The Role of Imaging in the Detection of Non-COVID-19 Pathologies during the Massive Screening of the First Pandemic Wave
Abstract
1. Introduction
2. Materials and Methods
2.1. Computerized Tomography Study
2.2. Statistical Analysis
3. Results
Risk Lesions
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| 0 Anomalies (N = 82) | ≥1 Anomalies (N = 280) | Comparison | |||
|---|---|---|---|---|---|
| N Non Missing | n (%) or Mean ± SD | N Non Missing | n (%) or Mean ± SD | Logistic Regression Adjusted for Age and Gender OR (95%CI), p-Value | |
| Age (years) | 82 | 52.3 ± 14.9 | 280 | 69.0 ± 14.0 | 1.1 (1.1; 1.1), <0.0001 |
| Gender, male | 82 | 41 (50.0) | 280 | 163 (58.2) | 1.8 (1.02; 3.2), 0.041 |
| BMI (kg/m2) | 65 | 30.0 ± 7.0 | 229 | 27.0 ± 5.7 | 0.96 (0.91; 1.003), 0.067 |
| Smoking (including stopped) | 74 | 5 (6.8) | 256 | 42 (16.4) | 3.5 (1.2; 10), 0.025 |
| Chronic renal failure | 76 | 1 (1.3) | 206 | 31 (15.0) | 9.4 (1.2; 72), 0.031 |
| Diabetes | 81 | 25 (30.9) | 271 | 113 (41.7) | 1.2 (0.65; 2.2), 0.59 |
| High blood pressure | 81 | 37 (45.7) | 272 | 169 (62.1) | 0.80 (0.43; 1.5), 0.50 |
| Obesity | 69 | 24 (34.8) | 238 | 59 (24.8) | 0.68 (0.35; 1.3), 0.25 |
| Cardiovascular pathology | 76 | 11 (14.5) | 203 | 76 (37.4) | 1.7 (0.77; 3.7), 0.20 |
| Chronic pulmonary pathology | 81 | 10 (12.3) | 265 | 53 (20.0) | 1.6 (0.72; 3.5), 0.26 |
| Immune suppression | 76 | 9 (11.8) | 203 | 13 (6.4) | 0.38 (0.14; 1.03), 0.058 |
| Asthma | 72 | 7 (9.7) | 233 | 16 (6.9) | 0.79 (0.28; 2.2), 0.65 |
| Oncologic patient | 82 | 5 (6.1) | 280 | 43 (15.4) | 1.8 (0.64; 4.9), 0.27 |
| 0 Unknown Anomalies (N = 143) | ≥1 Unknown Anomalies (N = 219) | Comparison | |||
|---|---|---|---|---|---|
| N Non Missing | n (%) or Mean ± SD | N Non Missing | n (%) or Mean ± SD | Logistic Regression Adjusted for Age and Gender OR (95%CI), p-Value | |
| Age (years) | 143 | 59.5 ± 16.9 | 219 | 68.9 ± 13.9 | 1.04 (1.03; 1.1), <0.0001 |
| Gender, male | 143 | 76 (53.1) | 219 | 128 (58.4) | 1.4 (0.91; 2.2), 0.12 |
| BMI (kg/m2) | 119 | 28.3 ± 6.6 | 175 | 27.2 ± 5.8 | 0.99 (0.95; 1.03), 0.59 |
| Smoking (including stopped) | 132 | 21 (15.9) | 198 | 26 (13.3) | 0.73 (0.38; 1.4), 0.36 |
| Chronic renal failure | 117 | 12 (10.3) | 165 | 20 (12.1) | 0.92 (0.42; 2.0), 0.84 |
| Diabetes | 139 | 58 (41.7) | 213 | 80 (38.6) | 0.70 (0.44; 1.1), 0.13 |
| High blood pressure | 140 | 83 (59.3) | 213 | 123 (57.8) | 0.52 (0.31; 0.88), 0.014 |
| Obesity | 124 | 36 (29.0) | 183 | 47 (25.7) | 0.97 (0.57; 1.7), 0.92 |
| Cardiovascular pathology | 115 | 26 (22.6) | 164 | 61 (37.2) | 1.2 (0.66; 2.2), 0.57 |
| Chronic pulmonary pathology | 138 | 19 (13.8) | 208 | 44 (21.1) | 1.7 (0.90; 3.1), 0.10 |
| Immune suppression | 115 | 16 (13.9) | 164 | 6 (3.7) | 0.20 (0.073; 0.55), 0.0019 |
| Asthma | 124 | 11 (8.9) | 181 | 12 (6.6) | 0.83 (0.34; 2.0), 0.67 |
| Oncologic patient | 143 | 20 (14.0) | 219 | 28 (12.8) | 0.69 (0.36; 1.3), 0.25 |
| Alive at Hospital Discharge (N = 313) | Death during Hospital Stay (N = 17) | Comparison | |||
|---|---|---|---|---|---|
| N Non Missing | n (%), Mean ± SD or Median(IQR) | N Non Missing | n (%), Mean ± SD or Median(IQR) | Logistic Regression Adjusted for Age OR (95%CI), p-Value | |
| Age (years) | 313 | 65.6 ± 15.0 | 17 | 71.5 ± 18.1 | 0.030 ± 0.018, 0.10 |
| Gender, male | 313 | 183 (58.5) | 17 | 11 (64.7) | 0.20 ± 0.27, 0.45 |
| BMI (kg/m2) | 261 | 27.8 ± 6.3 | 15 | 26.4 ± 5.5 | 0.97 (0.88; 1.1), 0.53 |
| Smoking (including stopped) | 288 | 40 (13.9) | 17 | 6 (35.3) | 3.5 (1.2; 10), 0.021 |
| Chronic renal failure | 245 | 31 (12.7) | 13 | 1 (7.7) | - |
| Diabetes | 306 | 125 (40.9) | 17 | 8 (47.1) | 1.3 (0.47; 3.4), 0.65 |
| High blood pressure | 306 | 182 (59.5) | 17 | 12 (70.6) | 1.3 (0.43; 3.9), 0.65 |
| Obesity | 270 | 79 (29.3) | 15 | 2 (13.3) | - |
| Cardiovascular pathology | 242 | 74 (30.6) | 13 | 8 (61.5) | 2.5 (0.74; 8.5), 0.14 |
| Chronic pulmonary pathology | 301 | 52 (17.3) | 17 | 4 (23.5) | 1.5 (0.46; 4.7), 0.52 |
| Immune suppression | 242 | 18 (7.4) | 13 | 0 (0.0) | - |
| Asthma | 269 | 22 (8.2) | 13 | 0 (0.0) | - |
| Oncologic patient | 313 | 38 (12.1) | 17 | 6 (35.3) | 3.6 (1.2; 10), 0.018 |
| ≥ 1 known anomalies on CT scan | 313 | 95 (30.3) | 17 | 10 (58.8) | 2.8 (1.0; 7.9), 0.046 |
| Suspicious nodule | 313 | 25 (8.0) | 17 | 2 (11.8) | - |
| Suspicious mass | 313 | 12 (3.8) | 17 | 2 (11.8) | - |
| COPD sign | 313 | 24 (7.7) | 17 | 4 (23.5) | 3.2 (0.96; 10.8), 0.059 |
| Sign of fibrosis | 313 | 1 (0.3) | 17 | 0 (0.0) | - |
| Calcified coronary | 313 | 75 (24.0) | 17 | 7 (41.2) | 1.8 (0.63; 5.1), 0.27 |
| Ascending aorta Aneurysm | 313 | 7 (2.2) | 17 | 1 (5.9) | - |
| Pericardial effusion | 313 | 3 (1.0) | 17 | 1 (5.9) | - |
| Thyroid goiter | 312 | 24 (7.7) | 17 | 4 (23.5) | 3.2 (0.96; 11), 0.059 |
| Vertebral collapse | 313 | 19 (6.1) | 17 | 0 (0.0) | - |
| Coef. ± SE | p-Value | |
|---|---|---|
| Age (years) | 0.0084 ± 0.0033 | 0.010 |
| Gender, male | 0.065 ± 0.10 | 0.51 |
| BMI (kg/m2) | 0.022 ± 0.010 | 0.014 |
| Smoking (including stopped) | −0.23 ± 0.14 | 0.11 |
| Chronic renal failure | 0.25 ± 0.17 | 0.13 |
| Diabetes | 0.31 ± 0.10 | 0.0017 |
| High blood pressure | 0.25 ± 0.11 | 0.020 |
| Obesity | 0.46 ± 0.12 | 0.0001 |
| Cardiovascular pathology | 0.11 ± 0.12 | 0.39 |
| Chronic pulmonary pathology | 0.29 ± 0.13 | 0.026 |
| Immune suppression | −0.0025 ± 0.21 | 0.99 |
| Asthma | 0.20 ± 0.20 | 0.33 |
| Oncologic patient | −0.038 ± 0.14 | 0.79 |
| ≥1 known anomalies on CT scan | 0.21 ± 0.11 | 0.054 |
| Suspicious nodule | −0.022 ± 0.18 | 0.90 |
| Suspicious mass | 0.29 ± 0.24 | 0.23 |
| COPD sign | 0.057 ± 0.18 | 0.75 |
| Sign of fibrosis | 1.9 ± 0.88 | 0.034 |
| Calcified coronary | 0.11 ± 0.12 | 0.36 |
| Ascending aorta aneurysm | −0.13 ± 0.32 | 0.69 |
| Pericardial effusion | −0.19 ± 0.45 | 0.68 |
| Thyroid goiter | 0.089 ± 0.18 | 0.61 |
| Vertebral collapse | 0.48 ± 0.21 | 0.022 |
References
- WHO Landing Page. Available online: https://www.who.int/ (accessed on 20 December 2020).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins University & Medicine Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 20 December 2020).
- Stang, A.; Robers, J.; Schonert, B.; Jöckel, K.H.; Spelsberg, A.; Keil, U.; Cullen, P. The performance of the SARS-CoV-2 RT-PCR test as a tool for detecting SARS-CoV-2 infection in the population. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ge, Y.; Zhao, K.; Zhu, X.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2). Virology 2020, 549, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Wang, Y.; Han, Y.; Wang, Y.; Wang, C.; Zhang, G.; Jin, J.; Jia, H.; Fan, F.; et al. Diagnostic value and key features of computed tomography in Coronavirus Disease 2019. Emerg. Microbes Infect. 2020, 9, 787–793. [Google Scholar] [CrossRef]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: A proposal based on the imaging data of 37 studies. Eur. Radiol. 2020, 30, 4930–4942. [Google Scholar] [CrossRef]
- Guillo, E.; Bedmar Gomez, I.; Dangeard, S.; Bennani, S.; Saab, I.; Tordjman, M.; Jilet, L.; Chassagnon, G.; Revel, M. COVID-19 pneumonia: Diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur. J. Radiol. 2020, 131, 109209. [Google Scholar] [CrossRef]
- Li, J.; Long, X.; Wang, X.; Fang, F.; Lv, X.; Zhang, D.; Sun, Y.; Hu, S.; Lin, Z.; Xiong, N. Radiology indispensable for tracking COVID-19. Diagn. Interv. Imaging 2021, 102, 69–75. [Google Scholar] [CrossRef]
- Lessmann, N.; Sánchez, C.I.; Beenen, L.; Boulogne, L.H.; Brink, M.; Calli, E.; Charbonnier, J.P.; Dofferhoff, T.; van Everdingen, W.M.; Gerke, P.K.; et al. Automated Assessment of COVID-19 Reporting and Data System and Chest CT Severity Scores in Patients Suspected of Having COVID-19 Using Artificial Intelligence. Radiology 2021, 298, E18–E28. [Google Scholar] [CrossRef]
- Prokop, M.; van Everdingen, W.; van Rees Vellinga, T.; van Ufford, J.Q.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef]
- Guiot, J.; Vaidyanathan, A.; Deprez, L.; Zerka, F.; Danthine, D.; Frix, A.N.; Thys, M.; Henket, M.; Canivet, G.; Mathieu, S.; et al. Development and Validation of an Automated Radiomic CT Signature for Detecting COVID-19. Diagnostics 2020, 11, 41. [Google Scholar] [CrossRef]
- Byrne, S.C.; Hammer, M.M. Malignant Nodules Detected on Lung Cancer Screening CT: Yield of Short-Term Follow-Up CT in Demonstrating Nodule Growth. AJR Am. J. Roentgenol. 2022, 218, 929–1112. [Google Scholar] [CrossRef] [PubMed]
- Couraud, S.; Ferretti, G.; Milleron, B.; Cortot, A.; Girard, N.; Gounant, V.; Laurent, F.; Leleu, O.; Quoix, E.; Revel, M.P.; et al. Intergroupe francophone de cancérologie thoracique, Société de pneumologie de langue française, and Société d’imagerie thoracique statement paper on lung cancer screening. Diagn. Interv. Imaging 2021, 102, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Mazzone, P.J.; Naidich, D.P.; Bach, P.B. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e78S–e92S. [Google Scholar] [CrossRef] [PubMed]
- Siemens Edge Plus. Available online: https://www.siemens-healthineers.com/fr-be/computed-tomography/single-source-ct-scanner/somatom-edge-plus (accessed on 8 June 2022).
- GE Revolution CT, GE Brightspeed. Available online: https://www.gehealthcare.com/ (accessed on 8 June 2022).
- Austin, J.H.; Muller, N.L.; Friedman, P.J.; Hansell, D.M.; Naidich, D.; Remy-Jardin, M.; Webb, W.R.; A Zerhouni, E. Glossary of terms for CT of the lungs: Recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996, 200, 327–331. [Google Scholar] [CrossRef]
- Corwin, M.T.; Schieda, N.; Remer, E.M.; Caoili, E.M. Management of incidental adrenal nodules: A survey of abdominal radiologists conducted by the Society of Abdominal Radiology Disease-Focused Panel on Adrenal Neoplasms. Abdom. Radiol. 2022, 47, 1360–1368. [Google Scholar] [CrossRef]
- Mori, M.; Bin Mahmood, S.U.; Yousef, S.; Shioda, K.; Vinholo, T.F.; Mangi, A.A.; Elefteriades, J.A.; Geirsson, A. Prevalence of Incidentally Identified Thoracic Aortic Dilations: Insights for Screening Criteria. Can. J. Cardiol. 2019, 35, 892–898. [Google Scholar] [CrossRef]
- Grenier, P.; Beigelman, C.; Lucidarme, O. Imagerie des BPCO [Imaging of COPD]. Rev. Prat. 1995, 45, 1233–1237. [Google Scholar]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Wang, Z.J.; Reddy, G.P.; Gotway, M.B.; Yeh, B.M.; Hetts, S.W.; Higgins, C.B. CT and MR imaging of pericardial disease. Radiographics 2003, 23, S167–S180. [Google Scholar] [CrossRef]
- Weber, A.L.; Randolph, G.; Aksoy, F.G. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol. Clin. N. Am. 2000, 38, 1105–1129. [Google Scholar] [CrossRef]
- Hoang, J.K.; Sosa, J.A.; Nguyen, X.V.; Galvin, P.L.; Oldan, J.D. Imaging thyroid disease: Updates, imaging approach, and management pearls. Radiol. Clin. N. Am. 2015, 53, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Allaire, B.T.; Lu, D.; Johannesdottir, F.; Kopperdahl, D.; Keaveny, T.M.; Jarraya, M.; Guermazi, A.; Bredella, M.A.; Samelson, E.J.; Kiel, D.P.; et al. Prediction of incident vertebral fracture using CT-based finite element analysis. Osteoporos. Int. 2019, 30, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Gould, M.K.; Arenberg, D.A.; Chen, A.C.; Choi, H.K.; Detterbeck, F.C.; Farjah, F.; Fong, K.M.; Iaccarino, J.M.; Janes, S.M.; et al. Management of Lung Nodules and Lung Cancer Screening During the COVID-19 Pandemic: CHEST Expert Panel Report. J. Am. Coll. Radiol. 2020, 17, 845. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228. [Google Scholar] [CrossRef] [PubMed]
- Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; Noncommunicable Diseases, World Health Organization; World Health Organization: Geneva, Switzerland, 2013; ISBN 978 92 4 150623 6.
- Storti, K.L.; Pettee Gabriel, K.K.; Underwood, D.A.; Kuller, L.H.; Kriska, A.M. Physical activity and coronary artery calcification in two cohorts of women representing early and late postmenopause. Menopause 2010, 17, 1146–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, S.L.; Buckley, B.J.R.; Rivera-Caravaca, J.M.; Zhang, J.; Lip, G.Y.H. Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews. Eur. Hear. J. Qual. Care Clin. Outcomes 2021, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gerayeli, F.V.; Milne, S.; Cheung, C.; Li, X.; Yang, C.W.T.; Tam, A.; Choi, L.H.; Bae, A.; Sin, D.D. COPD and the risk of poor outcomes in COVID-19: A systematic review and meta-analysis. eClinicalMedicine 2021, 33, 100789. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mahawar, K.; Xia, Z.; Yang, W.; El-Hasani, S. Obesity and mortality of COVID-19. Meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 295–300. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Sala, C. COVID-19 and diabetes: Is there enough evidence? J. Clin. Hypertens. 2020, 22, 943–948. [Google Scholar] [CrossRef]
- Wong, N.D.; Kouwabunpat, D.; Vo, A.N.; Detrano, R.C.; Eisenberg, H.; Goel, M.; Tobis, J.M. Coronary calcium and atherosclerosis by ultrafast computed tomography in asymptomatic men and women: Relation to age and risk factors. Am. Hear. J. 1994, 127, 422–430. [Google Scholar] [CrossRef]
- Jain, V.; Yuan, J.M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: A systematic review and meta-analysis. Int. J. Public Health 2020, 65, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Brattström, O.; Agvald-Öhman, C.; Grip, J.; Campoccia Jalde, F.; Strålin, K.; Nauclér, P.; Oldner, A.; Konrad, D.; Persson, B.P.; et al. Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden. Acta Anaesthesiol. Scand. 2021, 65, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
| Description | |
|---|---|
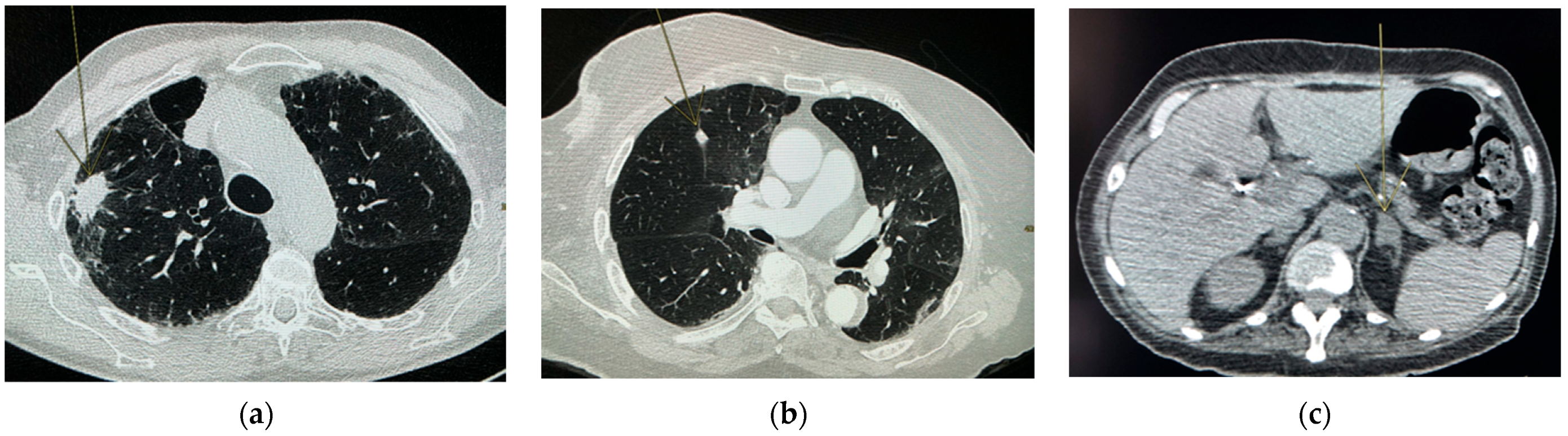
| Nodule and mass | -Mass is defined as >3 cm (as the mass definition in lung CT [18]) |
| -Nodule of variable origin (pulmonary, lymphadenopathy, thyroid, adrenal, breast, others) (for example, in lung [18] or in adrenal [19]) | |
| Pulmonary diseases | -Signs of COPD (inflation, sign of bronchopathy, emphysema) [20] |
| -Signs of pulmonary fibrosis (distribution of the attack, honeycomb, crosslinking, etc.) [21] | |
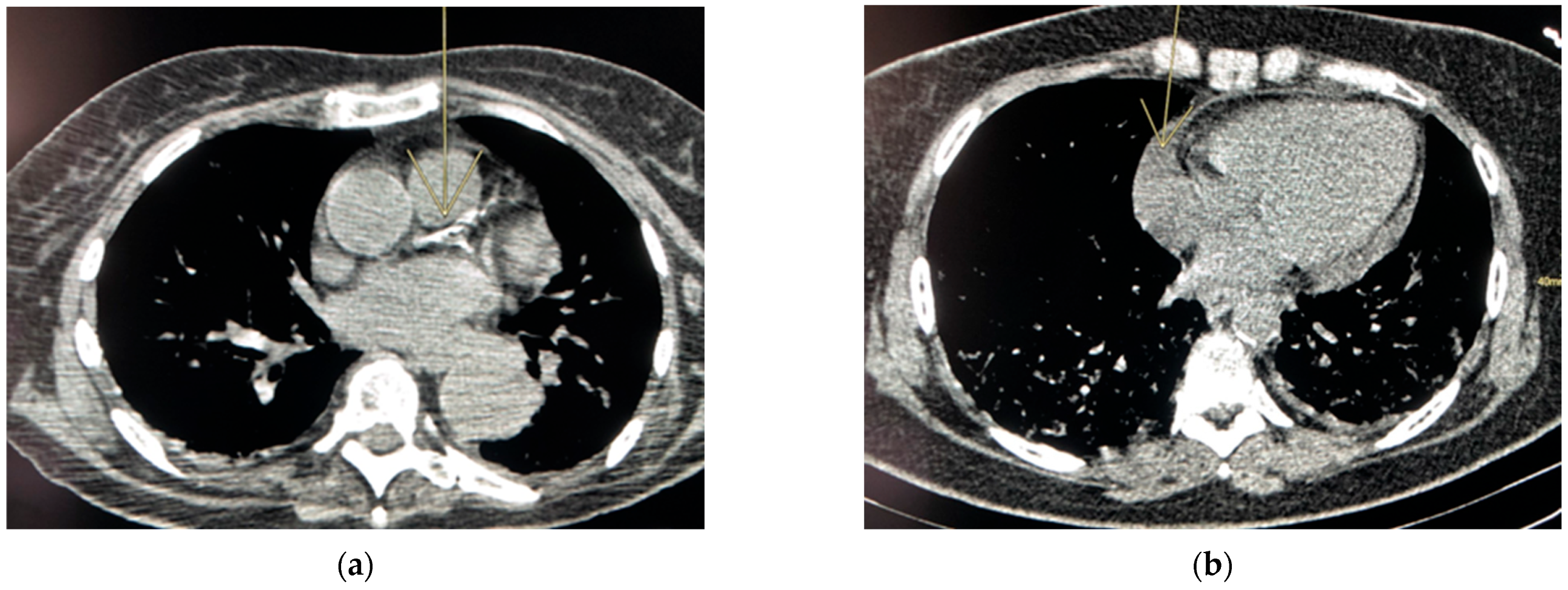
| Cardiovascular diseases | -Signs of calcifying atheromatosis (coronary calcification, presence of stent) |
| -Thoracic aortic aneurysm (diameter> 40 cm) [22] | |
| -Pericardial effusion (centimetric circumferential) [23] | |
| Thyroid lesions | -Thyroid goiter (large thyroid with submerging goiter, presence of thyroid nodule) [24,25] |
| Spinal lesions | -Vertebral compression with loss of height of the vertebral body of a vertebra of the dorsal or lumbar column (L1 and L2) [26] |
| n | Results | |
|---|---|---|
| Age (years) | 362 | 65.2 ± 15.8 |
| Gender, male | 362 | 204 (56.4) |
| Height (cm) | 316 | 170 ± 10 |
| Weight (kg) | 312 | 79.5 ± 19.1 |
| BMI (kg/m2) | 294 | 27.6 ± 6.1 |
| Smoking | 330 | |
| No | 283 (85.8) | |
| Stop > 6 months | 29 (8.8) | |
| Stop ≤ 6 months | 2 (0.6) | |
| Chronic | 5 (1.5) | |
| Occasional use | 3 (0.9) | |
| Yes | 8 (2.4) | |
| Chronic renal failure | 282 | 32 (11.3) |
| Diabetes | 352 | 138 (39.2) |
| High blood pressure | 353 | 206 (58.4) |
| Obesity | 307 | 83 (27.0) |
| Cardiovascular pathology | 279 | 87 (31.2) |
| Chronic pulm. pathology | 346 | 63 (18.2) |
| Immune suppression | 279 | 22 (7.9) |
| Asthma | 305 | 23 (7.5) |
| Oncologic patient | 362 | 48 (13.3) |
| Hospitalization (COVID) | 362 | 330 (91.2) |
| Length of stay (days) | 330 | 10 (6; 20) |
| Intensive care unit | 330 | 72 (21.8) |
| Deceased | 362 | 40 (11.0) |
| At hospital | 17 | |
| Not at hospital | 23 |
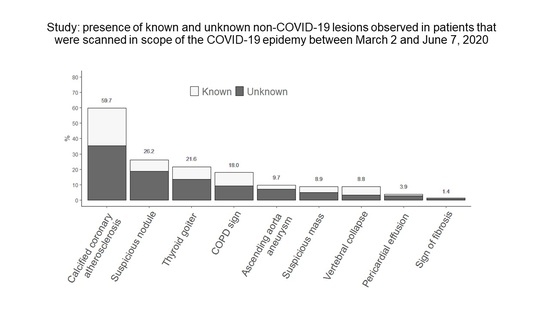
| Absent | Present | Present and Known Based on Data Collected in the PACs | Present and Unknown Based on Data Collected in the PACs | |
|---|---|---|---|---|
| Suspicious nodule | 267 (73.8) | 95 (26.2) | 27 (7.4) | 68 (18.8) |
| Suspicious mass | 330 (91.1) | 32 (8.9) | 14 (3.9) | 18 (5.0) |
| COPD sign | 297 (82.0) | 65 (18.0) | 31 (8.6) | 34 (9.4) |
| Sign of fibrosis | 357 (98.6) | 5 (1.4) | 1 (0.3) | 4 (1.1) |
| Calcified coronary atherosclerosis | 146 (40.3) | 216 (59.7) | 88 (24.3) | 128 (35.4) |
| Ascending aorta aneurysm | 327 (90.3) | 35 (9.7) | 9 (2.5) | 26 (7.2) |
| Pericardial effusion | 348 (96.1) | 14 (3.9) | 4 (1.1) | 10 (2.8) |
| Thyroid goiter | 283 (78.4) | 78 (21.6) | 29 (8.0) | 49 (13.6) |
| Vertebral collapse | 330 (91.2) | 32 (8.8) | 20 (5.5) | 12 (3.3) |
| Total number anomalies | 572 | 223 (39.0) | 349 (61.0) | |
| Number anomalies/patient, mean ± SD | 1.6 ± 1.3 | 0.62 ± 1.1 | 0.96 ± 1.0 | |
| 0 | 82 (22.6) | 251 (69.3) | 143 (39.5) | |
| 1 | 114 (31.5) | 51 (14.1) | 132 (36.5) | |
| 2 | 84 (23.2) | 26 (7.2) | 58 (16.0) | |
| 3 | 50 (13.8) | 19 (5.3) | 19 (5.2) | |
| 4 | 21 (5.8) | 12 (3.3) | 9 (2.5) | |
| 5 | 10 (2.8) | 3 (0.8) | 1 (0.3) | |
| 6 | 1 (0.3) | 0 (0.0) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canivet, P.; Desir, C.; Thys, M.; Henket, M.; Frix, A.-N.; Ernst, B.; Walsh, S.; Occhipinti, M.; Vos, W.; Maes, N.; et al. The Role of Imaging in the Detection of Non-COVID-19 Pathologies during the Massive Screening of the First Pandemic Wave. Diagnostics 2022, 12, 1567. https://doi.org/10.3390/diagnostics12071567
Canivet P, Desir C, Thys M, Henket M, Frix A-N, Ernst B, Walsh S, Occhipinti M, Vos W, Maes N, et al. The Role of Imaging in the Detection of Non-COVID-19 Pathologies during the Massive Screening of the First Pandemic Wave. Diagnostics. 2022; 12(7):1567. https://doi.org/10.3390/diagnostics12071567
Chicago/Turabian StyleCanivet, Perrine, Colin Desir, Marie Thys, Monique Henket, Anne-Noëlle Frix, Benoit Ernst, Sean Walsh, Mariaelena Occhipinti, Wim Vos, Nathalie Maes, and et al. 2022. "The Role of Imaging in the Detection of Non-COVID-19 Pathologies during the Massive Screening of the First Pandemic Wave" Diagnostics 12, no. 7: 1567. https://doi.org/10.3390/diagnostics12071567
APA StyleCanivet, P., Desir, C., Thys, M., Henket, M., Frix, A.-N., Ernst, B., Walsh, S., Occhipinti, M., Vos, W., Maes, N., Canivet, J. L., Louis, R., Meunier, P., & Guiot, J. (2022). The Role of Imaging in the Detection of Non-COVID-19 Pathologies during the Massive Screening of the First Pandemic Wave. Diagnostics, 12(7), 1567. https://doi.org/10.3390/diagnostics12071567