Diagnosis and Treatment of Adenomyosis with Office Hysteroscopy—A Narrative Review of Literature
Abstract
:1. Introduction
2. Classification and Diagnosis of Adenomyosis
3. Material and Methods
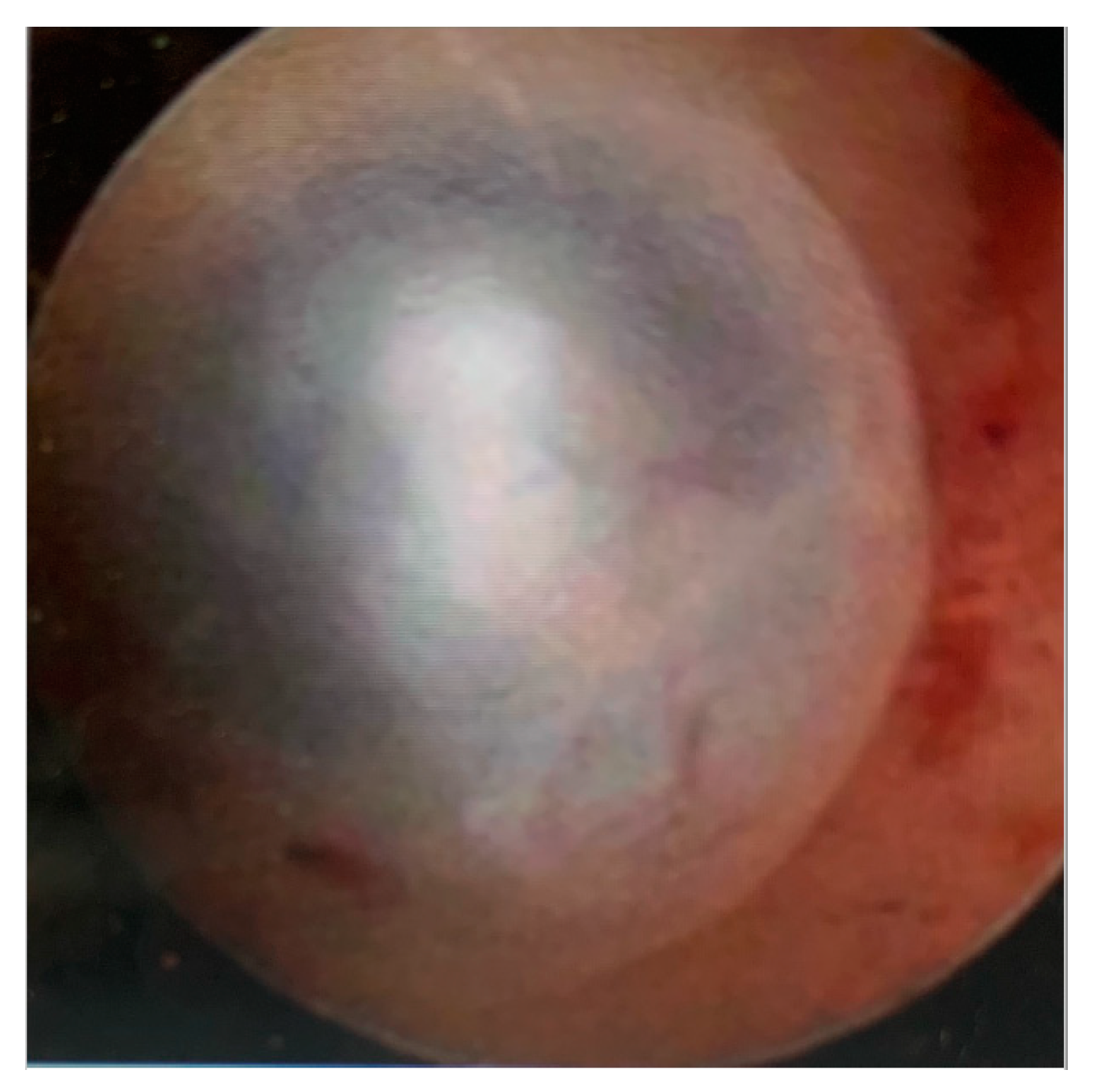
4. Hysteroscopic Diagnosis of Adenomyosis
- Irregular endometrium with tiny openings seen on the endometrial surface (Figure 1)
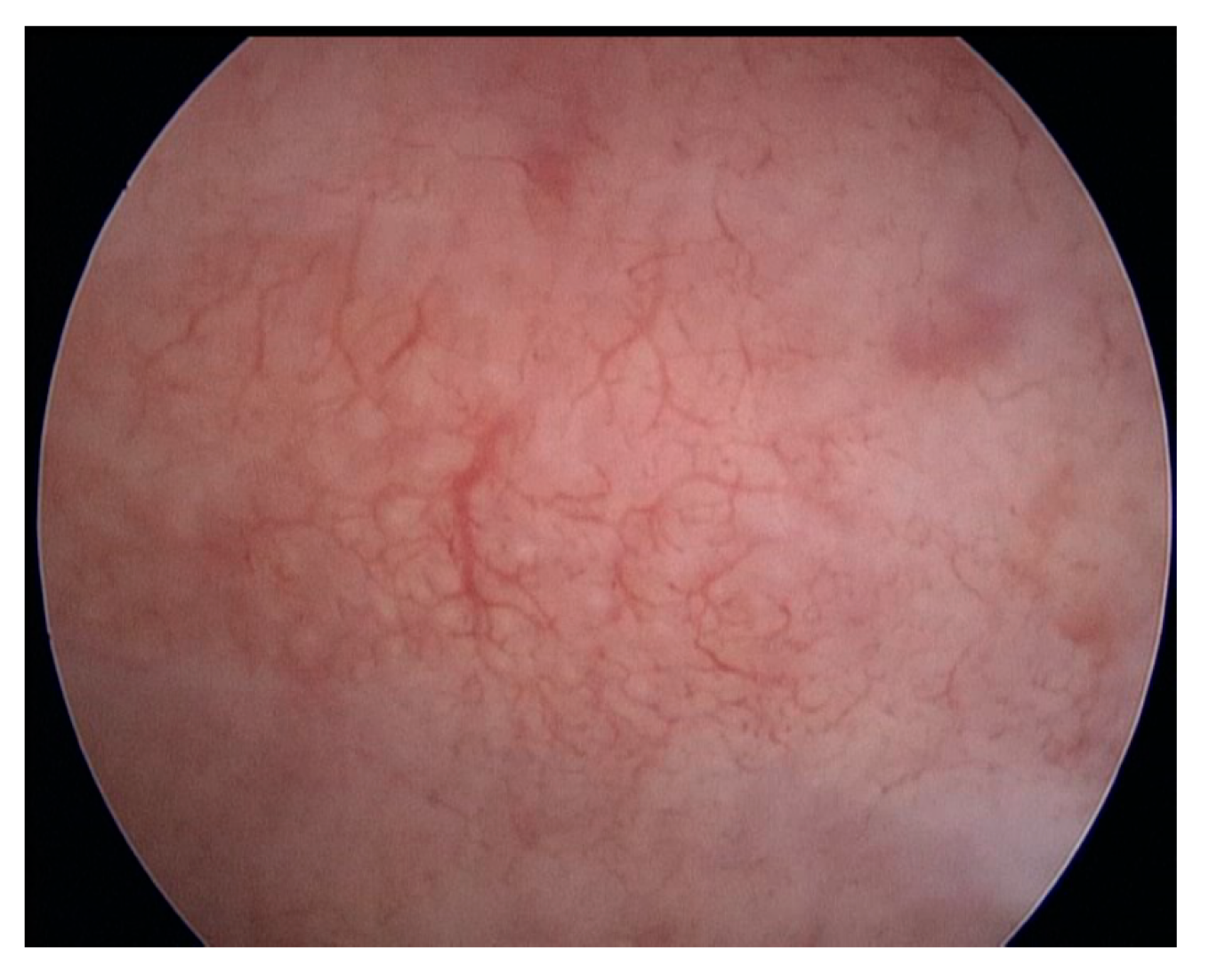
- Hypervascularisation (Figure 2)
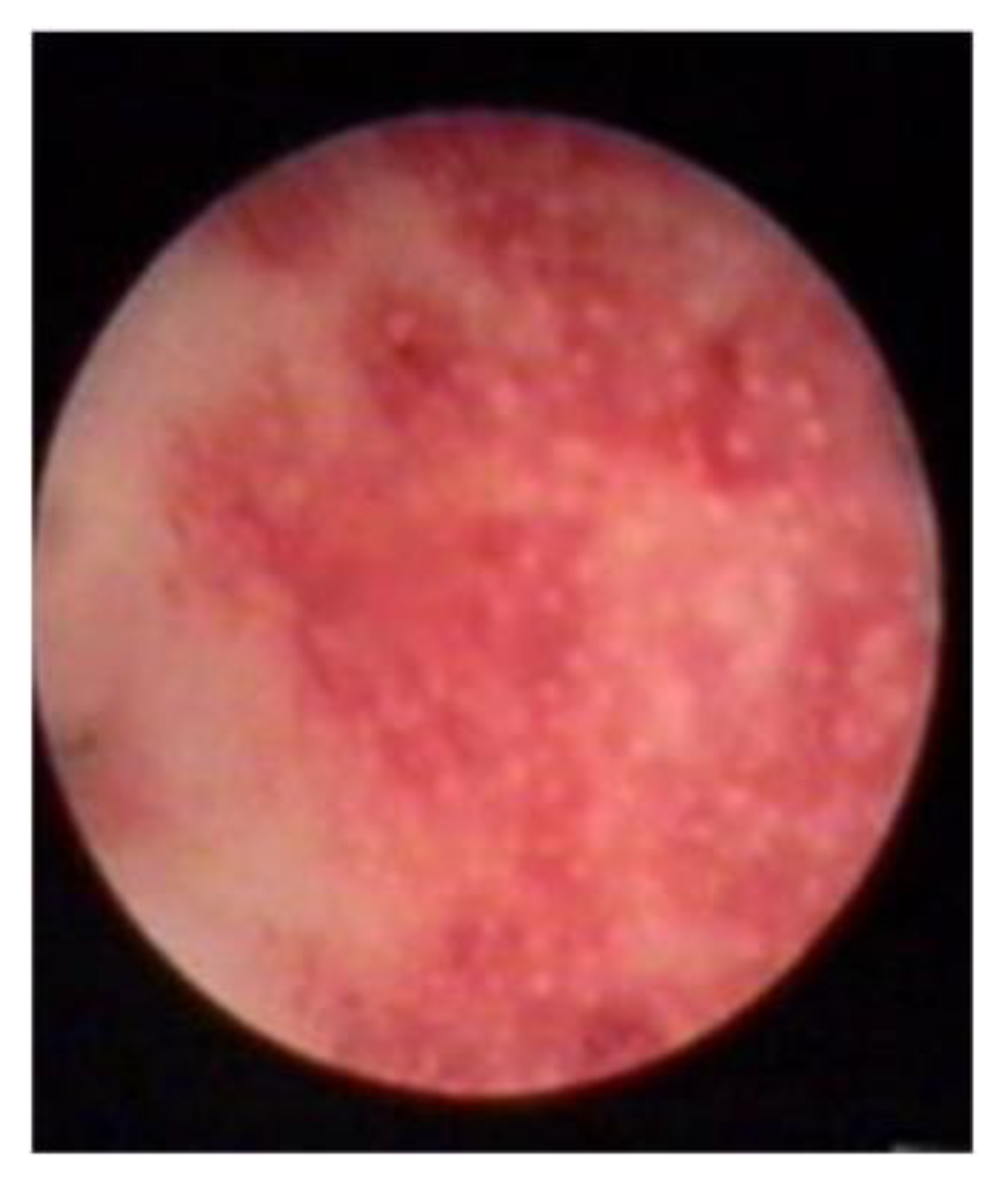
- An endometrial “strawberry” pattern (Figure 3)
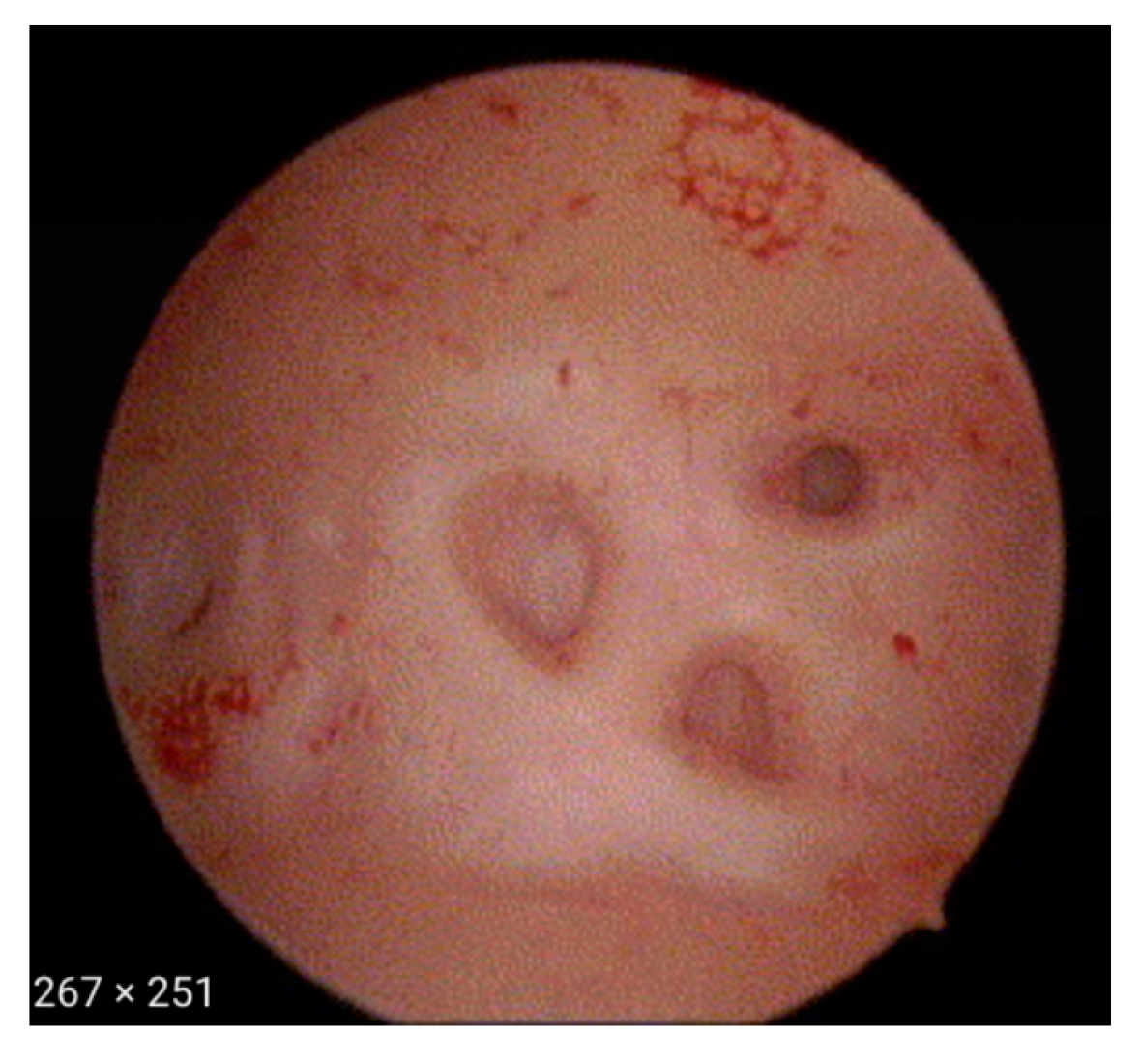
- Fibrous cystic appearance of intrauterine lesions (Figure 4)
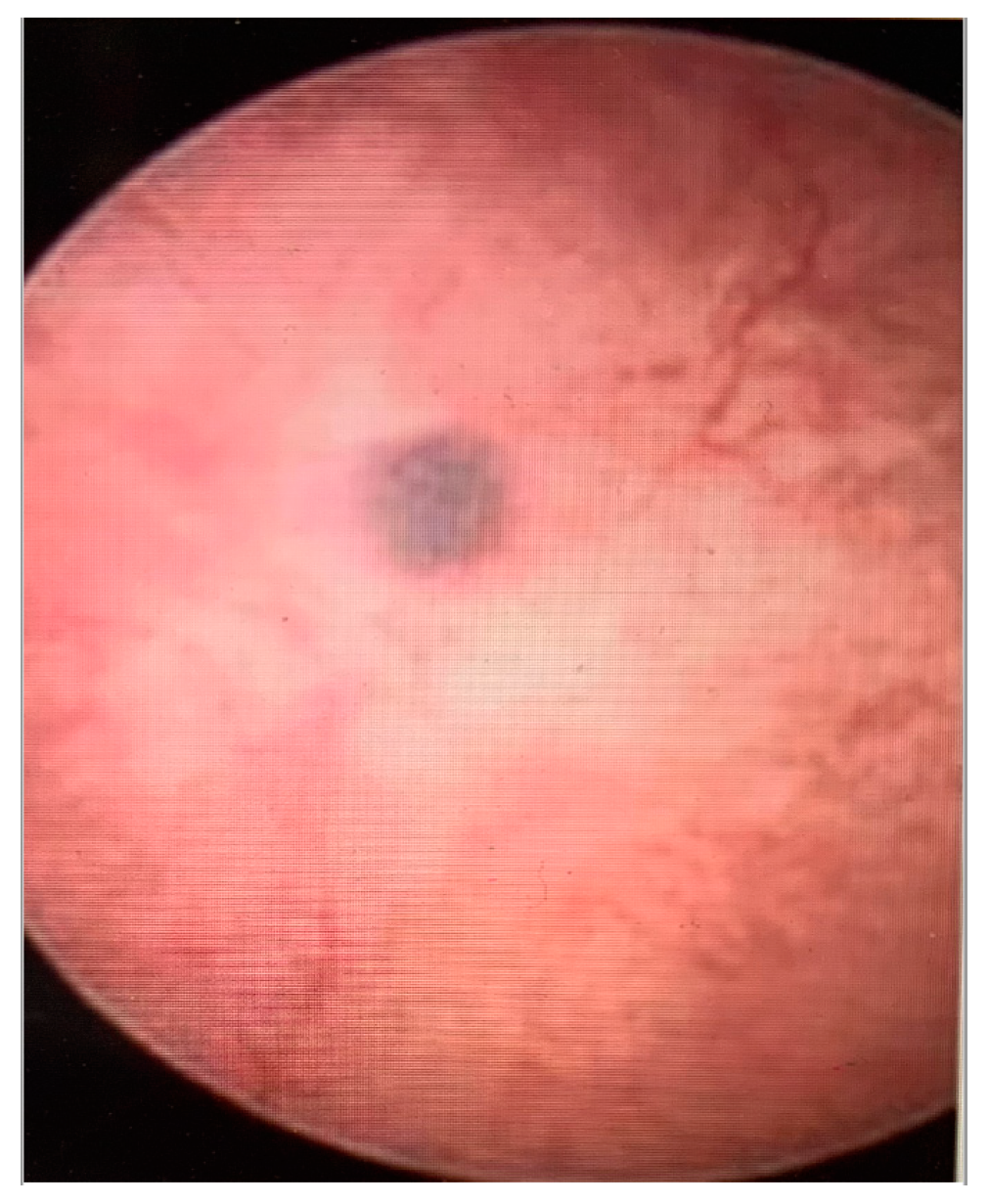
- Haemorrhagic cystic lesions presenting with a dark blue or chocolate brown appearance (Figure 5)
5. Office Hysteroscopy as a Treatment Option for Adenomyosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azziz, R. Adenomyosis: Current perspectives. Obstet. Gynecol. Clin. N. Am. 1989, 16, 221–235. [Google Scholar] [CrossRef]
- Habiba, M.; Benagiano, G. Uterine Adenomyosis; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ryo, E.; Takeshita, S.; Shiba, M.; Ayabe, T. Radiofrequency ablation for cystic adenomyosis: A case report. J. Reprod. Med. 2006, 51, 427–430. [Google Scholar] [PubMed]
- Peric, H.; Fraser, I.S. The symptomatology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388.e1. [Google Scholar] [CrossRef] [Green Version]
- Harmsen, M.J.; Bosch, T.V.D.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.J.K.; Groenman, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: Results of modified Delphi procedure. Ultrasound Obstet. Gynecol. 2021, 60, 118–131. [Google Scholar] [CrossRef]
- Ascher, S.M.; Arnold, L.L.; Patt, R.H.; Schruefer, J.J.; Bagley, A.S.; Semelka, R.C.; Zeman, R.K.; Simon, J.A. Adenomyosis: Prospective comparison of MR imaging and transvaginal sonography. Radiology 1994, 190, 803–806. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsubara, S.; Imanaka, S. Clinicopathological features of different subtypes in adenomyosis: Focus on early lesions. PLoS ONE 2021, 16, e0254147. [Google Scholar] [CrossRef]
- Keckstein, J. Hysteroscopy and adenomyosis. Contrib. Gynecol. Obstet. 2000, 20, 41–50. [Google Scholar]
- Van den Bosch, T.; De Bruijn, A.M.; De Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
- Bazot, M.; Cortez, A.; Darai, E.; Rouger, J.E.; Chopier, J.; Antoine, J.M.; Uzan, S. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: Correlation with histopathology. Hum. Reprod. 2001, 16, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Cody, R.F.J.; Ascher, S.M. Diagnostic value of radiological tests in chronic pelvic pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 433–466. [Google Scholar] [CrossRef]
- Bosch, T.V.D.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Lazzeri, L.; Morosetti, G.; Centini, G.; Monti, G.; Zupi, E.; Piccione, E.; Exacoustos, C. A sonographic classification of adenomyosis: Interobserver reproducibility in the evaluation of type and degree of the myometrial involvement. Fertil. Steril. 2018, 110, 1154–1161.e3. [Google Scholar] [CrossRef] [Green Version]
- Molinas, C.R.; Campo, R. Office hysteroscopy and adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 557–567. [Google Scholar] [CrossRef]
- McCausland, A.M. Hysteroscopic myometrial biopsy: Its use in diagnosing adenomyosis and its clinical application. Am. J. Obstet. Gynecol. 1992, 166 Pt 1, 1618–1619. [Google Scholar] [CrossRef]
- Bettocchi, S.; Selvaggi, L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J. Am. Assoc. Gynecol. Laparosc. 1997, 4, 255–258. [Google Scholar] [CrossRef]
- Brosens, I.; Gordts, S.; Habiba, M.; Benagiano, G. Uterine Cystic Adenomyosis: A Disease of Younger Women. J. Pediatr. Adolesc. Gynecol. 2015, 28, 420–426. [Google Scholar] [CrossRef]
- Sardo, A.D.S.; Calagna, G.; Santangelo, F.; Zizolfi, B.; Tanos, V.; Perino, A.; De Wilde, R.L. The Role of Hysteroscopy in the Diagnosis and Treatment of Adenomyosis. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Gordts, S.; Campo, R.; Brosens, I. Hysteroscopic diagnosis and excision of myometrial cystic adenomyosis. Gynecol. Surg. 2014, 11, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Tresserra, F.; Grases, P.; Ubeda, A.; Pascual, M.A.; Grases, P.J.; Labastida, R. Morphological changes in hysterectomies after endometrial ablation. Hum. Reprod. 1999, 14, 1473–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, A.M.; Makhlouf, A.M.; Youssof, A.A.; Gadalla, H.A. Hysteroscopic myometrial biopsy in unexplained abnormal uterine bleeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 86, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.; Khemani, M.; Logani, K.B.; Anand, R. Adenomyosis: Diagnosis by hysteroscopic endomyometrial biopsy, correlation of incidence and severity with menorrhagia. J. Obstet. Gynaecol. Res. 1998, 24, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Bianchi, S.; Dorta, M.; Zanotti, F.; Brioschi, D.; Carinelli, S. Transvaginal ultrasonography in the differential diagnosis of adenomyoma versus leiomyoma. Am. J. Obstet. Gynecol. 1992, 167, 603–606. [Google Scholar] [CrossRef]
- Dakhly, D.M.R.; Abdel Moety, G.A.F.; Saber, W.; Gad Allah, S.H.; Hashem, A.T.; Abdel Salam, L.O.E. Accuracy of Hysteroscopic Endomyometrial Biopsy in Diagnosis of Adenomyosis. J. Minim. Invasive Gynecol. 2016, 23, 364–371. [Google Scholar] [CrossRef]
- Champaneria, R.; Abedin, P.; Daniels, J.; Balogun, M.; Khan, K.S. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet. Gynecol. Scand. 2010, 89, 1374–1384. [Google Scholar] [CrossRef]
- Movilla, P.; Morris, S.; Isaacson, K. A Systematic Review of Tissue Sampling Techniques for the Diagnosis of Adenomyosis. J. Minim. Invasive Gynecol. 2020, 27, 344–351. [Google Scholar] [CrossRef]
- Luciano, D.E.; Exacoustos, C.; Albrecht, L.; LaMonica, R.; Proffer, A.; Zupi, E.; Luciano, A.A. Three-Dimensional Ultrasound in Diagnosis of Adenomyosis: Histologic Correlation With Ultrasound Targeted Biopsies of the Uterus. J. Minim. Invasive Gynecol. 2013, 20, 803–810. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.-W.; Just, P.-A.; Noël, J.-C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Updat. 2020, 26, 392–411. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.-M. Conservative Management of Uterine Adenomyosis: Medical vs. Surgical Approach. J. Clin. Med. 2021, 10, 4878. [Google Scholar] [CrossRef]
- Preutthipan, S.; Herabutya, Y. Hysteroscopic rollerball endometrial ablation as an alternative treatment for adenomyosis with menorrhagia and/or dysmenorrhea. J. Obstet. Gynaecol. Res. 2010, 36, 1031–1036. [Google Scholar] [CrossRef]
- Matsushima, T.; Akira, S.; Yoneyama, K.; Takeshita, T. Recurrence of uterine adenomyosis after administration of gonadotropin-releasing hormone agonist and the efficacy of dienogest. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 521–524. [Google Scholar] [CrossRef]
- De Silva, P.M.; Stevenson, H.; Smith, P.P.; Clark, T.J. Pain and Operative Technologies Used in Office Hysteroscopy: A Systematic Review of Randomized Controlled Trials. J. Minim. Invasive Gynecol. 2021, 28, 1699–1711. [Google Scholar] [CrossRef]
- McCausland, A.M.; McCausland, V.M. Long-term complications of endometrial ablation: Cause, diagnosis, treatment, and prevention. J. Minim. Invasive Gynecol. 2007, 14, 399–406. [Google Scholar] [CrossRef]
- Taran, F.A.; Stewart, E.A.; Brucker, S. Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe Frauenheilkd. 2013, 73, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Zhang, D.; Zhu, Q.; Zhang, H.; Yang, S.; Ma, J.; Pan, H.; Tong, T.; Sun, J.; Zhang, J. Hysteroscopic excision of symptomatic myometrial adenomyosis: Feasibility and effectiveness. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Nishida, M.; Takano, K.; Arai, Y.; Ozone, H.; Ichikawa, R. Conservative surgical management for diffuse uterine adenomyosis. Fertil. Steril. 2010, 94, 715–719. [Google Scholar] [CrossRef]
- Pontis, A.; D’Alterio, M.N.; Pirarba, S.; de Angelis, C.; Tinelli, R.; Angioni, S. Adenomyosis: A systematic review of medical treatment. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2016, 32, 696–700. [Google Scholar] [CrossRef]
- Giana, M.; Montella, F.; Surico, D.; Vigone, A.; Bozzola, C.; Ruspa, G. Large intramyometrial cystic adenomyosis: A hysteroscopic approach with bipolar resectoscope: Case report. Eur. J. Gynaecol. Oncol. 2005, 26, 462–463. [Google Scholar]
- Kamio, M.; Taguchi, S.; Oki, T.; Tsuji, T.; Iwamoto, I.; Yoshinaga, M.; Douchi, T. Isolated adenomyotic cyst associated with severe dysmenorrhea. J. Obstet. Gynaecol. Res. 2007, 33, 388–391. [Google Scholar] [CrossRef]
- Sun, W.; Guo, X.; Zhu, L.; Fei, X.; Zhang, Z.; Li, D. Hysteroscopic Treatment of a Uterine Cystic Adenomyosis. J. Minim. Invasive Gynecol. 2018, 25, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kitade, M.; Kikuchi, I.; Kumakiri, J.; Kuroda, K.; Jinushi, M. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: A review of nine cases. Fertil. Steril. 2010, 94, 862–868. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkrozou, F.; Vatopoulou, A.; Skentou, C.; Paschopoulos, M. Diagnosis and Treatment of Adenomyosis with Office Hysteroscopy—A Narrative Review of Literature. Diagnostics 2023, 13, 2182. https://doi.org/10.3390/diagnostics13132182
Gkrozou F, Vatopoulou A, Skentou C, Paschopoulos M. Diagnosis and Treatment of Adenomyosis with Office Hysteroscopy—A Narrative Review of Literature. Diagnostics. 2023; 13(13):2182. https://doi.org/10.3390/diagnostics13132182
Chicago/Turabian StyleGkrozou, Fani, Anastasia Vatopoulou, Chara Skentou, and Minas Paschopoulos. 2023. "Diagnosis and Treatment of Adenomyosis with Office Hysteroscopy—A Narrative Review of Literature" Diagnostics 13, no. 13: 2182. https://doi.org/10.3390/diagnostics13132182
APA StyleGkrozou, F., Vatopoulou, A., Skentou, C., & Paschopoulos, M. (2023). Diagnosis and Treatment of Adenomyosis with Office Hysteroscopy—A Narrative Review of Literature. Diagnostics, 13(13), 2182. https://doi.org/10.3390/diagnostics13132182





