Evaluation of Subcortical Structure Volumes in Patients with Non-Specific Digestive Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Scanning Protocol
2.2. Segmentation and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Oudenhove, L.; Levy, R.L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367.e2. [Google Scholar] [CrossRef] [PubMed]
- Ishak, W.W.; Pan, D.; Steiner, A.J.; Feldman, E.; Danovitch, I.; Melmed, G.Y.; Mann, A.; Mirocha, J. Patient-Reported Outcomes of Quality of Life, Functioning, and GI/Psychiatric Symptom Severity in Patients with Inflammatory Bowel Disease (IBD). Inflamm. Bowel Dis. 2017, 23, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, G.; Simren, M.; Jonefjäll, B.; Öhman, L.; Strid, H. Symptoms compatible with functional bowel disorders are common in patients with quiescent ulcerative colitis and influence the quality of life but not the course of the disease. Ther. Adv. Gastroenterol. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2019, 111, 104501. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e2. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- Talley, N.J.; Ford, A.C. Functional Dyspepsia. N. Engl. J. Med. 2015, 373, 1853–1863. [Google Scholar] [CrossRef]
- Pittet, V.; Vaucher, C.; Froehlich, F.; Maillard, M.H.; Michetti, P. Patient-reported healthcare expectations in inflammatory bowel diseases. PLoS ONE 2018, 13, e0197351. [Google Scholar] [CrossRef]
- Bickston, S.J.; Bloomfeld, R.S. Handbook of Inflammatory Bowel Disease 2009; Lippincott Wiliams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Bonaz, B. Inflammatory bowel diseases: A dysfunction of brain-gut interactions? Minerva Gastroenterol. Dietol. 2013, 59, 241–259. [Google Scholar] [CrossRef]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain–gut–microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef]
- Mayer, E.A.; Naliboff, B.D.; Craig, A.B. Neuroimaging of the Brain-Gut Axis: From Basic Understanding to Treatment of Functional GI Disorders. Gastroenterology 2006, 131, 1925–1942. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992, 267, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.E.; Rampton, D.S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, B.; Mazur-Bialy, A.; Pajdo, R.; Kwiecień, S.; Bilski, J.; Zwolinska-Wcislo, M.; Mach, T.; Brzozowski, T. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr. Neuropharmacol. 2016, 14, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Abautret-Daly, Á.; Dempsey, E.; Parra-Blanco, A.; Medina, C.; Harkin, A. Gut–brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2017, 30, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Tandon, R.K. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339. [Google Scholar] [CrossRef]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Oliván, C.M.C.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed “Gut/Feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef]
- Böhmelt, A.H.; Nater, U.; Franke, S.; Hellhammer, D.H.; Ehlert, U. Basal and Stimulated Hypothalamic-Pituitary-Adrenal Axis Activity in Patients With Functional Gastrointestinal Disorders and Healthy Controls. Psychosom. Med. 2005, 67, 288–294. [Google Scholar] [CrossRef]
- Chang, L.; Sundaresh, S.; Elliott, J.; Anton, P.A.; Baldi, P.; Licudine, A.; Mayer, M.; Vuong, T.; Hirano, M.; Naliboff, B.D.; et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009, 21, 149–159. [Google Scholar] [CrossRef]
- Weaver, K.R.; Sherwin, L.B.; Walitt, B.; Melkus, G.D.; Henderson, W. A Neuroimaging the brain-gut axis in patients with irritable bowel syndrome. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 320–333. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical Surface-Based Analysis. II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Hobson, A.R. Brain imaging and functional gastrointestinal disorders: Has it helped our understanding? Gut 2004, 53, 1198–1206. [Google Scholar] [CrossRef][Green Version]
- Agostini, A.; Benuzzi, F.; Filippini, N.; Bertani, A.; Scarcelli, A.; Farinelli, V.; Marchetta, C.; Calabrese, C.; Rizzello, F.; Gionchetti, P.; et al. New insights into the brain involvement in patients with Crohn’s disease: A voxel-based morphometry study. Neurogastroenterol. Motil. 2012, 25, 147-e82. [Google Scholar] [CrossRef]
- Bao, C.H.; Liu, P.; Liu, H.R.; Wu, L.Y.; Shi, Y.; Chen, W.F.; Qin, W.; Lu, Y.; Zhang, J.Y.; Jin, X.M.; et al. Alterations in Brain Grey Matter Structures in Patients with Crohn’s Disease and Their Correlation With Psychological Distress. J. Crohn’s Colitis 2015, 9, 532–540. [Google Scholar] [CrossRef]
- Lu, C.-L.; Wu, Y.-T.; Yeh, T.-C.; Chen, L.-F.; Chang, F.-Y.; Lee, S.-D.; Ho, L.-T.; Hsieh, J.-C. Neuronal correlates of gastric pain induced by fundus distension: A 3T-fMRI study. Neurogastroenterol. Motil. 2004, 16, 575–587. [Google Scholar] [CrossRef]
- Vandenbergh, J.; DuPont, P.; Fischler, B.; Bormans, G.; Persoons, P.; Janssens, J.; Tack, J. Regional brain activation during proximal stomach distention in humans: A positron emission tomography study. Gastroenterology 2005, 128, 564–573. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Dupont, P.; Van Oudenhove, L.; Bormans, G.; Demyttenaere, K.; Fischler, B.; Geeraerts, B.; Janssens, J.; Tack, J. Regional Cerebral Blood Flow During Gastric Balloon Distention in Functional Dyspepsia. Gastroenterology 2007, 132, 1684–1693. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, P.; Zeng, F.; Yuan, K.; Yu, D.; Von Deneen, K.M.; Liang, F.; Qin, W.; Tian, J. Increased interhemispheric resting-state functional connectivity in functional dyspepsia: A pilot study. NMR Biomed. 2012, 26, 410–415. [Google Scholar] [CrossRef]
- Herrero, M.-T.; Barcia, C.; Navarro, J. Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst. 2002, 18, 386–404. [Google Scholar] [CrossRef]
- Davis, K.D.; Pope, G.; Chen, J.; Kwan, C.L.; Crawley, A.P.; Diamant, N.E. Cortical thinning in IBS: Implications for homeostatic, attention, and pain processing. Neurology 2007, 70, 153–154. [Google Scholar] [CrossRef]
- Nair, V.A.; Beniwal-Patel, P.; Mbah, I.; Young, B.M.; Prabhakaran, V.; Saha, S. Structural Imaging Changes and Behavioral Correlates in Patients with Crohn’s Disease in Remission. Front. Hum. Neurosci. 2016, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.P.; Chen, F.R.; Sun, H.H.; Shi, M.J.; Yang, H.J.; Li, X.H.; Ding, D. Larger regional volume of the thalamus in diarrhea-predominant irritable bowel syndrome: A cross-sectional study. Brain Imaging Behav. 2019, 14, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bao, C.; Wei, Y.; Wu, J.; Zhao, Y.; Zeng, X.; Qin, W.; Wu, H.; Liu, P. Altered functional connectivity of the amygdala in Crohn’s disease. Brain Imaging Behav. 2019, 14, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Van Oudenhove, L.; Vandenberghe, J.; Dupont, P.; Geeraerts, B.; Vos, R.; Dirix, S.; Van Laere, K.; Bormans, G.; Vanderghinste, D.; Demyttenaere, K.; et al. Regional Brain Activity in Functional Dyspepsia: A H215O-PET Study on the Role of Gastric Sensitivity and Abuse History. Gastroenterology 2010, 139, 36–47. [Google Scholar] [CrossRef]
- Goodyear, B.G.; Heidari, F.; Ingram, R.J.M.; Cortese, F.; Sharifi, N.; Kaplan, G.G.; Ma, C.; Panaccione, R.; A Sharkey, K.; Swain, M.G. Multimodal Brain MRI of Deep Gray Matter Changes Associated with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Lin, S.; Gao, T.; Sun, C.; Jia, M.; Liu, C.; Ma, A. The association between functional dyspepsia and depression: A meta-analysis of observational studies. Eur. J. Gastroenterol. Hepatol. 2019, 31, 911–918. [Google Scholar] [CrossRef]
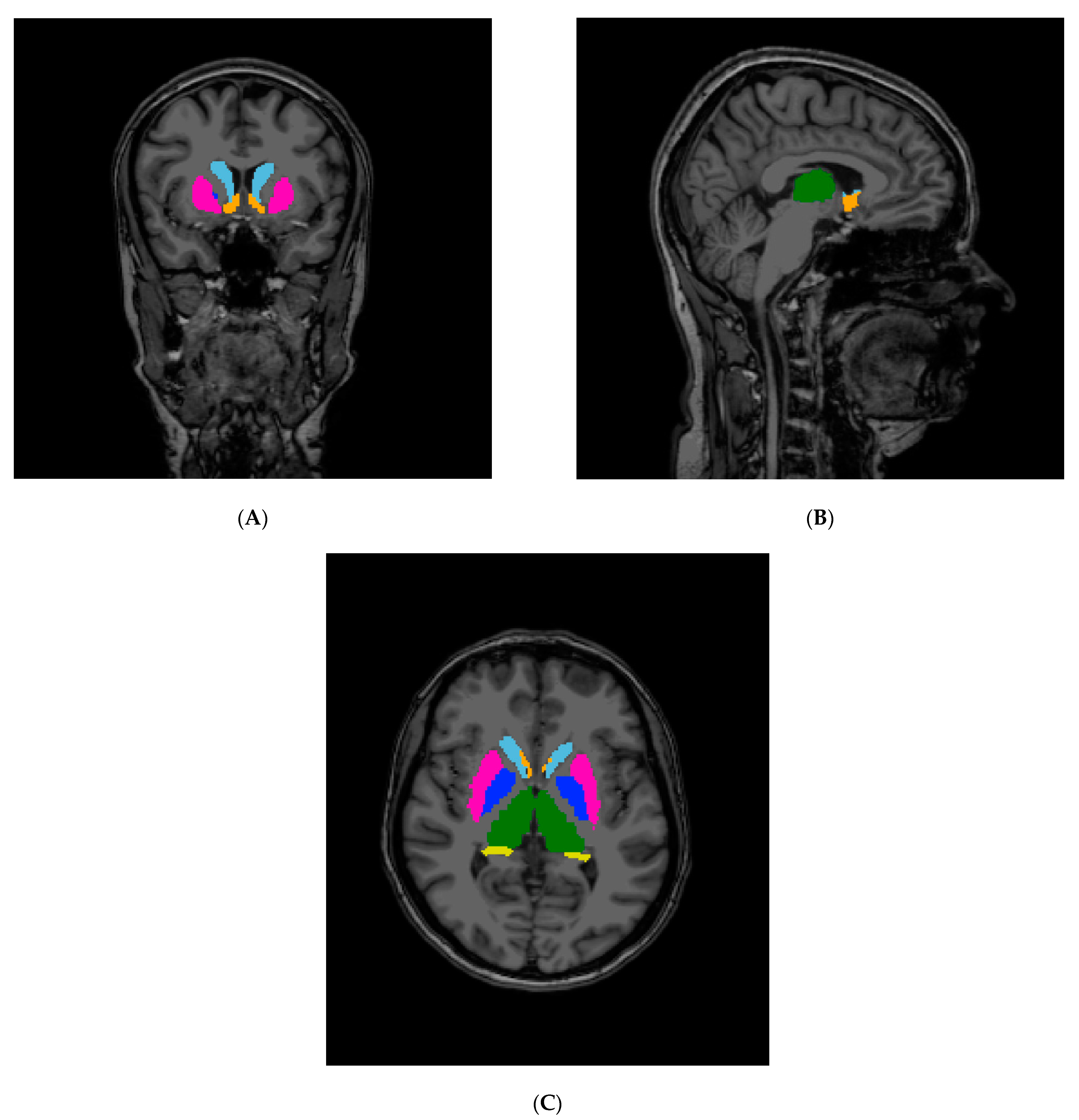
| Group 1 (CON) | Group 2 (FD) | Group 3 (IBD) | Group 4 (IBS) | |
|---|---|---|---|---|
| N (71) | 19 | 18 | 19 | 20 |
| Age [years] | mean 34.15 (min. 24, max. 47) SD 8.01 | mean 25.73 (min. 20, max. 40) SD 5.21 | mean 31.73 (min. 21, max. 43) SD 5.82 | mean 35.5 (min. 17, max. 62) SD 10.3 |
| Sex (F=, M=) | F = 9, M = 10 | F = 13, M = 5 | F = 9, M = 10 | F = 14, M = 6 |
| Volumes Normalized to eTIV | Levene’a p | ANOVA F | ANOVA p | Post-Hoc Contrast | Comments |
|---|---|---|---|---|---|
| Left cerebellum white matter | >>0.1 | 0.323 | 0.809 | - | |
| Left cerebellum cortex | >>0.1 | 1.106 | 0.352 | - | |
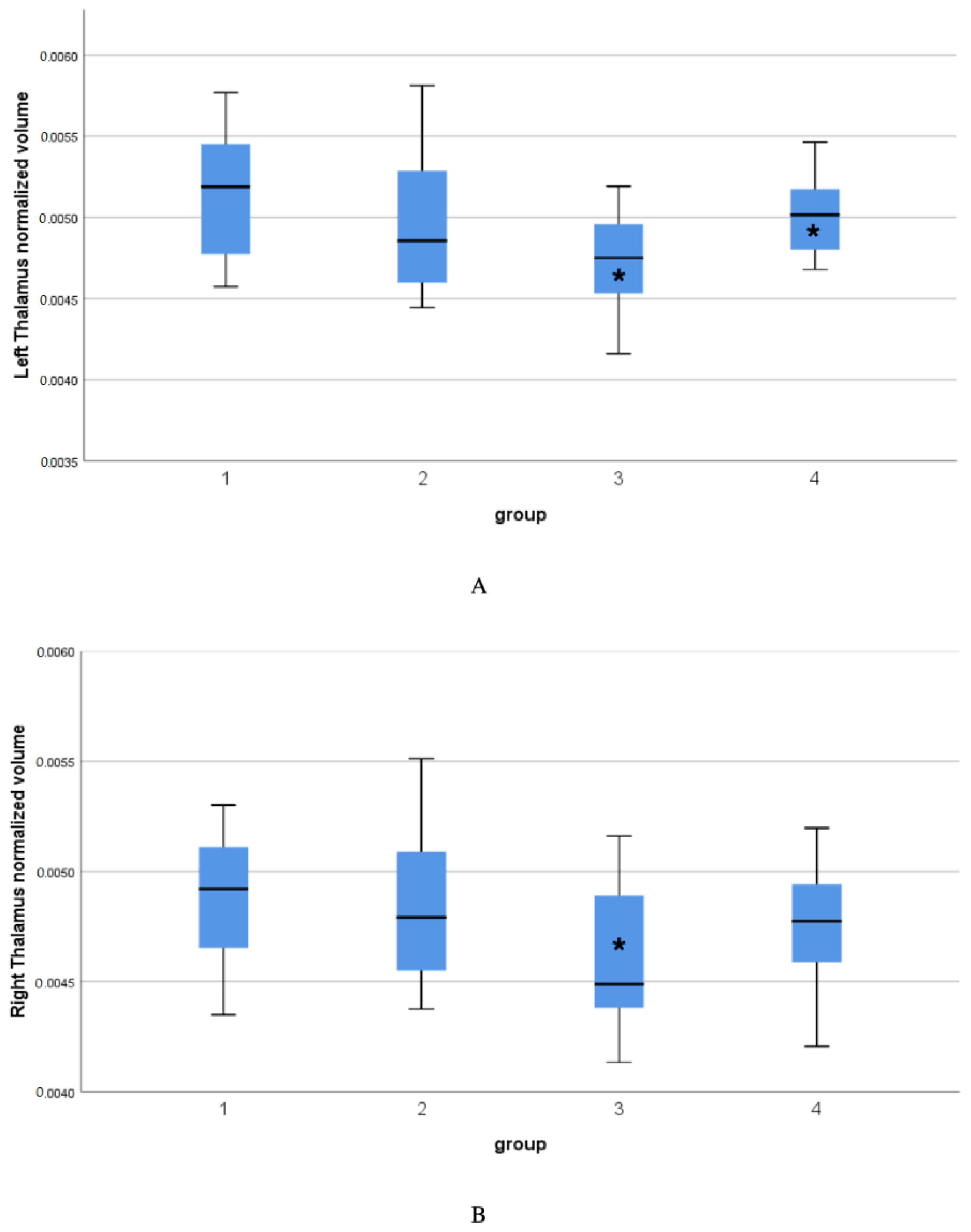
| Left thalamus | 0.006—Kruskall–Wallis test performed | KW 10.611 | KW p = 0.014 | 3->4 p = 0.015 3->1 p = 0.002 | Non-parametric test |
| Left caudate | >>0.1 | 1.037 | 0.366 | - | |
| Left putamen | >>0.1 | 0.849 | 0.472 | - | |
| Left pallidum | >>0.1 | 2.346 | 0.08 | - | |
| Left Hippocamp | >>0.1 | 0.421 | 0.738 | - | |
| Left Amygdala | >>0.1 | 2.169 | 0.099 | - | |
| Left Accumbens | >>0.1 | 0.664 | 0.577 | - | |
| CSF | >>0.1 | 0.504 | 0.681 | - | |
| Right cerebellum white matter | >>0.1 | 0.346 | 0.792 | - | |
| Right cerebellum cortex | >>0.1 | 0.346 | 0.792 | - | |
| Right thalamus | >>0.1 | 3.384 | 0.023 | 1->3 p = 0.022 | |
| Right caudate | 0.091 | 1.864 | 0.143 | - | |
| Right putamen | >>0.1 | 0.887 | 0.452 | - | |
| Right pallidum | >>0.1 | 1.648 | 0.186 | - | |
| Right amygdala | >>0.1 | 1.381 | 0.256 | - | |
| Right accumbens | >>0.1 | 0.858 | 0.467 | ||
| Right hippocamp | >>0.1 | 0.073 | 0.974 | - | |
| CerebralWhiteMatter vol | >>0.1 | 0.687 | 0.563 | - | |
| SubCortGrayVol | >>0.1 | 1.920 | 0.134 | - | |
| TotalGrayVol | >>0.1 | 1.607 | 0.195 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrobisz, K.; Piotrowicz, G.; Rudnik, A.; Naumczyk, P.; Sabisz, A.; Markiet, K.; Szurowska, E. Evaluation of Subcortical Structure Volumes in Patients with Non-Specific Digestive Diseases. Diagnostics 2022, 12, 2199. https://doi.org/10.3390/diagnostics12092199
Skrobisz K, Piotrowicz G, Rudnik A, Naumczyk P, Sabisz A, Markiet K, Szurowska E. Evaluation of Subcortical Structure Volumes in Patients with Non-Specific Digestive Diseases. Diagnostics. 2022; 12(9):2199. https://doi.org/10.3390/diagnostics12092199
Chicago/Turabian StyleSkrobisz, Katarzyna, Grazyna Piotrowicz, Agata Rudnik, Patrycja Naumczyk, Agnieszka Sabisz, Karolina Markiet, and Edyta Szurowska. 2022. "Evaluation of Subcortical Structure Volumes in Patients with Non-Specific Digestive Diseases" Diagnostics 12, no. 9: 2199. https://doi.org/10.3390/diagnostics12092199
APA StyleSkrobisz, K., Piotrowicz, G., Rudnik, A., Naumczyk, P., Sabisz, A., Markiet, K., & Szurowska, E. (2022). Evaluation of Subcortical Structure Volumes in Patients with Non-Specific Digestive Diseases. Diagnostics, 12(9), 2199. https://doi.org/10.3390/diagnostics12092199








