Elucidation of Ischemic Mechanisms of Early Pressure Injury during Post-Decompression and Detecting Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Confirmation, Assessment, and Histological Observation of Ischemia
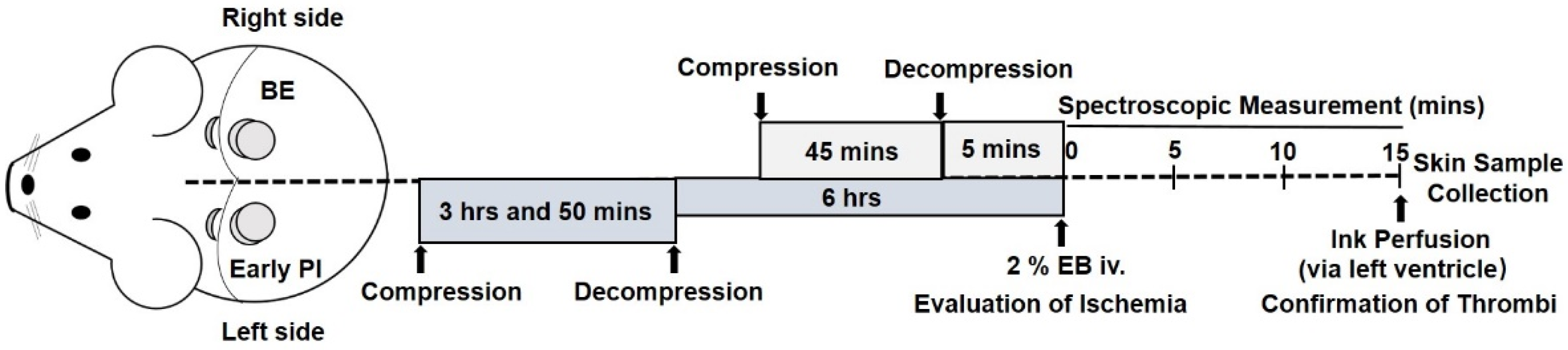
2.1.1. Generation of BE and Early PI Models
2.1.2. Macroscopic Observations
2.1.3. Evaluation of Ischemia
2.1.4. Pathological Analysis
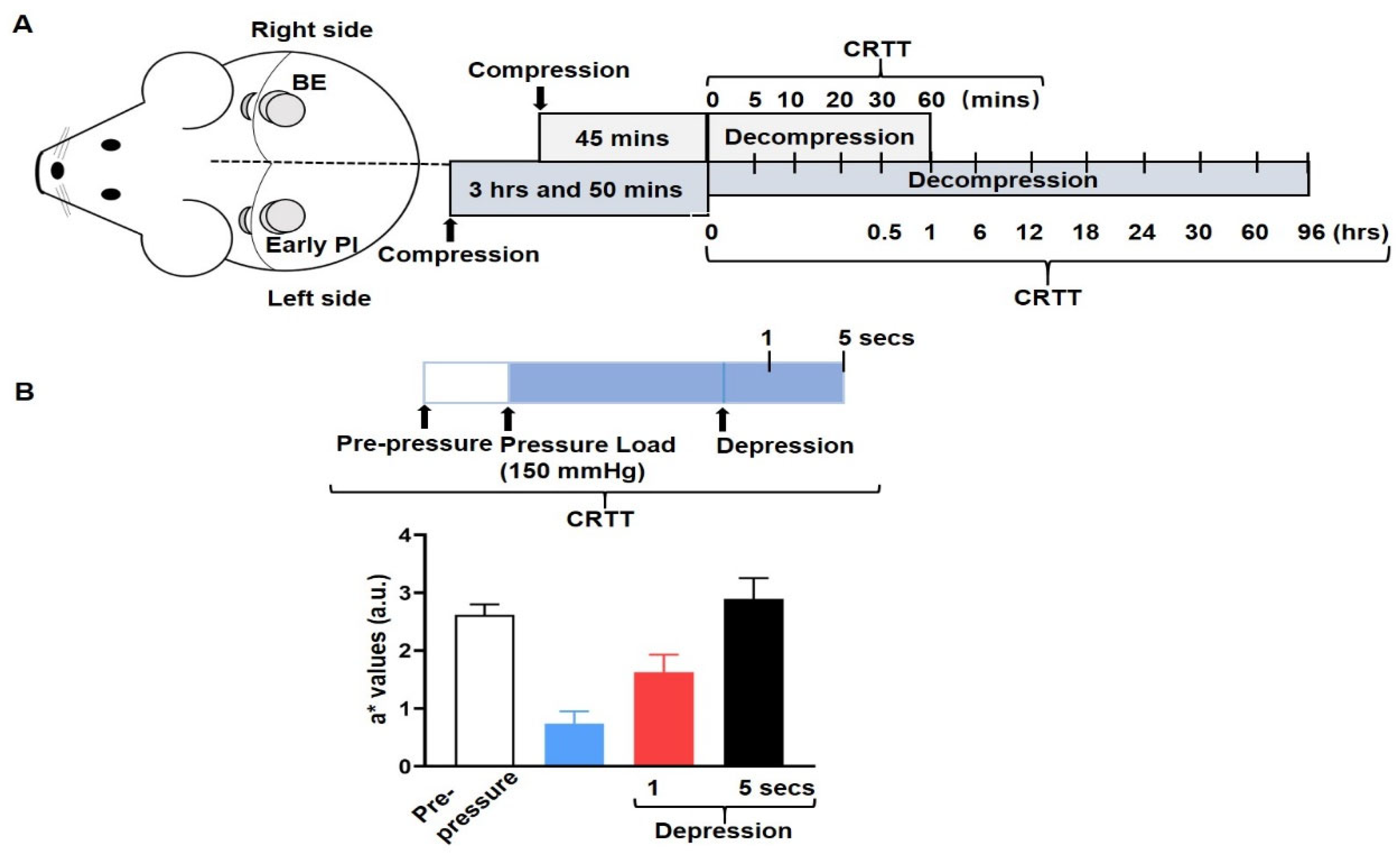
2.2. Measurement of Ischemia—CRTT
Capillary Refill Time Test (CRTT)
2.3. Statistical Analysis
3. Results
3.1. Confirmation of Ischemia after Early PI Decompression
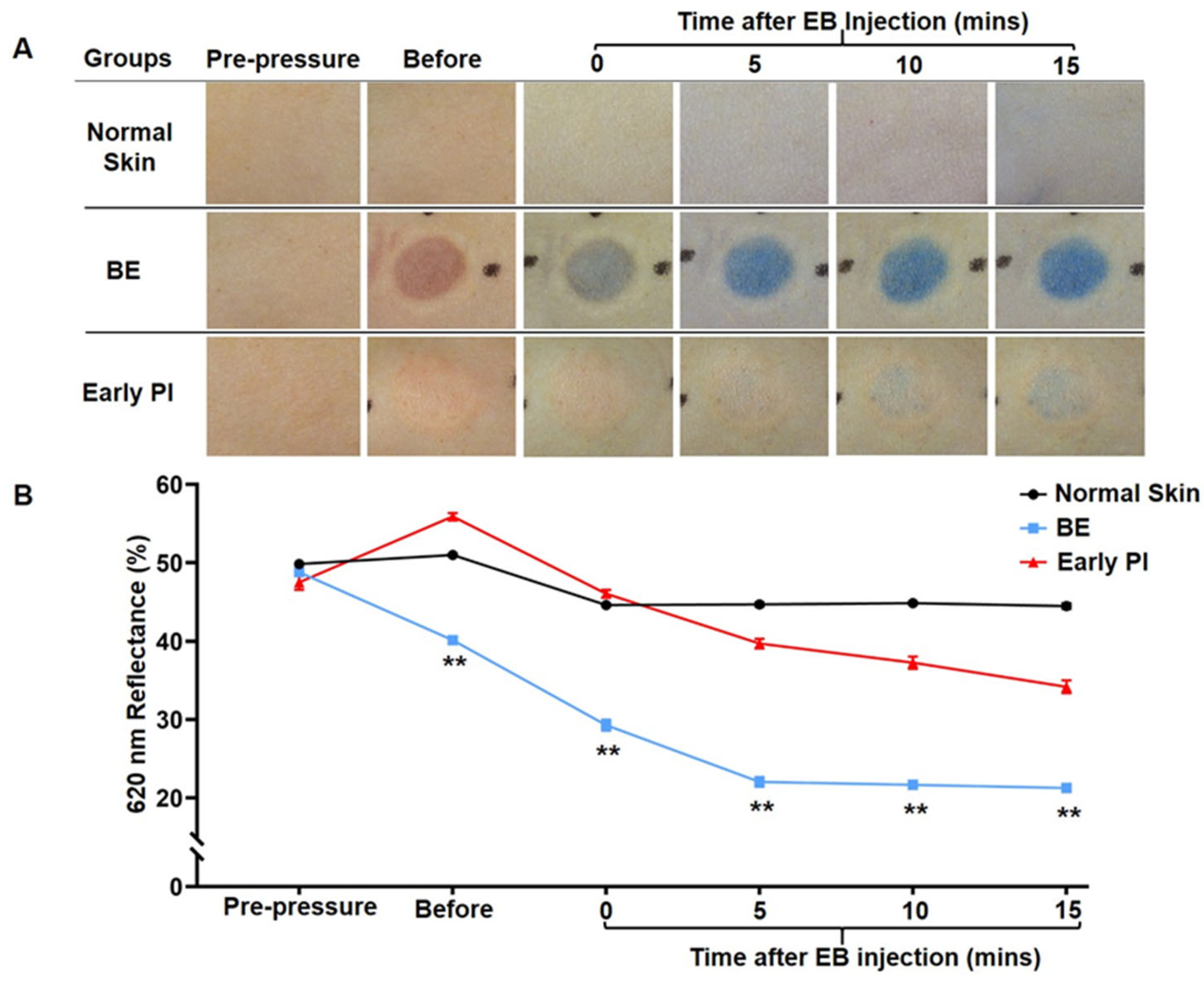
3.1.1. Macroscopic Observation and Spectroscopic Measurement of EB Injection
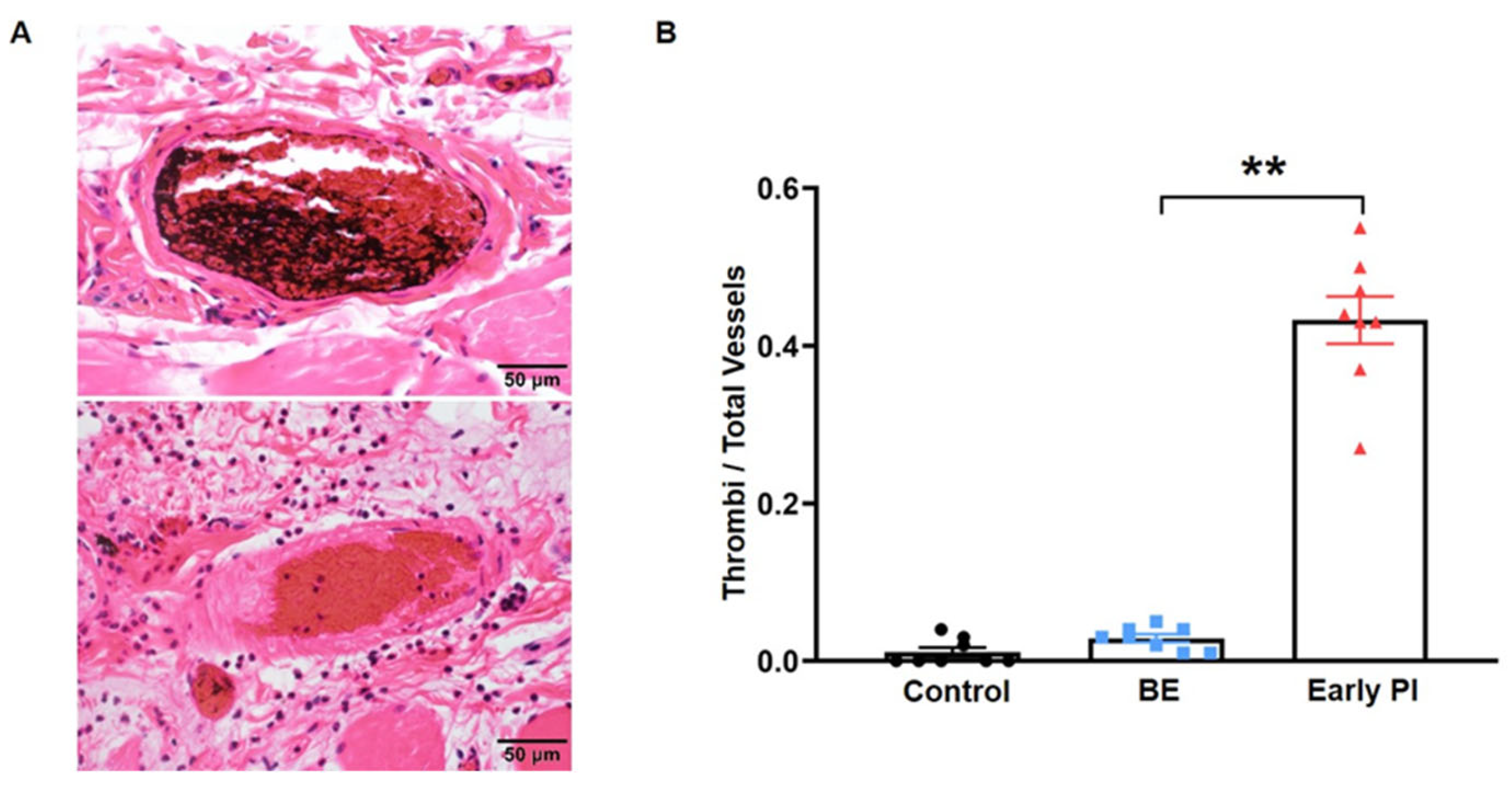
3.1.2. The Early PI Group Had More Thrombi
3.1.3. Observation of Ischemia
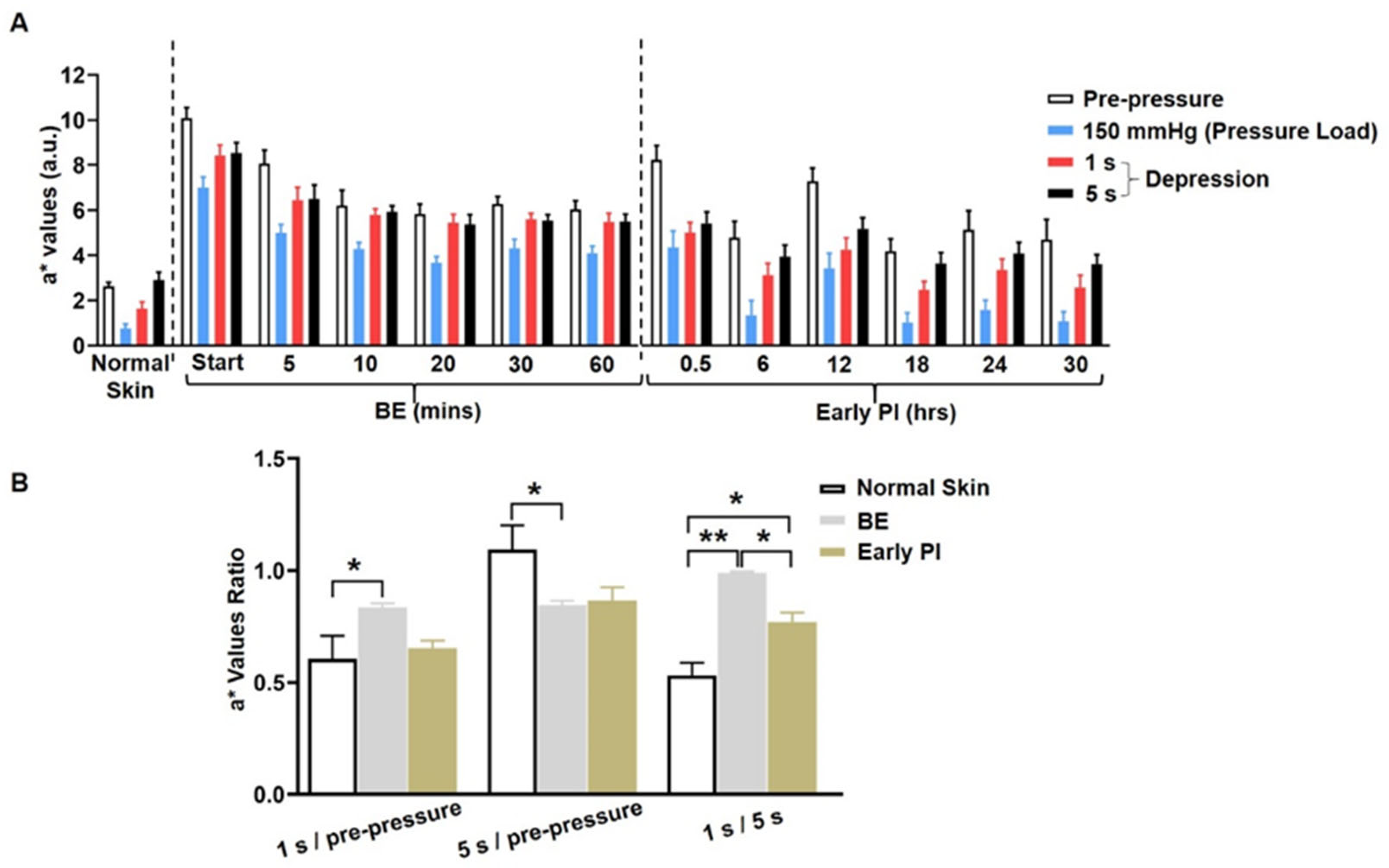
3.2. Measurement of Ischemia
Quantitation of Ischemia by CRTT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline; National Pressure Ulcer Advisory Panel: Washington, DC, USA, 2019. [Google Scholar]
- Reichel, S.M. Shearing force as a factor in decubitus ulcers in paraplegics. J. Am. Med. Assoc. 1958, 166, 762–763. [Google Scholar] [CrossRef] [PubMed]
- Parish, L.C.; Lowthian, P.; Witkowski, J.A. The decubitus ulcer: Many questions but few definitive answers. Clin. Dermatol. 2007, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A.; Brienza, D.M.; Cuddigan, J.; Haesler, E.; Kottner, J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int. Wound J. 2022, 19, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.A.; Barton, M. ACTH and decubitus ulceration: An experimental study. Br. J. Pharmacol. 1969, 36, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.A. The pathogenesis of skin wounds due to pressure. J. Tissue Viability 2006, 16, 12–15. [Google Scholar] [CrossRef]
- Uchiyama, A.; Yamada, K.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Ishikawa, O.; Motegi, S. Topical betamethasone butyrate propionate exacerbates pressure ulcers after cutaneous ischemia-reperfusion injury. Exp. Dermatol. 2016, 25, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, Y.; Takashi, E.; Kamijo, A.; Liang, J.; Fan, J. Establishing an Appropriate Pressure for the Transparent Disc Method to Distinguish Early Pressure Injury and Blanchable Erythema. Diagnostics 2022, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xue, X.; Yu, P.; Ni, Y.; Chen, F. Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol. Imaging 2018, 2018, 7628037. [Google Scholar] [CrossRef] [PubMed]
- Akyurek, M.; Safak, T.; Sonmez, E.; Ozkan, O.; Kecik, A. A new flap design: Neural-island flap. Plast. Reconstr. Surg. 2004, 114, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.X., Jr.; Sonntag, B.V. The axillary tree as a source of musculocutaneous and fasciocutaneous flaps in a fixed-skin porcine model. Ann. Plast. Surg. 1998, 40, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Keenan, A.-M. An evaluation of the reliability and validity of capillary refill time test. Foot 2007, 17, 15–20. [Google Scholar] [CrossRef]
- Lowthian, P.T. Trauma and thrombosis in the pathogenesis of pressure ulcers. Clin. Dermatol. 2005, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.; Karlen, W.; Ansermino, J.M. Capillary refill time: Is it still a useful clinical sign? Anesth. Analg. 2011, 113, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Takashi, E.; Kamijo, A.; Miura, D.; Karasawa, K.; Kitayama, A.; Lu, J.; Zhang, L. Predicting the different progressions of early pressure injury by ultraviolet photography in rat models. Int. Wound J. 2022, 19, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Schriger, D.L.; Baraff, L. Defining normal capillary refill: Variation with age, sex, and temperature. Ann. Emerg. Med. 1988, 17, 932–935. [Google Scholar] [CrossRef]
- Loerakker, S.; Oomens, C.W.; Manders, E.; Schakel, T.; Bader, D.L.; Baaijens, F.P.; Nicolay, K.; Strijkers, G.J. Ischemia-reperfusion injury in rat skeletal muscle assessed with T2-weighted dynamic contrast-enhanced, M.R.I. Magn. Reason. Med. 2011, 66, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Roller, B.T.; Munson, J.M.; Brahma, B.; Santangelo, P.J.; Pai, S.B.; Bellamkonda, R.V. Evans blue nanocarriers visually demarcate margins of invasive gliomas. Drug Deliv. Transl. Res. 2015, 5, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Huang, Z.-J.; Guo, Z.-L.; Feng, Y.-W.; Zhang, C.-Y.; He, G.-Y.; Tang, A.-Z. Plasma volume, cell volume, total blood volume and F factor in the tree shrew. PLoS ONE 2020, 15, e0234835. [Google Scholar] [CrossRef] [PubMed]
- Sosa, T.; Loncarić, L.; Boljesić, S. Capillary refill test—A possible auxiliary diagnostic method in obliterative arteriosclerosis. Cor Vasa 1991, 33, 404–413. [Google Scholar] [PubMed]
- Shinozaki, K.; Saeki, K.; Jacobson, L.S.; Falotico, J.M.; Li, T.; Hirahara, H.; Horie, K.; Kobayashi, N.; Weisner, S.; Lampe, J.W.; et al. Evaluation of accuracy of capillary refill index with pneumatic fingertip compression. J. Clin. Monit. Comput. 2021, 35, 135–145. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Takashi, E.; Hou, P.; Kamijo, A.; Miura, D.; Fan, J. Elucidation of Ischemic Mechanisms of Early Pressure Injury during Post-Decompression and Detecting Methods. Diagnostics 2022, 12, 2198. https://doi.org/10.3390/diagnostics12092198
Chen L, Takashi E, Hou P, Kamijo A, Miura D, Fan J. Elucidation of Ischemic Mechanisms of Early Pressure Injury during Post-Decompression and Detecting Methods. Diagnostics. 2022; 12(9):2198. https://doi.org/10.3390/diagnostics12092198
Chicago/Turabian StyleChen, Lu, En Takashi, Ping Hou, Akio Kamijo, Daiji Miura, and Jianglin Fan. 2022. "Elucidation of Ischemic Mechanisms of Early Pressure Injury during Post-Decompression and Detecting Methods" Diagnostics 12, no. 9: 2198. https://doi.org/10.3390/diagnostics12092198
APA StyleChen, L., Takashi, E., Hou, P., Kamijo, A., Miura, D., & Fan, J. (2022). Elucidation of Ischemic Mechanisms of Early Pressure Injury during Post-Decompression and Detecting Methods. Diagnostics, 12(9), 2198. https://doi.org/10.3390/diagnostics12092198






