Proposal of Modified Lung-RADS in Assessing Pulmonary Nodules of Patients with Previous Malignancies: A Primary Study
Abstract
:1. Introduction
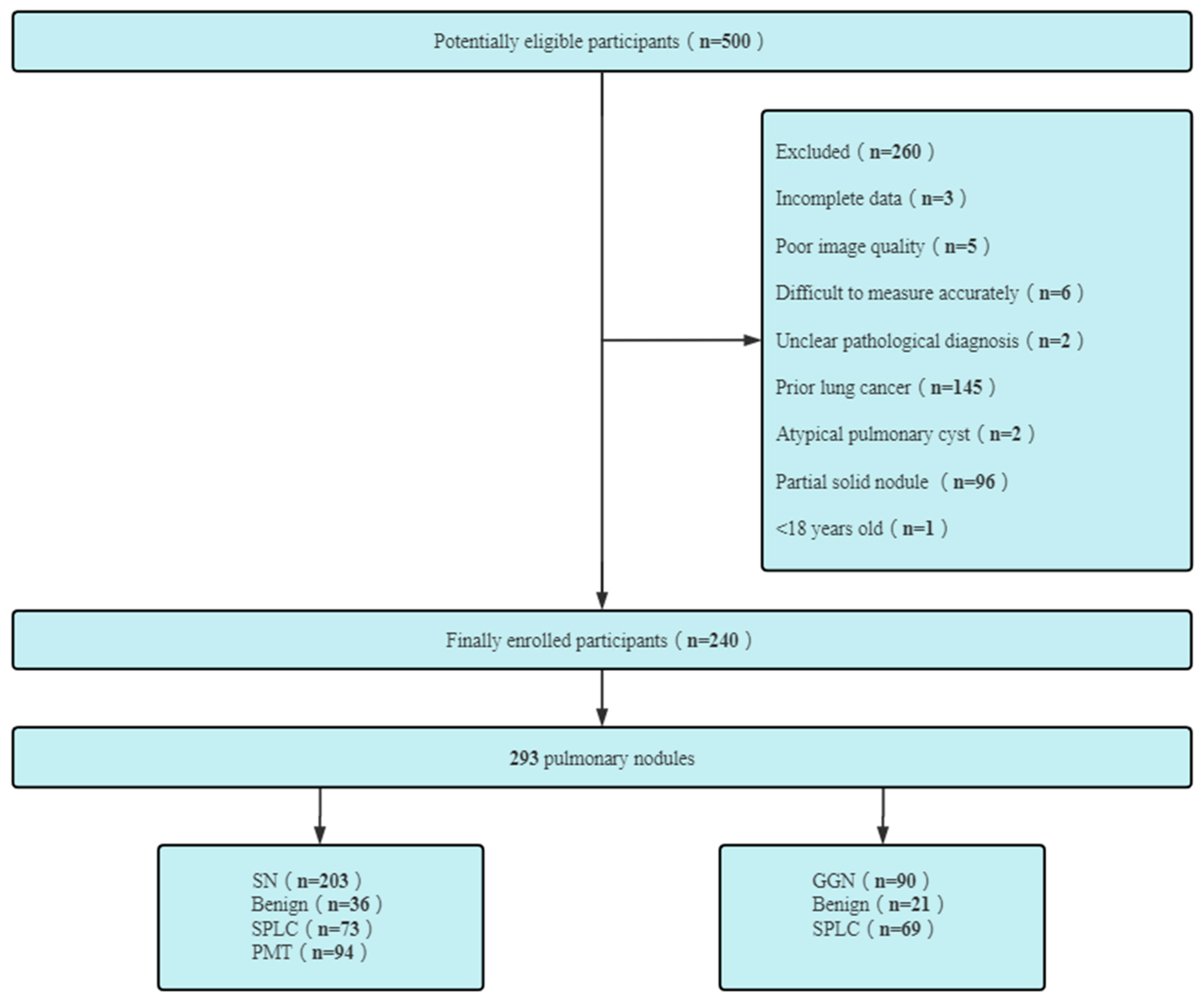
2. Patients and Methods
2.1. Patients
2.2. CT Protocol
2.3. Image Analysis
2.3.1. Observation and Measurement of Nodules
2.3.2. Category of Pulmonary Nodules
2.4. Statisticalanalysis
3. Results
3.1. General Data Statistics of Patients
3.1.1. Consistency between Observers
3.1.2. Comparison of Diagnostic Accuracy between Original and Modified Versions for pGGN
3.1.3. Difference of Diagnostic Performance of SNs between Original and Modified Versions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Msc, M.L.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Hsieh, M.-S.; Liao, H.-C.; Chen, P.-H.; Chiang, X.-H.; Tsou, K.-C.; Tsai, T.-M.; Chuang, J.-H.; Lin, M.-W.; Hsu, H.-H.; et al. Previous Extrapulmonary Malignancies Impact Outcomes in Patients With Surgically Resected Lung Cancer. Front. Surg. 2021, 8, 747249. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Feng, Y.; Zeng, S.; Zhong, M. Problems to affect long-term survival for breast cancer patients: An observational study of subsequent lung/bronchus malignancies. Medicine 2018, 97, e12603. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, S.; Lyons, G.; Colt, H.; Chimondeguy, D.; Silva, C. Lung cancer as a second primary malignancy: Increasing prevalence and its influence on survival. Ann. Surg. Oncol. 2009, 16, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Deng, L.; Harðardottír, H.; Song, H.; Xiao, Z.; Jiang, C.; Wang, Q.; Valdimarsdóttir, U.; Cheng, H.; Loo, B.W.; Lu, D. Mortality of lung cancer as a second primary malignancy: A population-based cohort study. Cancer Med. 2019, 8, 3269–3277. [Google Scholar] [CrossRef] [Green Version]
- Laccetti, A.L.; Pruitt, S.L.; Xuan, L.; Halm, E.A.; Gerber, D.E. Prior cancer does not adversely affect survival in locally advanced lung cancer: A national SEER-medicare analysis. Lung Cancer 2016, 98, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung cancer LDCT screening and mortality reduction-evidence, pitfalls and future perspectives. Nat. Rev. Clin. Oncol. 2021, 18, 135–151. [Google Scholar] [CrossRef]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.-U.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Qu, G.; Wang, L.; Wu, W.; Sun, Y. Low-dose CT screening can reduce cancer mortality: A meta-analysis. Rev. Assoc. Med. Bras. 2019, 65, 1508–1514. [Google Scholar] [CrossRef]
- American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS) 2022 Assessment Categories. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf (accessed on 21 November 2022).
- Hammer, M.M.; Byrne, S.C. Cancer Risk in Nodules Detected at Follow-Up Lung Cancer Screening CT. AJR Am. J. Roentgenol. 2022, 218, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Kanne, J.P.; Broderick, L.S.; Kazerooni, E.A.; Meyer, C.A. Lung-RADS: Pushing the Limits. Radiographics 2017, 37, 1975–1993. [Google Scholar] [CrossRef]
- White, C.S.; Dharaiya, E.; Dalal, S.; Chen, R.; Haramati, L.B. Vancouver Risk Calculator Compared with ACR Lung-RADS in Predicting Malignancy: Analysis of the National Lung Screening Trial. Radiology 2019, 291, 205–211. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Lam, L. Evaluating the Patient with a Pulmonary Nodule: A Review. JAMA 2022, 327, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.M.; Palazzo, L.L.; Kong, C.Y.; Hunsaker, A.R. Cancer Risk in Subsolid Nodules in the National Lung Screening Trial. Radiology 2019, 293, 441–448. [Google Scholar] [CrossRef]
- Kim, H.; Goo, J.M.; Kim, T.J.; Kim, H.Y.; Gu, G.; Gil, B.; Kim, W.; Park, S.Y.; Park, J.; Park, J.; et al. Effectiveness of radiologist training in improving reader agreement for Lung-RADS 4X categorization. Eur. Radiol. 2021, 31, 8147–8159. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Jacobs, C.; Scholten, E.T.; Goo, J.M.; Prosch, H.; Sverzellati, N.; Ciompi, F.; Mets, O.M.; Gerke, P.K.; Prokop, M.; et al. Lung-RADS Category 4X: Does It Improve Prediction of Malignancy in Subsolid Nodules? Radiology 2017, 284, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Xu, X.-Q.; Xu, H.; Yuan, M.; Zhang, W.; Shi, Z.-F.; Yu, T.-F. Using the CT features to differentiate invasive pulmonary adenocarcinoma from pre-invasive lesion appearing as pure or mixed ground-glass nodules. Br. J. Radiol. 2015, 88, 20140811. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.-R.; Han, R.; Xue, F.; Xu, L.; Ren, W.-G.; Li, M.; Feng, Z.; Hu, B.-C.; Peng, Z.-M. Expression and clinical significance of CD31, CD34, and CD105 in pulmonary ground glass nodules with different vascular manifestations on CT. Front. Oncol. 2022, 12, 956451. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Ge, X.; Zheng, X.; Ren, Q.; Chen, Y.; Lv, F.; Hua, Y. Multi-detector spiral CT study of the relationships between pulmonary ground-glass nodules and blood vessels. Eur. Radiol. 2013, 23, 3271–3277. [Google Scholar] [CrossRef]
- Meng, Q.; Ren, P.; Gao, P.; Dou, X.; Chen, X.; Guo, L.; Song, Y. Effectiveness and Feasibility of Complementary Lung-RADS version 1.1 in Risk Stratification for pGGN in LDCT Lung Cancer Screening in a Chinese Population. Cancer Manag. Res. 2020, 12, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, M.; Romano, J.; Ara Jo, D.; Pereira, J.M.; Ramos, I.; Hespanhol, V. Predicting lung nodules malignancy. Pulmonology 2022, 28, 454–460. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B. Application of CT Postprocessing Reconstruction Technique in Differential Diagnosis of Benign and Malignant Solitary Pulmonary Nodules and Analysis of Risk Factors. Comput. Math. Methods Med. 2022, 2022, 9739047. [Google Scholar] [CrossRef]
- Bankier, A.A.; MacMahon, H.; Goo, J.M.; Rubin, G.D.; Schaefer-Prokop, C.M.; Naidich, D.P. Recommendations for Measuring Pulmonary Nodules at CT: A Statement from the Fleischner Society. Radiology 2017, 285, 584–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinmuth, N.; Stumpf, P.; Stumpf, A.; Muley, T.; Kobinger, S.; Hoffmann, H.; Herth, F.; Schnabel, P.A.; Bischoff, H.; Thomas, M. Characteristics of lung cancer after a previous malignancy. Respir. Med. 2014, 108, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.X.; Nelson, R.A.; Kim, J.Y.; Raz, D.J. Non-Small Cell Lung Cancer as a Second Primary Among Patients With Previous Malignancy: Who Is at Risk? Clin. Lung Cancer 2017, 18, 543–550.e3. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, P.; Ventura, L.; Aprile, V.; Cattoni, M.A.; Nachira, D.; Lococo, F.; Perez, M.R.; Guerrera, F.; Minervini, F.; Gnetti, L.; et al. Pathological and clinical features of multiple cancers and lung adenocarcinoma: A multicentre study. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac047. [Google Scholar] [CrossRef]
- Chinese Alliance Against Lung Cancer; Chinese Medical Association of Respiratory Disease Branch Lung Cancer Study Group; Chinese Medical Doctor Association of Respiratory Doctor Branch Lung Cancer Working Committee. Chinese expert consensus on screening and management of lung cancer. Int. J. Respir. 2019, 39, 1604–1615. [Google Scholar]
- McKee, B.J.; Hashim, J.A.; French, R.J.; McKee, A.B.; Hesketh, P.J.; Lamb, C.R.; Williamson, C.; Flacke, S.; Wald, C. Experience with a CT screening program for individuals at high risk for developing lung cancer. J. Am. Coll. Radiol. 2015, 12, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Halpenny, D.F.; Cunningham, J.D.; Long, N.M.; Sosa, R.E.; Ginsberg, M.S. Patients with a Previous History of Malignancy Undergoing Lung Cancer Screening: Clinical Characteristics and Radiologic Findings. J. Thorac. Oncol. 2016, 11, 1447–1452. [Google Scholar] [CrossRef] [Green Version]
- Copur, M.S.; Manapuram, S. Multiple Primary Tumors over a Lifetime. Oncology 2019, 33, 629384. [Google Scholar]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, E.; Halpenny, D.F.; Ginsberg, M.S. Lung cancer screening in patients with previous malignancy: Is this cohort at increased risk for malignancy? Eur. Radiol. 2021, 31, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Gould, M.K.; Nair, V.S. A Comparison of the PanCan Model and Lung-RADS to Assess Cancer Probability among People with Screening-Detected, Solid Lung Nodules. Chest 2021, 159, 1273–1282. [Google Scholar] [CrossRef]
- Dyer, S.C.; Bartholmai, B.J.; Koo, C.W. Implications of the updated Lung CT Screening Reporting and Data System (Lung-RADS version 1.1) for lung cancer screening. J. Thorac. Dis. 2020, 12, 6966–6977. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Mazzone, P.J.; Naidich, D.P.; Bach, P.B. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), e78S–e92S. [Google Scholar] [CrossRef] [Green Version]



| Category | Solid Nodule (SN) | Pure Ground Glass Nodule (pGGN) | ||
|---|---|---|---|---|
| OV | MV | OV | MV | |
| 2 | <6 mm at baseline OR new < 4 mm Juxtapleural nodule: <10 mm mean diameter at baseline or new AND Solid; smooth margins; oval, lentiform, or triangular shape | <6 mm at baseline OR new < 4 mm; Category 3 or 4 nodules without spiculation and with additional features (≥1 sign) that indicate the suspicion of benign disease; Category 3 nodules unchanged for ≥6 months Juxtapleural nodule: <10 mm mean diameter at baseline or new AND solid; smooth margins; oval, lentiform, or triangular shape | <30 mm at baseline, new, or growing OR ≥30 mm stable or slow-growing | <30 mm and type I GVR; Category 3 or 4 nodules stable ≥ 5 years; Category 3 or 4 nodules decreased in size and there was an absence of solid components OR this was resolved on a follow-up |
| 3 | ≥6 to <8 mm at baseline; new 4 mm to <6 mm; Category 4A nodule that is stable or decreased in size at 3-month follow-up CT (excluding airway nodules) | ≥6 to <8 mm at baseline; new 4 mm to <6 mm Category 4A nodules unchanged for ≥3 months | ≥30 mm at baseline or new | ≥30 mm and type I GVR; any size with type II GVR |
| 4A | ≥8 to <15 mm at baseline OR Growing < 8 mm OR New 6 to <8 mm | ≥8 to <15 mm at baseline; growing < 8 mm; new 6 to <8 mm | - | any size with type III GVR |
| 4B | ≥15 mm at baseline OR New or growing ≥ 8 mm | ≥15 mm at baseline; new or growing, and ≥8 mm | - | any size with type IV GVR |
| 4X | Category 3 or 4 nodules with additional features or imaging findings that increase suspicion for lung cancer | Category 3 or 4 nodules with additional features or imaging findings that increase the suspicion of malignancy and lack benign signs; Category 3 or 4 nodules with spiculation sign with or without benign signs | Category 3 or 4 nodules with additional features or imaging findings that increase suspicion for lung cancer | Category 3 or 4 nodules with additional features or imaging findings that increases the suspicion of malignancy |
| OV | MV | χ2 | p | |
|---|---|---|---|---|
| TP | 0 | 67 | - | - |
| FP | 0 | 5 | - | - |
| TN | 21 | 16 | - | - |
| FN | 69 | 2 | - | - |
| Sensitivity (%) | 0 | 97.10 | - | - |
| Specificity (%) | 100 | 76.19 | - | - |
| AR (%) | 23.33 | 92.22 | 87.54 | <0.001 # |
| OV | MV | χ2 | p | |
|---|---|---|---|---|
| TP | 164 | 164 | - | - |
| FP | 20 | 9 | - | - |
| TN | 16 | 27 | - | - |
| FN | 3 | 3 | - | - |
| Sensitivity (%) | 98.20 | 98.20 | - | - |
| Specificity (%) | 44.44 | 75.00 | - | 0.002 * |
| AR (%) | 88.67 | 94.09 | 3.78 | 0.052 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.; Fu, B.; Liu, M.; Liu, X.; Liu, S.; Lv, F. Proposal of Modified Lung-RADS in Assessing Pulmonary Nodules of Patients with Previous Malignancies: A Primary Study. Diagnostics 2023, 13, 2210. https://doi.org/10.3390/diagnostics13132210
Song F, Fu B, Liu M, Liu X, Liu S, Lv F. Proposal of Modified Lung-RADS in Assessing Pulmonary Nodules of Patients with Previous Malignancies: A Primary Study. Diagnostics. 2023; 13(13):2210. https://doi.org/10.3390/diagnostics13132210
Chicago/Turabian StyleSong, Feipeng, Binjie Fu, Mengxi Liu, Xiangling Liu, Sizhu Liu, and Fajin Lv. 2023. "Proposal of Modified Lung-RADS in Assessing Pulmonary Nodules of Patients with Previous Malignancies: A Primary Study" Diagnostics 13, no. 13: 2210. https://doi.org/10.3390/diagnostics13132210
APA StyleSong, F., Fu, B., Liu, M., Liu, X., Liu, S., & Lv, F. (2023). Proposal of Modified Lung-RADS in Assessing Pulmonary Nodules of Patients with Previous Malignancies: A Primary Study. Diagnostics, 13(13), 2210. https://doi.org/10.3390/diagnostics13132210






