Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
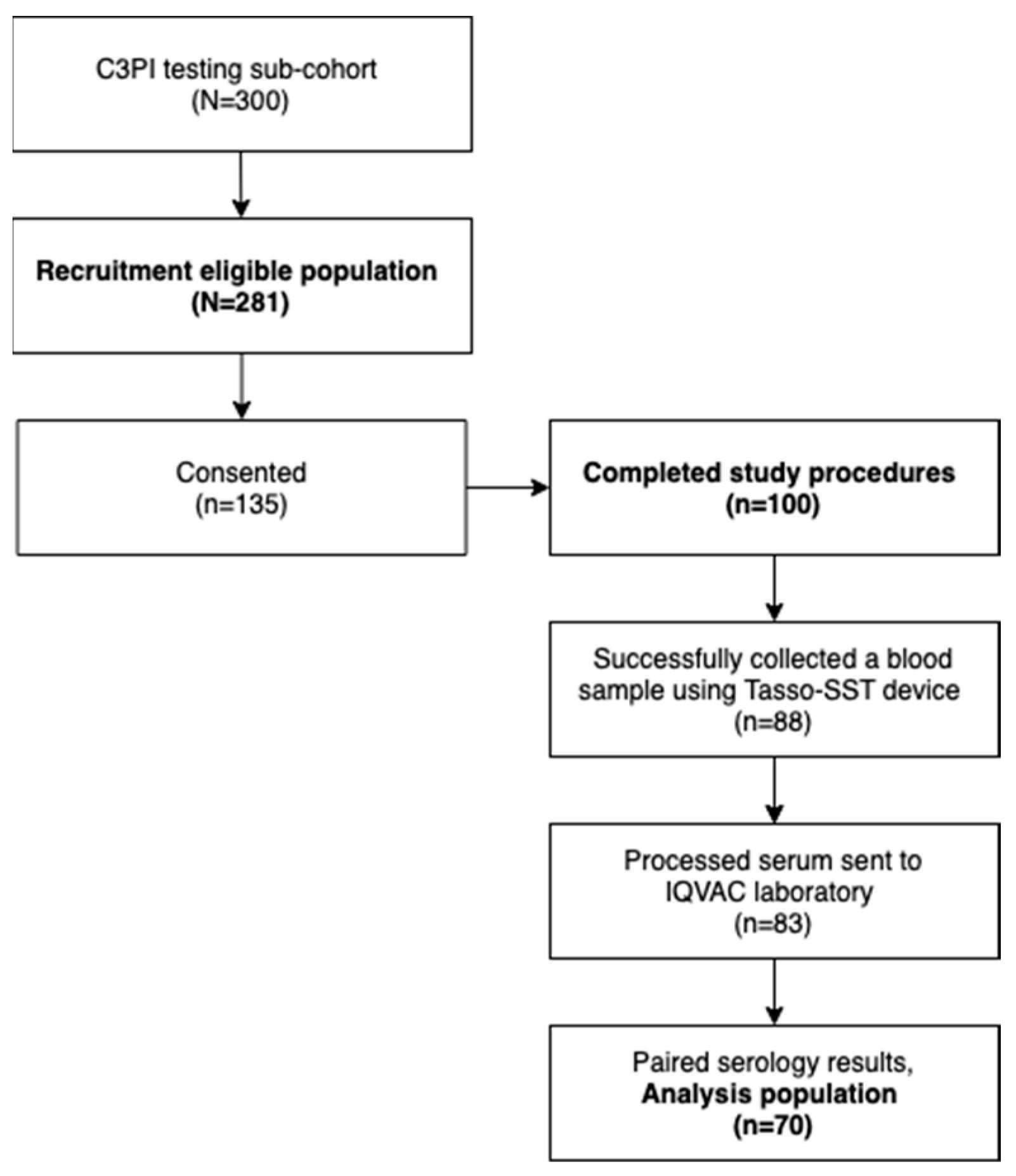
2.2. Participant Recruitment
2.3. Study Visit Procedures
2.4. SARS-CoV-2 Antibody Testing
2.5. After-Visit Survey
- Did you find the instructions for use of the Tasso-SST device easy to follow?
- Do you think you could use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the C3PI Study?
- Would you be willing to use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the C3PI Study?
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Characteristics
3.3. Comparison of SARS-CoV-2 IgG Antibody Testing Results
3.4. User Acceptance of the Tasso-SST Device
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Enrolled Study Population (N = 100) | |
|---|---|
| Did you find the instructions for use of the Tasso-SST device easy to follow? | |
| Yes | 95 (95.0%) |
| No, or do not know/not sure | 5 (5.0%) |
| Do you think you could use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the MURDOCK C3PI Study? | |
| Yes | 91 (91.0%) |
| No, or do not know/not sure | 9 (9.0%) |
| Would you be willing to use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the MURDOCK C3PI Study? | |
| Yes | 90 (90.0%) |
| No, or do not know/not sure | 10 (10.0%) |
| Did you find the instructions for use of the Tasso-SST device easy to follow? | |
| No | Once I saw the demonstration [video], it was simple. |
| No | Needed to be a bit more detailed about what to expect. |
| No | Too much detail for simple procedure. |
| Do not know or not sure | Just was not totally clear what the overall objective was, in order to clearly understand the detailed instructions. |
| Do not know or not sure | Need to emphasize that pushing the red button requires more effort than you might expect. |
| Do you think you could use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the MURDOCK C3PI Study? | |
| No | SAFETY concern. |
| No | NULL |
| No | After 5 min, I only had a small drop of blood in the tube. |
| No | No. I’m not into self-inflicted pain. |
| No | No blood in the collection tube. |
| Do not know or not sure | I was not able to collect blood with supervision. |
| Do not know or not sure | Test did not work on me. |
| Do not know or not sure | Direction were very easy to follow however the device failed to draw blood. |
| Do not know or not sure | I followed the instructions, but blood did not collect in my Tasso device. |
| Would you be willing to use the Tasso-SST device to collect a blood sample at home and send the sample to the lab, if it was an option for the MURDOCK C3PI Study? | |
| No | I am local and it is very easy and convenient to go to the lab. |
| No | Do not think I could collect an adequate sample. |
| No | No, not into self-inflicted pain. |
| No | Blood did not collect in te tube. |
| Do not know or not sure | Same as above [I was not able to collect blood with supervision.] |
| Do not know or not sure | NULL |
| Do not know or not sure | I was not able to collect blood other than at the point of needle entry. Would try again sometime. |
| Do not know or not sure | NULL |
| Do not know or not sure | Not as familiar with the device as the current system. |
| Do not know or not sure | What would happen if it did not work? |
| NULL is listed when participants provided no specific details regarding their response. | |
References
- Elbadawy, H.M.; Khattab, A.; Alalawi, A.; Dakilallah Aljohani, F.; Sundogji, H.; Mahmoud, A.S.; Abouzied, M.; Eltahir, H.M.; Alahmadey, Z.; Bahashwan, S.; et al. The detection of SARS-CoV-2 in outpatient clinics and public facilities during the COVID-19 pandemic. J. Med. Virol. 2021, 93, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Jung, S.E.; Kim, M.S.; Oh, H.S.; Kwon, Y.M.; Lee, J.; Choi, J.H. Analysis of a COVID-19 Prescreening Process in an Outpatient Clinic at a University Hospital during the COVID-19 Pandemic. J. Korean Med. Sci. 2021, 36, e295. [Google Scholar] [CrossRef] [PubMed]
- Jinadatha, C.; Jones, L.D.; Choi, H.; Chatterjee, P.; Hwang, M.; Redmond, S.N.; Navas, M.E.; Zabarsky, T.F.; Bhullar, D.; Cadnum, J.L.; et al. Transmission of SARS-CoV-2 in Inpatient and Outpatient Settings in a Veterans Affairs Health Care System. Open Forum Infect. Dis. 2021, 8, ofab328. [Google Scholar] [CrossRef] [PubMed]
- Neprash, H.T.; Sheridan, B.; Jena, A.B.; Grad, Y.H.; Barnett, M.L. Evidence of Respiratory Infection Transmission Within Physician Offices Could Inform Outpatient Infection Control. Health Aff. 2021, 40, 1321–1327. [Google Scholar] [CrossRef]
- Conserve, D.F.; Mathews, A.; Choko, A.T.; Nelson, L.E. Preparing for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Self-Testing Implementation: Lessons Learned from HIV Self-Testing. Front. Med. 2020, 7, 599521. [Google Scholar] [CrossRef] [PubMed]
- Hoth, A.B.; Shafer, C.; Dillon, D.B.; Mayer, R.; Walton, G.; Ohl, M.E. Iowa TelePrEP: A Public-Health-Partnered Telehealth Model for Human Immunodeficiency Virus Preexposure Prophylaxis Delivery in a Rural State. Sex. Transm. Dis. 2019, 46, 507–512. [Google Scholar] [CrossRef]
- Knitza, J.; Tascilar, K.; Vuillerme, N.; Eimer, E.; Matusewicz, P.; Corte, G.; Schuster, L.; Aubourg, T.; Bendzuck, G.; Korinth, M.; et al. Accuracy and tolerability of self-sampling of capillary blood for analysis of inflammation and autoantibodies in rheumatoid arthritis patients-results from a randomized controlled trial. Arthritis Res. Ther. 2022, 24, 125. [Google Scholar] [CrossRef]
- Norelli, J.; Zlotorzynska, M.; Sanchez, T.; Sullivan, P.S. Scaling Up CareKit: Lessons Learned from Expansion of a Centralized Home HIV and Sexually Transmitted Infection Testing Program. Sex. Transm. Dis. 2021, 48, S66–S70. [Google Scholar] [CrossRef]
- Liao, W.T.; Hsu, M.Y.; Shen, C.F.; Hung, K.F.; Cheng, C.M. Home Sample Self-Collection for COVID-19 Patients. Adv. Biosyst. 2020, 4, e2000150. [Google Scholar] [CrossRef]
- Hall, E.W.; Luisi, N.; Zlotorzynska, M.; Wilde, G.; Sullivan, P.; Sanchez, T.; Bradley, H.; Siegler, A.J. Willingness to Use Home Collection Methods to Provide Specimens for SARS-CoV-2/COVID-19 Research: Survey Study. J. Med. Internet. Res. 2020, 22, e19471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, Y.; Feng, Z.; Zhang, J. Recent advances of functional nucleic acid-based sensors for point-of-care detection of SARS-CoV-2. Mikrochim. Acta 2022, 189, 128. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; De Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef] [PubMed]
- Randriamahazo, T.R.; Andrianarivelo, A.M.; Rakotoarivo, A.T.; Raheritiana, T.M.; Rakotovao, L.A.; Randriamanantany, Z.A.; Rakoto Alson, A.O.; Rasamindrakotroka, A. Evaluation of antigen-based rapid detection test for the diagnosis of SARS CoV-2 in low-income countries. J. Virol. Methods 2022, 300, 114409. [Google Scholar] [CrossRef] [PubMed]
- Asghar, R.; Rasheed, M.; Ul Hassan, J.; Rafique, M.; Khan, M.; Deng, Y. Advancements in Testing Strategies for COVID-19. Biosensors 2022, 12, 410. [Google Scholar] [CrossRef]
- Chau, C.H.; Strope, J.D.; Figg, W.D. COVID-19 Clinical Diagnostics and Testing Technology. Pharmacotherapy 2020, 40, 857–868. [Google Scholar] [CrossRef]
- Mardian, Y.; Kosasih, H.; Karyana, M.; Neal, A.; Lau, C.Y. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front. Med. 2021, 8, 615099. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Babady, E.; Theel, E.S.; Storch, G.A.; Pinsky, B.A.; St George, K.; Smith, T.C.; Bertuzzi, S. Report from the American Society for Microbiology COVID-19 International Summit, 23 March 2020: Value of Diagnostic Testing for SARS-CoV-2/COVID-19. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. COVID-19 Testing: What You Need to Know. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html (accessed on 24 July 2022).
- U.S. Food and Drug Administration. In Vitro Diagnostics EUAs—Serology and Other Adaptive Immune Response Tests for SARS-CoV-2. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2 (accessed on 24 July 2022).
- Klumpp-Thomas, C.; Kalish, H.; Drew, M.; Hunsberger, S.; Snead, K.; Fay, M.P.; Mehalko, J.; Shunmugavel, A.; Wall, V.; Frank, P.; et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat. Commun. 2021, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Brown, B.; Brooks, T.; Semper, A.; Machin, N.; Linley, E.; Borrow, R.; Wyllie, D.; Investigators, E.-H.S. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys (R) high throughput immunoassay. J. Clin. Virol. 2021, 136, 104739. [Google Scholar] [CrossRef]
- McDade, T.W.; McNally, E.M.; Zelikovich, A.S.; D’Aquila, R.; Mustanski, B.; Miller, A.; Vaught, L.A.; Reiser, N.L.; Bogdanovic, E.; Fallon, K.S.; et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS ONE 2020, 15, e0237833. [Google Scholar] [CrossRef]
- Warszawski, J.; Beaumont, A.L.; Seng, R.; de Lamballerie, X.; Rahib, D.; Lydie, N.; Slama, R.; Durrleman, S.; Raynaud, P.; Sillard, P.; et al. Prevalence of SARS-Cov-2 antibodies and living conditions: The French national random population-based EPICOV cohort. BMC Infect. Dis. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Warszawski, J.; Meyer, L.; Franck, J.E.; Rahib, D.; Lydie, N.; Gosselin, A.; Counil, E.; Kreling, R.; Novelli, S.; Slama, R.; et al. Trends in social exposure to SARS-Cov-2 in France. Evidence from the national socio-epidemiological cohort-EPICOV. PLoS ONE 2022, 17, e0267725. [Google Scholar] [CrossRef] [PubMed]
- Mustanski, B.; Saber, R.; Ryan, D.T.; Benbow, N.; Madkins, K.; Hayford, C.; Newcomb, M.E.; Schrock, J.M.; Vaught, L.A.; Reiser, N.L.; et al. Geographic disparities in COVID-19 case rates are not reflected in seropositivity rates using a neighborhood survey in Chicago. Ann. Epidemiol. 2022, 66, 44–51. [Google Scholar] [CrossRef]
- Dube, W.C.; Kellogg, J.T.; Adams, C.; Collins, M.H.; Lopman, B.A.; Johnson, T.M., 2nd; Amin, A.B.; Weitz, J.S.; Fridkin, S.K. Quantifying Risk for SARS-CoV-2 Infection Among Nursing Home Workers for the 2020–2021 Winter Surge of the COVID-19 Pandemic in Georgia, USA. J. Am. Med. Dir. Assoc. 2022, 23, 942–946.e941. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, P.T. Thinking about dried blood spots for pharmacokinetic assays and therapeutic drug monitoring. Bioanalysis 2011, 3, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Coralei, E.; Neighbors, A.W.; Wixted, D.; Heidenfelder, B.; Kingsbury, C.; Register, H.; Louzao, R.; Sloane, R.; Eckstrand, J.; Pieper, C.; et al. The Cabarrus County COVID-19 Prevalence and Immunity (C3PI) Study: Design, Methods, and Baseline Characteristics. Am. J. Transl. Res. 2022, in press. [Google Scholar]
- Bhattacharya, S.; Dunham, A.A.; Cornish, M.A.; Christian, V.A.; Ginsburg, G.S.; Tenenbaum, J.D.; Nahm, M.L.; Miranda, M.L.; Califf, R.M.; Dolor, R.J.; et al. The Measurement to Understand Reclassification of Disease of Cabarrus/Kannapolis (MURDOCK) Study Community Registry and Biorepository. Am. J. Transl. Res. 2012, 4, 458–470. [Google Scholar]
- Tenenbaum, J.D.; Christian, V.; Cornish, M.A.; Dolor, R.J.; Dunham, A.A.; Ginsburg, G.S.; Kraus, V.B.; McHutchison, J.G.; Nahm, M.L.; Newby, L.K.; et al. The MURDOCK Study: A long-term initiative for disease reclassification through advanced biomarker discovery and integration with electronic health records. Am. J. Transl. Res. 2012, 4, 291–301. [Google Scholar]
- Abbott Laboratories. Alinity i SARS-CoV-2 IgG; Abbott Laboratories: Chicago, IL, USA, 2020. [Google Scholar]
- Bryan, A.; Pepper, G.; Wener, M.H.; Fink, S.L.; Morishima, C.; Chaudhary, A.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020, 58, e00941-20. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments; Willey: New York, NY, USA, 1986. [Google Scholar]
- Bland, J.M.; Altman, D.G. Difference versus mean plots. Ann. Clin. Biochem. 1997, 34 Pt 5, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.B.; Gervasi, S.; Song, H.; Bond, A.M.; Chen, A.T.; Bergman, A.; David, G.; Bailey, J.M.; Brooks, R.; Smith-McLallen, A. Telemedicine catches on: Changes in the utilization of telemedicine services during the COVID-19 pandemic. Am. J. Manag. Care. 2022, 28, e1–e6. [Google Scholar] [CrossRef]
- Koonin, L.M.; Hoots, B.; Tsang, C.A.; Leroy, Z.; Farris, K.; Jolly, T.; Antall, P.; McCabe, B.; Zelis, C.B.R.; Tong, I.; et al. Trends in the Use of Telehealth During the Emergence of the COVID-19 Pandemic—United States, January–March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Hendelman, T.; Chaudhary, A.; LeClair, A.C.; van Leuven, K.; Chee, J.; Fink, S.L.; Welch, E.J.; Berthier, E.; Quist, B.A.; Wald, A.; et al. Self-collection of capillary blood using Tasso-SST devices for Anti-SARS-CoV-2 IgG antibody testing. PLoS ONE 2021, 16, e0255841. [Google Scholar] [CrossRef] [PubMed]
- Vusirikala, A.; Whitaker, H.; Jones, S.; Tessier, E.; Borrow, R.; Linley, E.; Hoschler, K.; Baawuah, F.; Ahmad, S.; Andrews, N.; et al. Seroprevalence of SARS-CoV-2 antibodies in university students: Cross-sectional study, December 2020, England. J. Infect. 2021, 83, 104–111. [Google Scholar] [CrossRef]

| Variable 1 | Primary Analysis Population (N = 70) | Enrolled Study Population (N = 100) | Recruitment Eligible Population (N = 281) |
|---|---|---|---|
| Age (in years) | 58.0 (45.0, 68.0) | 56.0 (49.0, 67.0) | 57.0 (49.0, 68.0) |
| Male | 27 (38.6) | 40 (40.0) | 108 (38.4) |
| Race | |||
| White/Caucasian | 57 (81.4) | 85 (85.0) | 226 (80.4) |
| Black or African American | 8 (11.4) | 10 (10.0) | 46 (16.4) |
| American Indian or Alaska Native | 1 (1.4) | 1 (1.0) | 1 (0.4) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 1 (1.4) | 1 (1.0) | 4 (1.4) |
| Multiple | 3 (4.3) | 3 (3.0) | 4 (1.4) |
| Don’t know, not sure, prefer not to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hispanic | 7 (10.0) | 7 (7.0) | 18 (6.4) |
| Medical Comorbidities 2 | |||
| Osteoarthritis | 23 (32.9) | 36 (36.0) | 89 (31.7) |
| Rheumatoid arthritis | 9 (12.9) | 15 (15.0) | 27 (9.6) |
| Diabetes | 7 (10.0) | 11 (11.0) | 34 (12.1) |
| COVID-19 Infection Status | |||
| Positive study test | 7 (10.0) | 8 (8.0) | 33 (11.7) |
| Days since positive study test | 42.5 (37.0, 120.0) | 43.0 (37.0, 168.0) | N/A |
| Highest Education Level | |||
| Less than high school graduate | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| High school graduate/GED | 4 (5.7) | 7 (7.0) | 15 (5.3) |
| Some college/associate’s degree | 26 (37.1) | 30 (30.0) | 86 (30.6) |
| Bachelor’s degree | 24 (34.3) | 36 (36.0) | 95 (33.8) |
| Master’s or higher professional degree | 16 (22.9) | 27 (27.0) | 84 (29.9) |
| Tasso-SST Device | ||||
| Negative | Positive | Total | ||
| Phlebotomy | Negative | 65 (92.9%) | 0 (0.0%) | 65 (92.9%) |
| Positive | 1 (1.4%) | 4 (5.7%) | 5 (7.1%) | |
| Total | 66 (94.3%) | 4 (5.7%) | 70 (100.0%) | |
| Continuous Index | Samples Collected via Tasso-SST (n = 70) | Samples Collected via Routine Phlebotomy (n = 70) |
|---|---|---|
| Median (25th, 75th percentile) | 0.02 (0.02, 0.07) | 0.04 (0.02, 0.09) |
| Mean (SD) | 0.43 (1.42) | 0.50 (1.48) |
| Minimum, Maximum | 0.00, 7.44 | 0.01, 7.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wixted, D.; Neighbors, C.E.; Pieper, C.F.; Wu, A.; Kingsbury, C.; Register, H.; Petzold, E.; Newby, L.K.; Woods, C.W. Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing. Diagnostics 2022, 12, 1857. https://doi.org/10.3390/diagnostics12081857
Wixted D, Neighbors CE, Pieper CF, Wu A, Kingsbury C, Register H, Petzold E, Newby LK, Woods CW. Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing. Diagnostics. 2022; 12(8):1857. https://doi.org/10.3390/diagnostics12081857
Chicago/Turabian StyleWixted, Douglas, Coralei E. Neighbors, Carl F. Pieper, Angie Wu, Carla Kingsbury, Heidi Register, Elizabeth Petzold, L. Kristin Newby, and Christopher W. Woods. 2022. "Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing" Diagnostics 12, no. 8: 1857. https://doi.org/10.3390/diagnostics12081857
APA StyleWixted, D., Neighbors, C. E., Pieper, C. F., Wu, A., Kingsbury, C., Register, H., Petzold, E., Newby, L. K., & Woods, C. W. (2022). Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing. Diagnostics, 12(8), 1857. https://doi.org/10.3390/diagnostics12081857







