Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway
Abstract


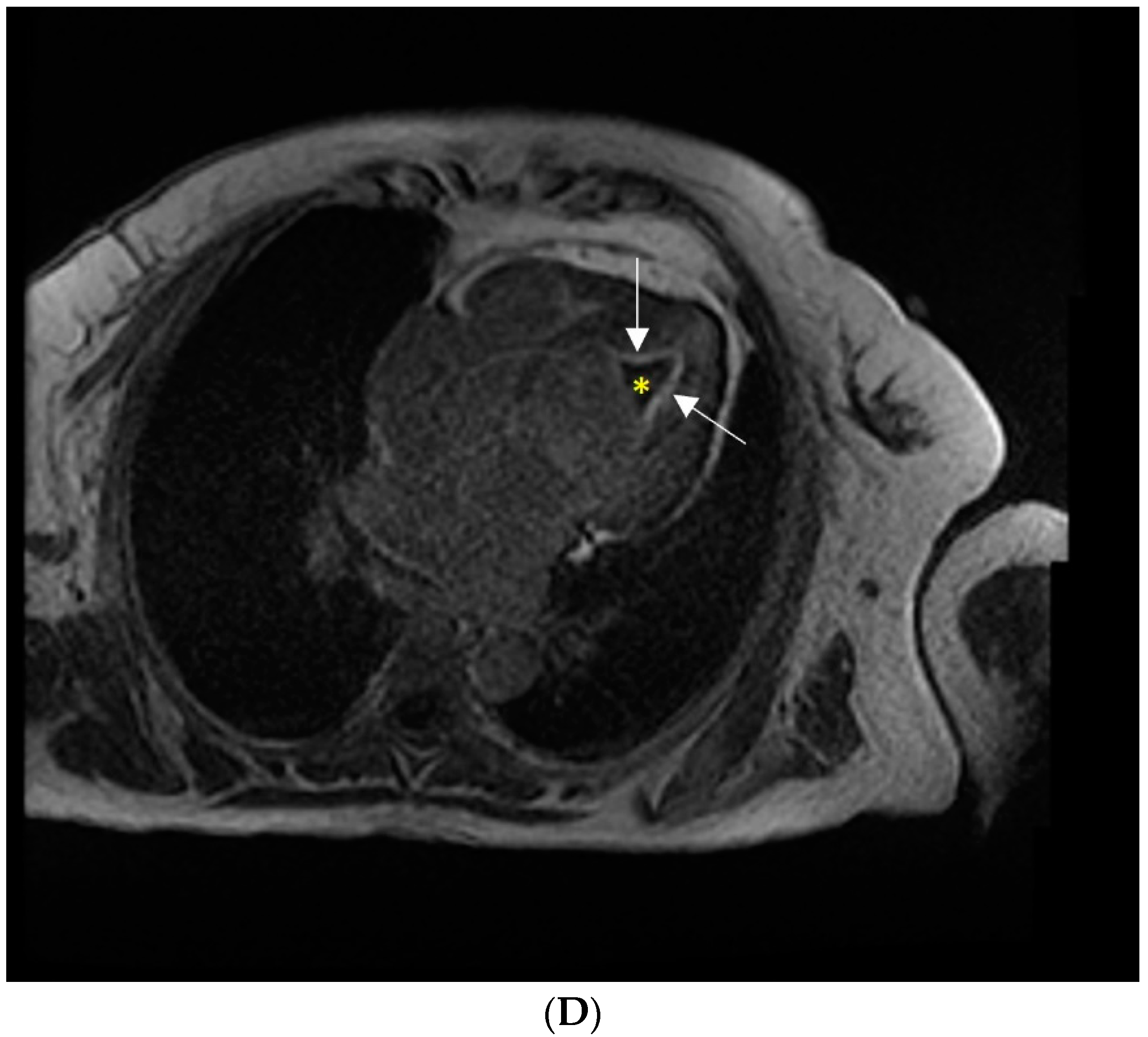
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleinfeldt, T.; Nienaber, C.A.; Kische, S.; Akin, I.; Turan, R.G.; Körber, T.; Schneider, H.; Ince, H. Cardiac Manifestation of the Hypereosinophilic Syndrome: New Insights. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2010, 99, 419–427. [Google Scholar] [CrossRef]
- Park, J.; Hemu, M.; Kalra, D. Loeffler’s Endocarditis: A Diagnosis Made With Cardiac Magnetic Resonance (CMR) Imaging. J. Am. Coll. Cardiol. 2019, 73, 2267. [Google Scholar] [CrossRef]
- Roufosse, F.; Weller, P.F. Practical Approach to the Patient with Hypereosinophilia. J. Allergy Clin. Immunol. 2010, 126, 39–44. [Google Scholar] [CrossRef]
- Habib, G.; Bucciarelli-Ducci, C.; Caforio, A.L.P.; Cardim, N.; Charron, P.; Cosyns, B.; Dehaene, A.; Derumeaux, G.; Donal, E.; Dweck, M.; et al. Multimodality Imaging in Restrictive Cardiomyopathies: An EACVI expert consensus document In collaboration with the “Working Group on myocardial and pericardial diseases” of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur. Heart J. -Cardiovasc. Imaging 2017, 18, 1090–1121. [Google Scholar] [CrossRef] [PubMed]
- Gastl, M.; Behm, P.; Jacoby, C.; Kelm, M.; Bönner, F. Multiparametric Cardiac Magnetic Resonance Imaging (CMR) for the Diagnosis of Loeffler’s Endocarditis: A Case Report. BMC Cardiovasc. Disord. 2017, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, M.; Jevtic, D.; Calvin, A.D.; Petrovic, M.; Paulson, M.; Rueda Prada, L.; Sprecher, L.; Savic, I.; Dumic, I. “Heart in DRESS”: Cardiac Manifestations, Treatment and Outcome of Patients with Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Zuern, C.S.; Gawaz, M. Loeffler Endocarditis: Findings on Magnetic Resonance Imaging. Heart 2007, 93, 354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allderdice, C.; Marcu, C.; Kabirdas, D. Intracardiac Thrombus in Leukemia: Role of Cardiac Magnetic Resonance Imaging in Eosinophilic Myocarditis. CASE Cardiovasc. Imaging Case Rep. 2018, 2, 114–117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, S.; Pop, M.; Mitre, A. Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway. Diagnostics 2022, 12, 2157. https://doi.org/10.3390/diagnostics12092157
Lupu S, Pop M, Mitre A. Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway. Diagnostics. 2022; 12(9):2157. https://doi.org/10.3390/diagnostics12092157
Chicago/Turabian StyleLupu, Silvia, Marian Pop, and Adriana Mitre. 2022. "Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway" Diagnostics 12, no. 9: 2157. https://doi.org/10.3390/diagnostics12092157
APA StyleLupu, S., Pop, M., & Mitre, A. (2022). Loeffler Endocarditis Causing Heart Failure with Preserved Ejection Fraction (HFpEF): Characteristic Images and Diagnostic Pathway. Diagnostics, 12(9), 2157. https://doi.org/10.3390/diagnostics12092157




