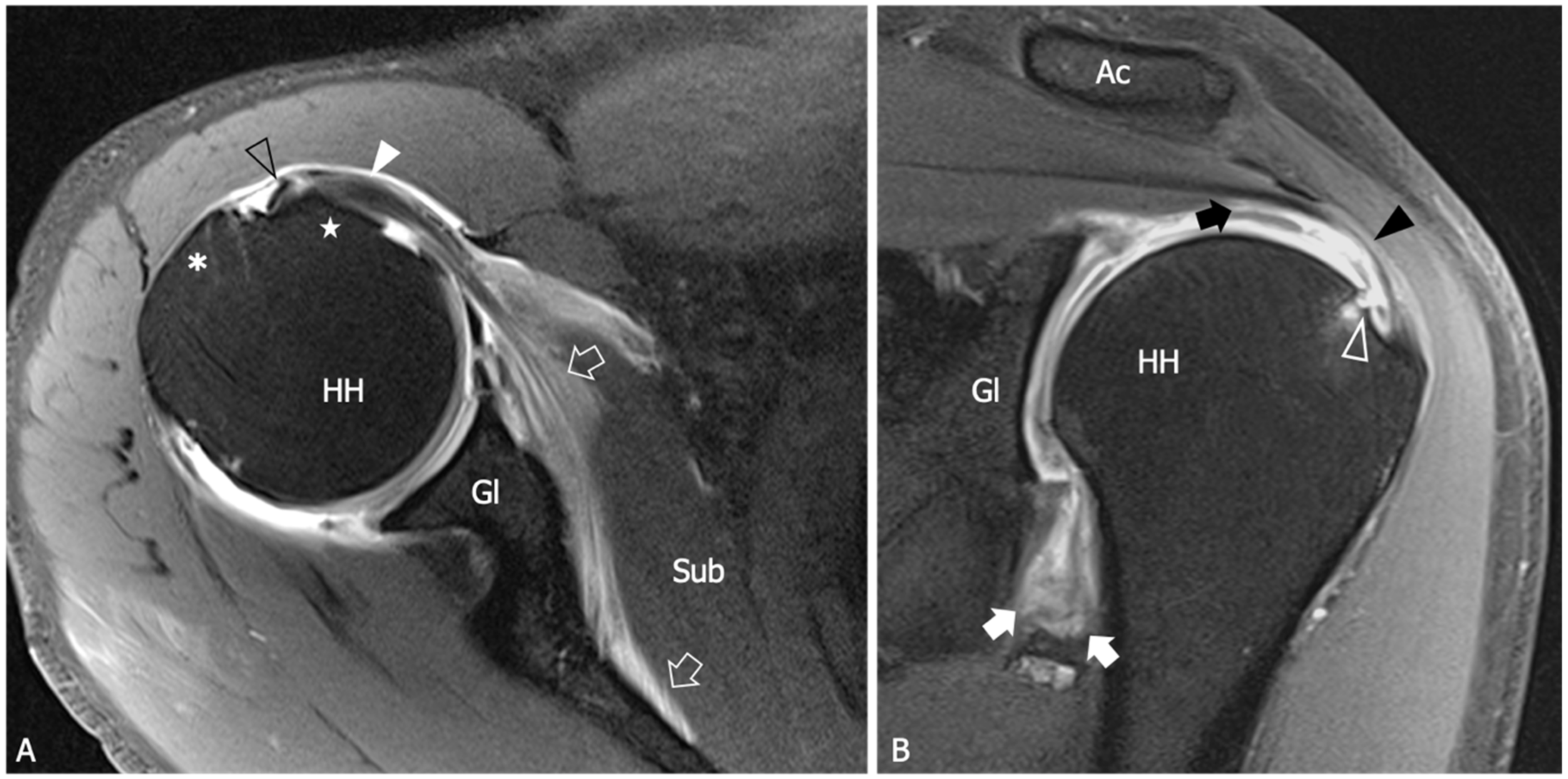
9. Ultrasound
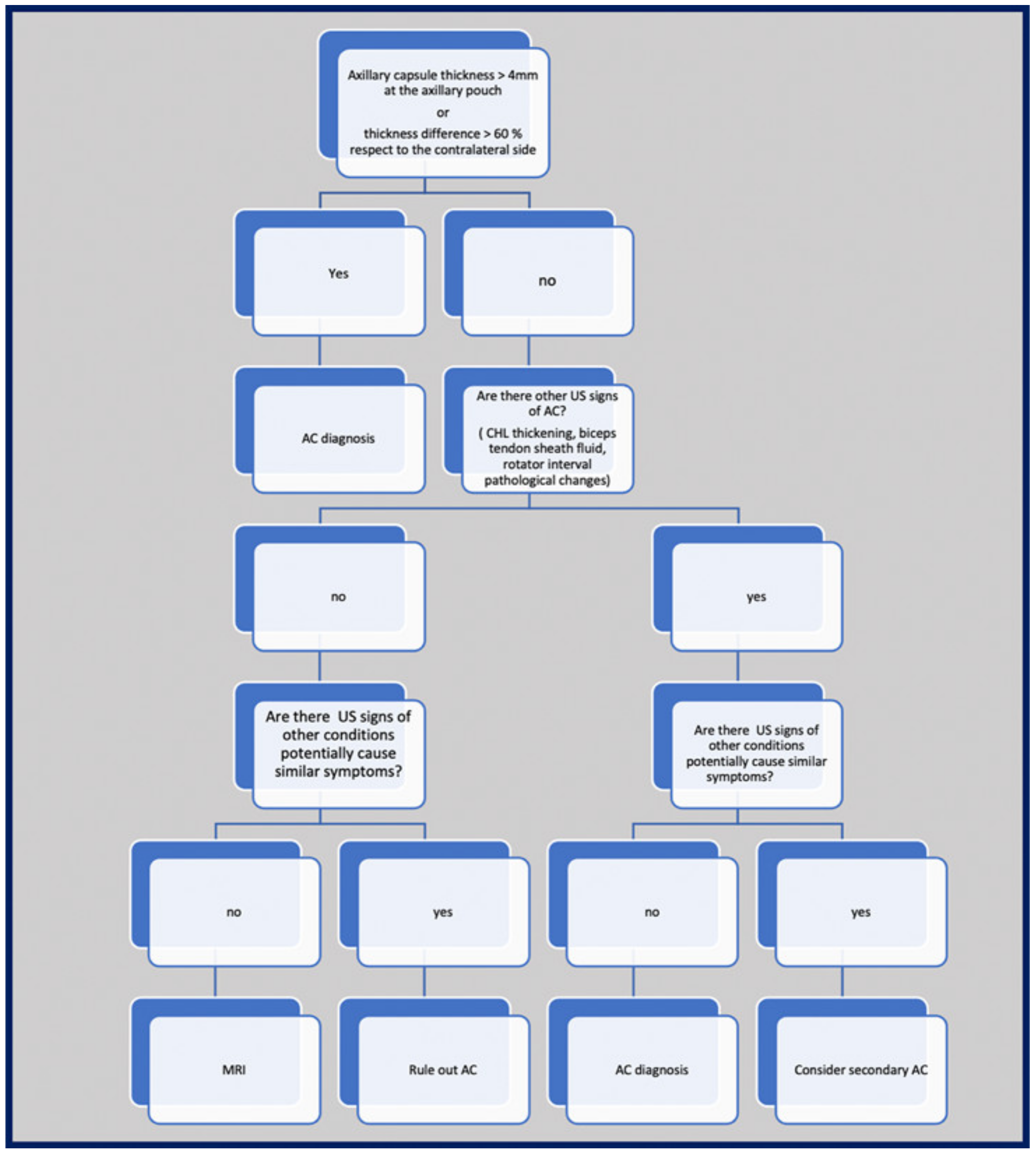
10. Suggested Imaging Protocol for Patients with Clinically Suspected AC
11. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zappia, M.; Di Pietto, F.; Aliprandi, A.; Pozza, S.; De Petro, P.; Muda, A.; Sconfienza, L.M. Multi-modal imaging of adhesive capsulitis of the shoulder. Insights Imaging 2016, 7, 365–371. [Google Scholar] [CrossRef]
- Codman, E.A. The Shoulder: Rupture of the Supraspinatus Tendon and Other Lesions in or about the Subacromial Bursa; Thomas Todd Co.: Boston, MA, USA, 1934; pp. 216–222. [Google Scholar]
- Neviaser, J.S. Adhesive capsulitis of the shoulder (the frozen shoulder). Med. Times 1962, 90, 783–807. [Google Scholar]
- Papalexis, N.; Parmeggiani, A.; Facchini, G.; Miceli, M.; Carbone, G.; Cavallo, M.; Spinnato, P. Current concepts in the diagnosis and treatment of adhesive capsulitis: Role of diagnostic imaging and ultrasound-guided interventional procedures. Radiol. Med. 2022, 127, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Fields, B.K.K.; Skalski, M.R.; Patel, D.B.; White, E.A.; Tomasian, A.; Gross, J.S.; Matcuk, G.R. Adhesive capsulitis: Review of imaging findings, pathophysiology, clinical presentation, and treatment options. Skelet. Radiol. 2019, 48, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, M.; Hafe, M.; Kamal, H. MR imaging biomarkers for evaluation of adhesive capsulitis of the shoulder. Additive value of anterior capsule abnormality as a reliable criterion for diagnosis of adhesive capsulitis: A cross sectional analytic study. Egypt. J. Radiol. Nucl. Med. 2022, 53, 232. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef]
- Fodor, D.; Rodriguez-Garcia, S.C.; Cantisani, V.; Hammer, H.B.; Hartung, W.; Klauser, A.; Martinoli, C.; Terslev, L.; Alfageme, F.; Bong, D.; et al. The EFSUMB Guidelines and Recommendations for Musculoskeletal Ultrasound—Part I: Extraarticular Pathologies. Ultraschall Med. 2022, 43, 34–57. [Google Scholar]
- Naredo, E.; Rodriguez-Garcia, S.C.; Terslev, L.; Martinoli, C.; Klauser, A.; Hartung, W.; Hameer, H.B.; Cantisani, V.; Zaottini, F.; Vlad, V.; et al. The EFSUMB Guidelines and Recommendations for Musculoskeletal Ultrasound—Part II: Joint Pathologies, Pediatric Applications, and Guided Procedures. Ultraschall Med. 2022, 43, 252–273. [Google Scholar]
- Wu, H.; Tian, H.; Dong, F.; Liang, W.; Song, D.; Zeng, J.; Ding, Z.; Shi, Y.; Luo, H.; Xu, J. The role of grey-scale ultrasound in the diagnosis of adhesive capsulitis of the shoulder: A systematic review and meta-analysis. Med. Ultrason. 2020, 22, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Al Khayyat, S.G.; Falsetti, P.; Conticini, E.; Fredian, B.; Galletti, S.; Stella, S.M. Adhesive capsulitis and ultrasound diagnosis, an inseparable pair: A novel review. J. Ultrasound. 2023, 26, 369–384. [Google Scholar] [CrossRef]
- Do, J.G.; Hwang, J.T.; Yoon, K.J.; Lee, Y.T. Correlation of Ultrasound Findings With Clinical Stages and Impairment in Adhesive Capsulitis of the Shoulder. Orthop. J. Sports Med. 2021, 9, 23259671211003675. [Google Scholar] [CrossRef]
- Ladd, L.M.; Crews, M.; Maertz, N.A. Glenohumeral Joint Instability: A Review of Anatomy, Clinical Presentation, and Imaging. Clin. Sports Med. 2021, 40, 585–599. [Google Scholar] [CrossRef]
- Momma, D.; Nimura, A.; Muro, S.; Fujishiro, H.; Miyamoto, T.; Funakoshi, T.; Mochizuki, T.; Iwasaki, N.; Akita, K. Anatomic analysis of the whole articular capsule of the shoulder joint, with reference to the capsular attachment and thickness. J. Exp. Orthop. 2018, 5, 16. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Fox, O.J.K.; Schär, M.O.; Chadbury, S.; Warren, R.F.; Rodeo, S.A. The glenohumeral ligaments: Superior, middle, and inferior: Anatomy, biomechanics, injury, and diagnosis. Clin. Anat. 2021, 34, 283–296. [Google Scholar] [CrossRef]
- Kask, K.; Põldoja, E.; Lont, T.; Norit, R.; Merila, M.; Busch, L.C.; Kolts, I. Anatomy of the superior glenohumeral ligament. J. Shoulder Elbow. Surg. 2010, 19, 908–916. [Google Scholar] [CrossRef]
- Williams, M.M.; Snyder, S.J.; Buford, D., Jr. The Buford complex—The “cord-like” middle glenohumeral ligament and absent anterosuperior labrum complex: A normal anatomic capsulolabral variant. Arthroscopy 1994, 10, 241–247. [Google Scholar] [CrossRef]
- Ide, J.; Maeda, S.; Takagi, K. Normal variations of the glenohumeral ligament complex: An anatomic study for arthroscopic Bankart repair. Arthroscopy 2004, 20, 164–168. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.J.; Neves, M.C.; Arnoczky, S.P.; Rozbruck, S.R.; Dicarlo, E.F.; Warren, R.F.; Schwartz, R.; Wickiewicz, T.L. The anatomy and histology of the inferior glenohumeral ligament complex of the shoulder. Am. J. Sports Med. 1990, 18, 449–456. [Google Scholar] [CrossRef]
- Bigliani, L.U.; Pollock, R.G.; Soslowsky, L.J. Tensile properties of the inferior glenohumeral ligament. J. Orthop. Res. 1992, 10, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Tang, K.L.; Chen, W.; Dong, S.; Jin, T.; Gong, J.; Li, J.; Wang, H.; Wang, J.; Xu, J. An anatomic and histologic study of the coracohumeral ligament. J. Shoulder Elbow. Surg. 2009, 18, 305–310. [Google Scholar] [CrossRef]
- Arai, R.; Nimura, A.; Yamaguchi, K.; Yoshimura, H.; Sugaya, H.; Saji, T.; Matsuda, S.; Akita, K. The anatomy of the coracohumeral ligament and its relation to the subscapularis muscle. J. Shoulder Elbow. Surg. 2014, 23, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Esch, J.C.; Jolson, R.S. Re: The rotator crescent and rotator cable: An anatomic description of the shoulder’s “suspension bridge”. Arthroscopy 2010, 26, 256–257. [Google Scholar]
- Pouliart, N.; Somers, K.; Eid, S.; Gagey, O. Variations in the superior capsuloligamentous complex and description of a new ligament. J. Shoulder Elbow. Surg. 2007, 16, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Nakata, W.; Katou, S.; Fujita, A.; Nakata, M.; Lefor, A.T.; Sugimoto, H. Biceps pulley: Normal anatomy and associated lesions at MR arthrography. Radiographics 2011, 31, 791–810. [Google Scholar] [CrossRef]
- Izumi, T.; Aoki, M.; Tanaka, Y.; Uchiyama, E.; Suzuki, D.; Miyamoto, S.; Fujimiya, M. Stretching positions for the coracohumeral ligament: Strain measurement during passive motion using fresh/frozen cadaver shoulders. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, J.; Nakagawa, Y.; Sakurai, G.; Tamai, S. Recalcitrant chronic adhesive capsulitis of the shoulder. Role of contracture of the coracohumeral ligament and rotator interval in pathogenesis and treatment. J. Bone Joint. Surg. Am. 1989, 71, 1511–1515. [Google Scholar] [CrossRef]
- Binder, A.I.; Bulgen, D.Y.; Hazleman, B.L.; Roberts, S. Frozen shoulder: A long-term prospective study. Ann. Rheum. Dis. 1984, 43, 361–364. [Google Scholar] [CrossRef]
- Le, H.V.; Lee, S.J.; Nazarian, A.; Rodriguez, E.K. Adhesive capsulitis of the shoulder: Review of pathophysiology and current clinical treatments. Shoulder Elbow. 2017, 9, 75–84. [Google Scholar] [CrossRef]
- Prodromidis, A.D.; Charalambous, C.P. Is There a Genetic Predisposition to Frozen Shoulder?: A Systematic Review and Meta-Analysis. JBJS Rev. 2016, 4, e4. [Google Scholar] [CrossRef]
- Rkkila, P.E.; Kantola, I.M.; Viikari, J.S.; Ronnemaa, T. Shoulder capsulitis in type I and II diabetic patients: Association with diabetic complications and related diseases. Ann. Rheum. Dis. 1996, 55, 907–914. [Google Scholar] [CrossRef]
- Bruckner, F.E.; Nye, C.J. A prospective study of adhesive capsulitis of the shoulder (‘frozen shoulder’) in a high risk population. Q. J. Med. 1981, 50, 191–204. [Google Scholar] [PubMed]
- Spinnato, P.; Masuzzo, O.; Tuè, G.; Tucci, F.; Bevere, A.; Vita, F.; Cavallo, M.; Marinelli, A.; Micelli, M. A Novel Ultrasound-Guided Interventional Procedure for the Combined Treatment of Rotator Cuff Calcific Tendinopathy Complicated with Adhesive Capsulitis: The ‘Rizzoli’ Technique. Acad. Radiol. 2023, 30, 2437–2438. [Google Scholar] [CrossRef]
- Merolla, G.; Singh, S.; Paladini, P.; Porcellini, G. Calcific tendinitis of the rotator cuff: State of the art in diagnosis and treatment. J. Orthop. Traumatol. 2016, 17, 7–14. [Google Scholar] [CrossRef]
- Merolla, G.; Bhat, M.G.; Paladini, P.; Porcellini, G. Complications of calcific tendinitis of the shoulder: A concise review. J. Orthop. Traumatol. 2015, 16, 175–183. [Google Scholar] [CrossRef]
- Neviaser, A.S.; Neviaser, R.J. Adhesive capsulitis of the shoulder. J. Am. Acad. Orthop. Surg. 2011, 19, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Lubis, A.M.; Lubis, V.K. Matrix metalloproteinase, tissue inhibitor of metalloproteinase and transforming growth factor-beta 1 in frozen shoulder, and their changes as response to intensive stretching and supervised neglect exercise. J. Orthop. Sci. 2013, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bunker, T.D.; Reilly, J.; Baird, K.S.; Hamblen, D.L. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J. Bone Joint. Surg. Br. 2000, 82, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Peng, C.; Liu, C.; Zhang, N.; Yue, S. Gene polymorphism of IL-6 and MMP-3 decreases passive range of motion after rotator cuff repair. Int. J. Clin. Exp. Pathol. 2015, 8, 5709–5714. [Google Scholar]
- Kim, Y.S.; Kim, J.M.; Lee, Y.G.; Hong, O.K.; Kwon, H.S.; Ji, J.H. Intercellular adhesion molecule-1 (ICAM-1, CD54) is increased in adhesive capsulitis. J. Bone Joint. Surg. Am. 2013, 95, e181–e188. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, Z.; Li, J. Pathophysiology of adhesive capsulitis of shoulder and the physiological effects of hyaluronan. Eur. J. Inflamm. 2017, 15, 239–243. [Google Scholar]
- Date, A.; Rahman, L. Frozen shoulder: Overview of clinical presentation and review of the current evidence base for management strategies. Future Sci. OA 2020, 6, FSO647. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Levine, W.N.; Deo, K.; Kesting, R.S.; Mercer, E.A.; Schram, G.A.; Strang, B.L. Natural history of frozen shoulder: Fact or fiction? A systematic review. Physiotherapy 2017, 103, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hand, C.; Clipsham, K.; Rees, J.L.; Carr, A.J. Long-term outcome of frozen shoulder. J. Shoulder Elbow. Surg. 2008, 17, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.J. Frozen shoulder: Diagnosis and management. J. Am. Acad. Orthop. Surg. 1997, 5, 130–140. [Google Scholar] [CrossRef][Green Version]
- De Winter, A.F.; Jans, M.P.; Scholten, R.J.; Devillé, W.; Schaardenburg, D.; Bouter, L.M. Diagnostic classification of shoulder disorders: Interobserver agreement and determinants of disagreement. Ann. Rheum. Dis. 1999, 58, 272–277. [Google Scholar] [CrossRef]
- Schellingerhout, J.M.; Verhagen, A.P.; Thomas, S.; Koes, B.W. Shoulder pain: Diagnosis and management in primary care. BMJ 2005, 331, 1124–1128. [Google Scholar]
- Walmsley, S.; Rivett, D.A.; Osmotherly, P.G. Adhesive capsulitis: Establishing consensus on clinical identifiers for stage 1 using the DELPHI technique. Phys. Ther. 2009, 89, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Schellingerhout, J.M.; Verhagen, A.P.; Thomas, S.; Koes, B.W. Lack of uniformity in diagnostic labeling of shoulder pain: Time for a different approach. Man. Ther. 2008, 13, 478–483. [Google Scholar] [CrossRef]
- Rangan, A.; Brealey, S.D.; Keding, A.; Corbacho, B.; Northgraves, M.; Kottam, L.; Goodchild, L.; Srikesavan, C.; Rex, S.; Charalambous, P. UK FROST Study Group. Management of adults with primary frozen shoulder in secondary care (UK FROST): A multicentre, pragmatic, three-arm, superiority randomised clinical trial. Lancet 2020, 396, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Neviaser, J.S. Arthrography of the shoulder joint: Study of the findings in adhesive capsulitis of the shoulder. J. Bone Joint. Surg. Am. 1962, 44, 1321–1359. [Google Scholar] [CrossRef]
- Papalexis, N.; Ponti, F.; Rinaldi, R.; Pet, G.; Bruno, R.; Miceli, M.; Battaglia, M.; Marinelli, A.; Spinnato, P. Ultrasound-guided Treatments for the Painful Shoulder. Curr. Med. Imaging 2022, 18, 693–700. [Google Scholar] [CrossRef]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Park, J.S.; Ryu, K.N. Systematic review and meta-analysis of magnetic resonance imaging features for diagnosis of adhesive capsulitis of the shoulder. Eur. Radiol. 2019, 29, 566–577. [Google Scholar] [CrossRef]
- Mengiardi, B.; Pfirrmann, C.W.A.; Gerber, C.; Hodler, J.; Zanetti, M. Frozen shoulder: MR arthrographic findings. Radiology 2004, 233, 486–492. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, D.H.; Yi, J.; Kim, D.H.; Cho, C.H. Determination of magnetic resonance imaging criteria for diagnosis of adhesive capsulitis. Rheumatol. Int. 2019, 39, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sofka, C.M.; Ciavarra, G.A.; Hannafin, J.A.; Cordasco, F.A.; Potter, H.G. Magnetic resonance imaging of adhesive capsulitis: Correlation with clinical staging. HSS J. 2008, 4, 164–169. [Google Scholar] [CrossRef]
- Gondim Teixeira, P.A.; Balaj, C.; Chanson, A.; Lecocq, S.; Louis, M.; Blum, A. Adhesive capsulitis of the shoulder: Value of inferior glenohumeral ligament signal changes on T2-weighted fat-saturated images. AJR Am. J. Roentgenol. 2012, 198, 589–596. [Google Scholar] [CrossRef]
- Emig, E.W.; Schweitzer, M.E.; Karasick, D.; Lubowitz, J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am. J. Roentgenol. 1995, 164, 1457–1459. [Google Scholar] [CrossRef]
- Ahn, K.S.; Kang, C.H.; Oh, Y.W.; Jeong, W.K. Correlation between magnetic resonance imaging and clinical impairment in patients with adhesive capsulitis. Skelet. Radiol. 2012, 41, 1301–1308. [Google Scholar] [CrossRef]
- Erber, B.; Hesse, N.; Goller, S.; Gilbert, F.; Ricke, J.; Glaser, C.; Heuck, A. Diagnostic performance and interreader agreement of individual and combined non-enhanced and contrast-enhanced MR imaging parameters in adhesive capsulitis of the shoulder. Skelet. Radiol. 2023, 10, 1007. [Google Scholar] [CrossRef]
- Michelin, P.; Delarue, Y.; Duparc, F.; Dacher, J.N. Thickening of the inferior glenohumeral capsule: An ultrasound sign for shoulder capsular contracture. Eur. Radiol. 2013, 23, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, C.H.; Sung, D.H. Ultrasound measurements of axillary recess capsule thickness in unilateral frozen shoulder: Study of correlation with MRI measurements. Skelet. Radiol. 2018, 47, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.M.; Gualtierotti, R.; Ciampi, B.; Trentanni, C.; Sconfienza, L.M.; Del Chiaro, A.; Pacini, P.; Miccoli, M.; Galletti, S. Ultrasound Features of Adhesive Capsulitis. Rheumatol. Ther. 2022, 9, 481–495. [Google Scholar] [CrossRef]
- Homsi, C.; Bordalo-Rodrigues, M.; da Silva, J.J.; Stump, X.M.G.R.G. Ultrasound in adhesive capsulitis of the shoulder: Is assessment of the coracohumeral ligament a valuable diagnostic tool? Skelet. Radiol. 2006, 35, 673–678. [Google Scholar] [CrossRef]
- Tandon, A.; Dewan, S.; Bhatt, S.; Jain, A.K.; Kumari, R. Sonography in diagnosis of adhesive capsulitis of the shoulder: A case-control study. J. Ultrasound. 2017, 20, 227–236. [Google Scholar] [CrossRef]
- Lee, B.C.; Yeo, S.M.; Do, J.G.; Hwang, J.H. Sequential Ultrasound Assessment of Peri-Articular Soft Tissue in Adhesive Capsulitis of the Shoulder: Correlations with Clinical Impairments-Sequential Ultrasound in Adhesive Capsulitis. Diagnostics 2022, 12, 2231. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.J.; Kim, S.E.; Bae, S.E.; Lee, K.Y.; Park, K.S.; Kim, Y.S. Evaluation of the Effusion within Biceps Long Head Tendon Sheath Using Ultrasonography. Clin. Orthop. Surg. 2015, 7, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Yi, J.H.; Lee, J.; Bae, J.H.; Lim, J.K.; Yoon, J.P.; Jeon, I.H. Limited subacromial gliding of the supraspinatus tendon during dynamic ultrasonography can predict a decrease in capacity and MR arthrographic features of the shoulder joint. Eur. Radiol. 2012, 22, 2365–2370. [Google Scholar] [CrossRef]
- Lee, J.C.; Sykes, C.; Saifuddin, A.; Connel, D. Adhesive capsulitis: Sonographic changes in the rotator cuff interval with arthroscopic correlation. Skelet. Radiol. 2005, 34, 522–527. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, Y.H.; Oh, S.; Kim, H.J.; Chai, J.W. Ultrasound Microflow Imaging Technology for Diagnosis of Adhesive Capsulitis of the Shoulder. J. Ultrasound. Med. 2020, 39, 967–976. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Z.; Xuanyan, G.; Li, T.; Li, J.; Yin, L.; Lu, M. Adhesive Capsulitis of the Shoulder: Evaluation with US-Arthrography Using a Sonographic Contrast Agent. Sci. Rep. 2017, 7, 5551. [Google Scholar] [CrossRef] [PubMed]
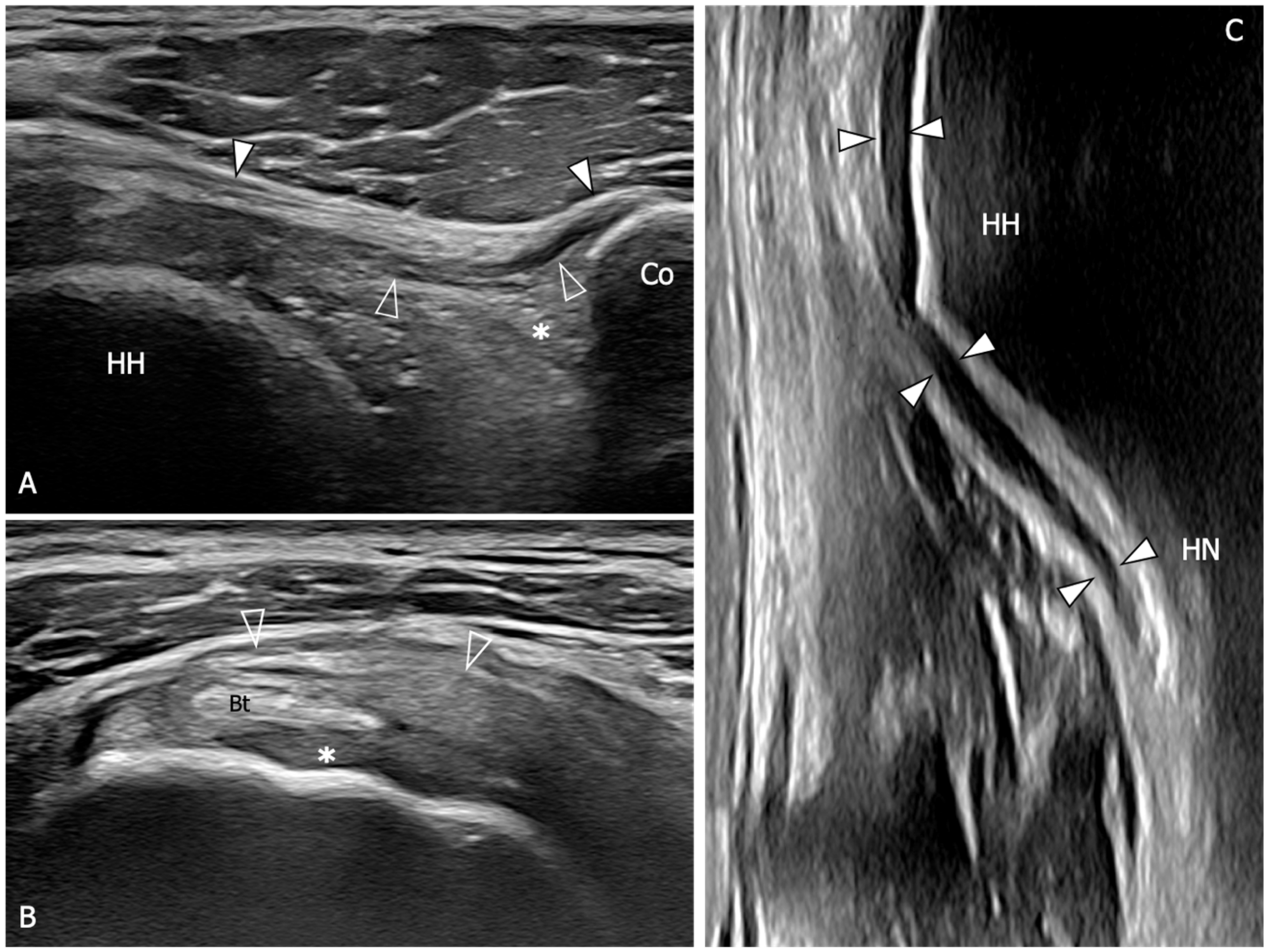
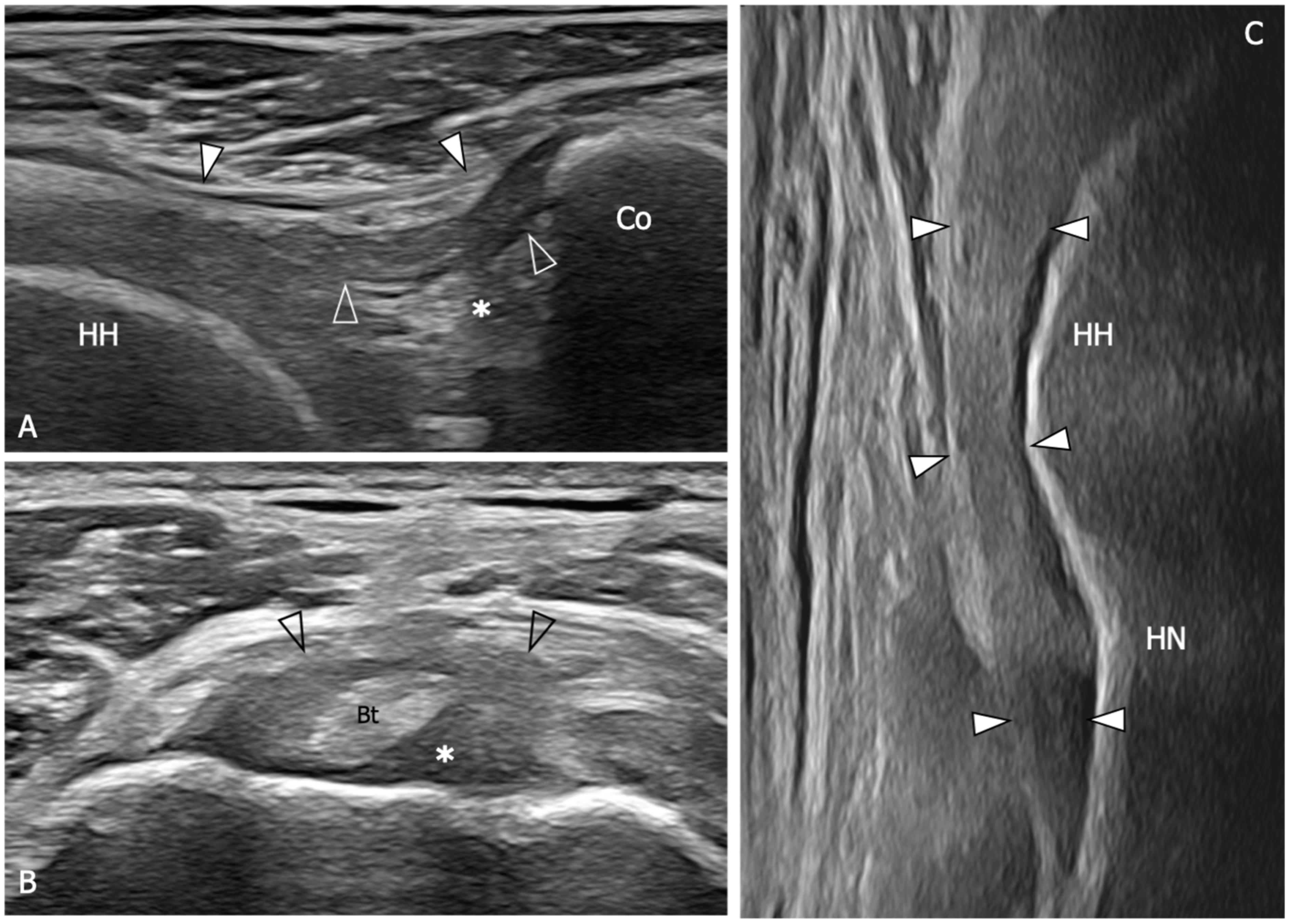
| References | |
|---|---|
| Thickening of the coracohumeral ligament, fat obliteration of the rotator interval, hyperintensity and thickening of the inferior glenohumeral ligament, and contrast enhancement of the axillary joint capsule and the rotator interval are the most accurate signs of AC. The sensitivity and specificity of inferior glenohumeral thickening detected on conventional MRI are not significantly different from those detected on direct MR Arthrogram: consequently, the non-arthrogram MRI is recommended for AC diagnosis. | [53] |
| The rotator interval capsule thickness ≥7 mm has a specificity of 86% and a sensitivity of 64% for AC diagnosis. A coracohumeral ligament thickness ≥4 mm has high specificity (95%) but lower sensitivity (59%) for AC. Obliteration of the triangular fat pad inferior to the coracohumeral ligament has high specificity (100%) and poor sensitivity (32%). | [54] |
| Thickening of the rotator interval over 6 mm on sagittal oblique proton-density images may correlate with the patient’s range of rotational motion. An axillary recess capsule thickness of more than 4.5 mm measured on T1 oblique coronal images demonstrated the greatest diagnostic accuracy for AC diagnosis, with a sensitivity, specificity, and overall accuracy of 91%, 90%, and 90%, respectively. | [55] |
| Obliteration of the subcoracoid fat triangle has been more frequently observed in early stages of AC. Capsule thickness and hyperintensity on proton density sequence correlate with clinical stages. | [56] |
| Hyperintensity in the axillary pouch/inferior glenohumeral ligament complex on MRI using non-arthrography T2-weighted fat-suppressed sequences demonstrated high sensitivity (85.3–88.2%) and specificity (88.2%) and low variability among different observers with a kappa value of 0.85. | [57] |
| An axillary recess capsule thicker than 4 mm on T1 oblique coronal MR images suggests a diagnosis of AC with a sensitivity of 70% and a specificity of 95%. | [58] |
| A positive linear correlation is demonstrated between the grade of axillary recess capsule enhancement, the thickness of the joint capsule, and the intensity of pain in individuals with AC. No association was observed between the aforementioned parameters and the severity of range of motion limitation. | [59] |
| No differences in the accuracy of AC diagnosis emerged between conventional MRI and gadolinium-enhanced MRI despite the intravenous administration of contrast agent demonstrated to have some effects in increasing the reader’s confidence in measuring the joint capsule. | [60] |
| References | |
|---|---|
| An 88% sensitivity (95%CI: 74–95) and a 96% specificity (95%CI: 88–99) are demonstrated when US detect inferior capsule and coracohumeral thickening, rotator interval abnormality, and restricted range of motion. | [10] |
| The mean thickness of the axillary pouch capsule in patients with AC measured with US is 4 mm versus 1.3 mm in asymptomatic controls. | [61] |
| The axillary capsule thickness measured at its widest portion is 4.4 mm in the affected shoulder and 2.2 mm in the unaffected shoulder (p < 0.001). US measurements demonstrated good correlation with MR (p < 0.001, r = 0.83). | [62] |
| A cutoff value of 4 mm for axillary pouch thickness yielded a sensitivity of 93.8% and a specificity of 98% in diagnosing AC. A difference of 60% between the affected and the unaffected side may help in disclosing this condition also in patients with suggestive symptoms but axillary recess thickness less than 4 mm. | [63] |
| The average CHL thickness measure both in short and long axis was 3 mm in shoulders affected by AC. Painful shoulders without AC diagnosis exhibited an average coracohumeral ligament thickness of 1.39 mm; asymptomatic shoulders had an average coracohumeral ligament thickness of 1.34 mm. | [64] |
| Patients with AC exhibited a significantly thicker coracohumeral ligament (1.2 mm) compared to both subjects with painful shoulders (0.54 mm) and healthy volunteers (0.4 mm). | [65] |
| Patients with AC demonstrated a higher prevalence of effusion in the long head of biceps tendon sheath in the affected shoulder with respect to the contralateral side. | [66] |
| A greater amount of the long head of the biceps sheath effusion was found in patients with AC compared to patients with other causes of a painful shoulder. A negative correlation between the amount of effusion was found within the long head of the biceps tendon sheath and the glenohumeral range of motion. | [67] |
| Limitation in subacromial gliding of the supraspinatus tendon is found in 70.1% of patients with AC. The limitation of the supraspinatus tendon gliding beneath the acromion was demonstrated to be inversely correlated with the maximum amount of intra-articular injection for MRA. | [68] |
| US evidence of hypervascular soft tissue within the rotator interval demonstrated high sensitivity (97%) and specificity (100%) for the diagnosis of AC. | [69] |
| Microvascular analysis of the subcoracoid triangle demonstrated a sensitivity of 76.92% and a specificity of 91.43% in diagnosing AC. The cutoff for the area of vascular flow detected during the examination of the subcoracoid triangle was set at 1.31 mm2. | [70] |
| The injected volume of sonographic contrast did not differ significantly between healthy controls and patients with AC (19.0 ± 0.22 mL vs. 18.3 ± 0.29 mL, p = 0.07). However, the latter had a significantly smaller axillary recess volume compared to controls and showed more frequent filling defects in the joint cavity and synovitis-like abnormalities (91.1% vs. 13.3% and 75.6% vs. 22.2%, respectively). | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picasso, R.; Pistoia, F.; Zaottini, F.; Marcenaro, G.; Miguel-Pérez, M.; Tagliafico, A.S.; Martinoli, C. Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation. Diagnostics 2023, 13, 3410. https://doi.org/10.3390/diagnostics13223410
Picasso R, Pistoia F, Zaottini F, Marcenaro G, Miguel-Pérez M, Tagliafico AS, Martinoli C. Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation. Diagnostics. 2023; 13(22):3410. https://doi.org/10.3390/diagnostics13223410
Chicago/Turabian StylePicasso, Riccardo, Federico Pistoia, Federico Zaottini, Giovanni Marcenaro, Maribel Miguel-Pérez, Alberto Stefano Tagliafico, and Carlo Martinoli. 2023. "Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation" Diagnostics 13, no. 22: 3410. https://doi.org/10.3390/diagnostics13223410
APA StylePicasso, R., Pistoia, F., Zaottini, F., Marcenaro, G., Miguel-Pérez, M., Tagliafico, A. S., & Martinoli, C. (2023). Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation. Diagnostics, 13(22), 3410. https://doi.org/10.3390/diagnostics13223410








