Melanoma Detection by AFM Indentation of Histological Specimens
Abstract
:1. Introduction
2. Results
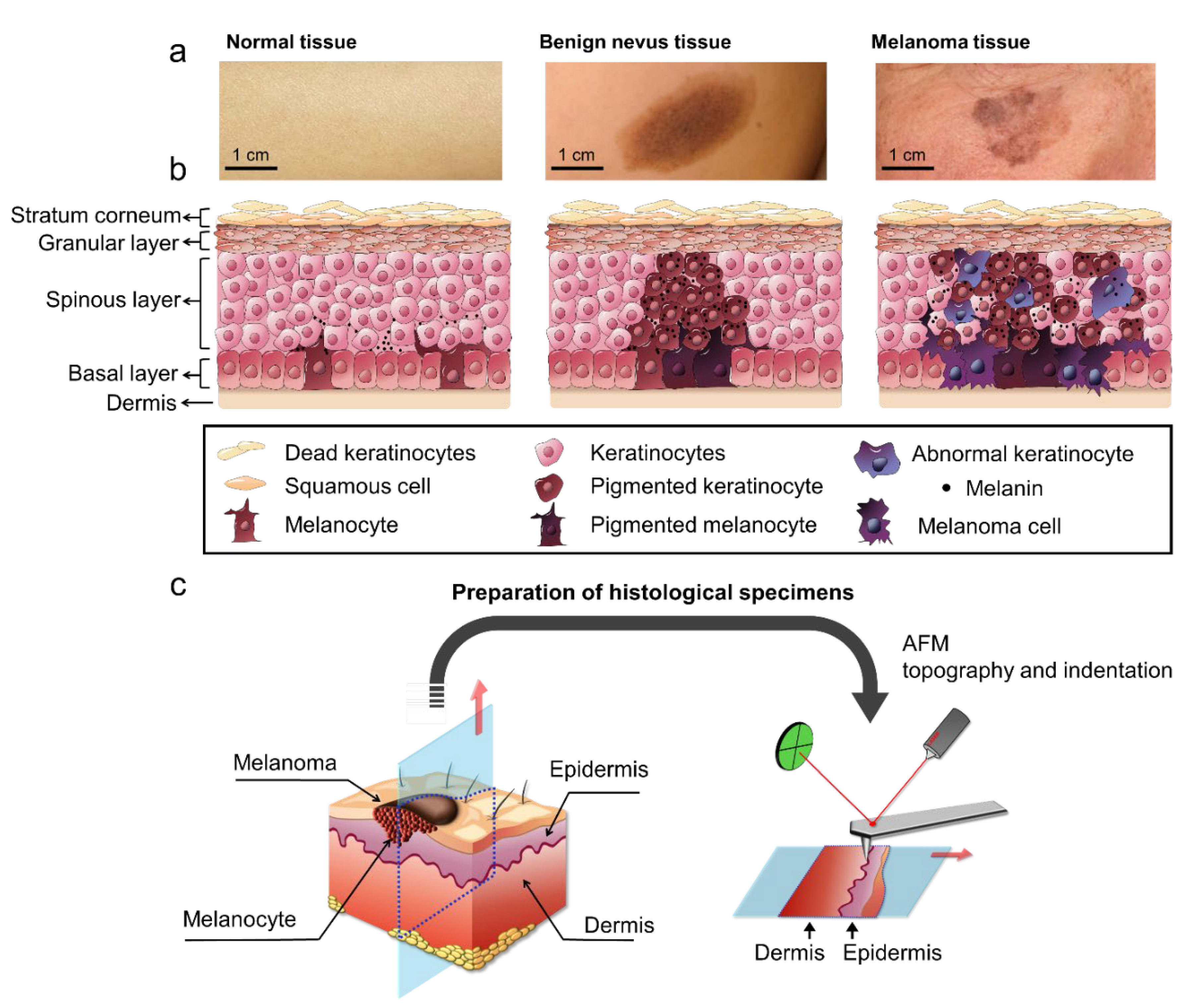
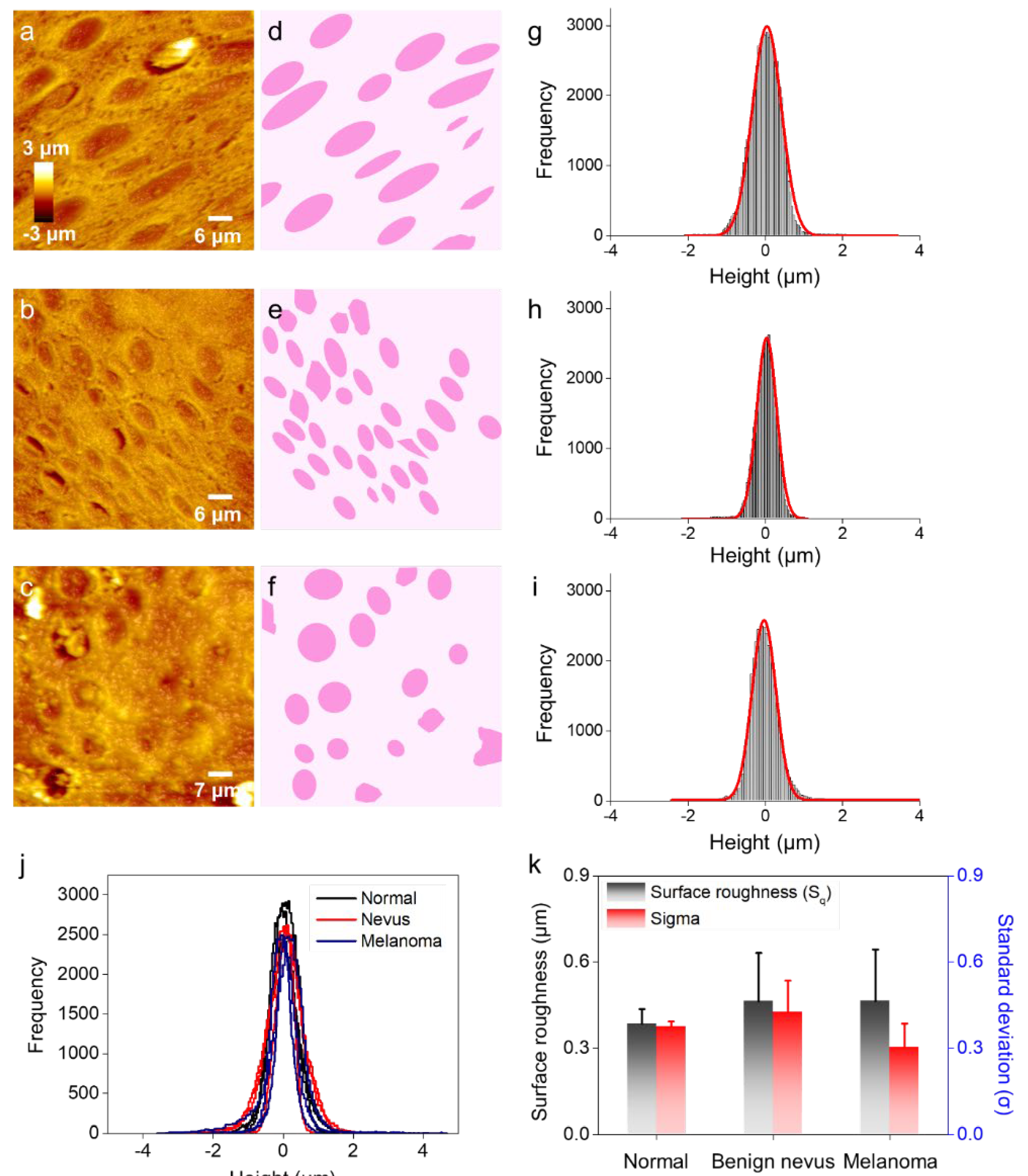
2.1. Surface Profiling of Histological Specimens by Using AFM
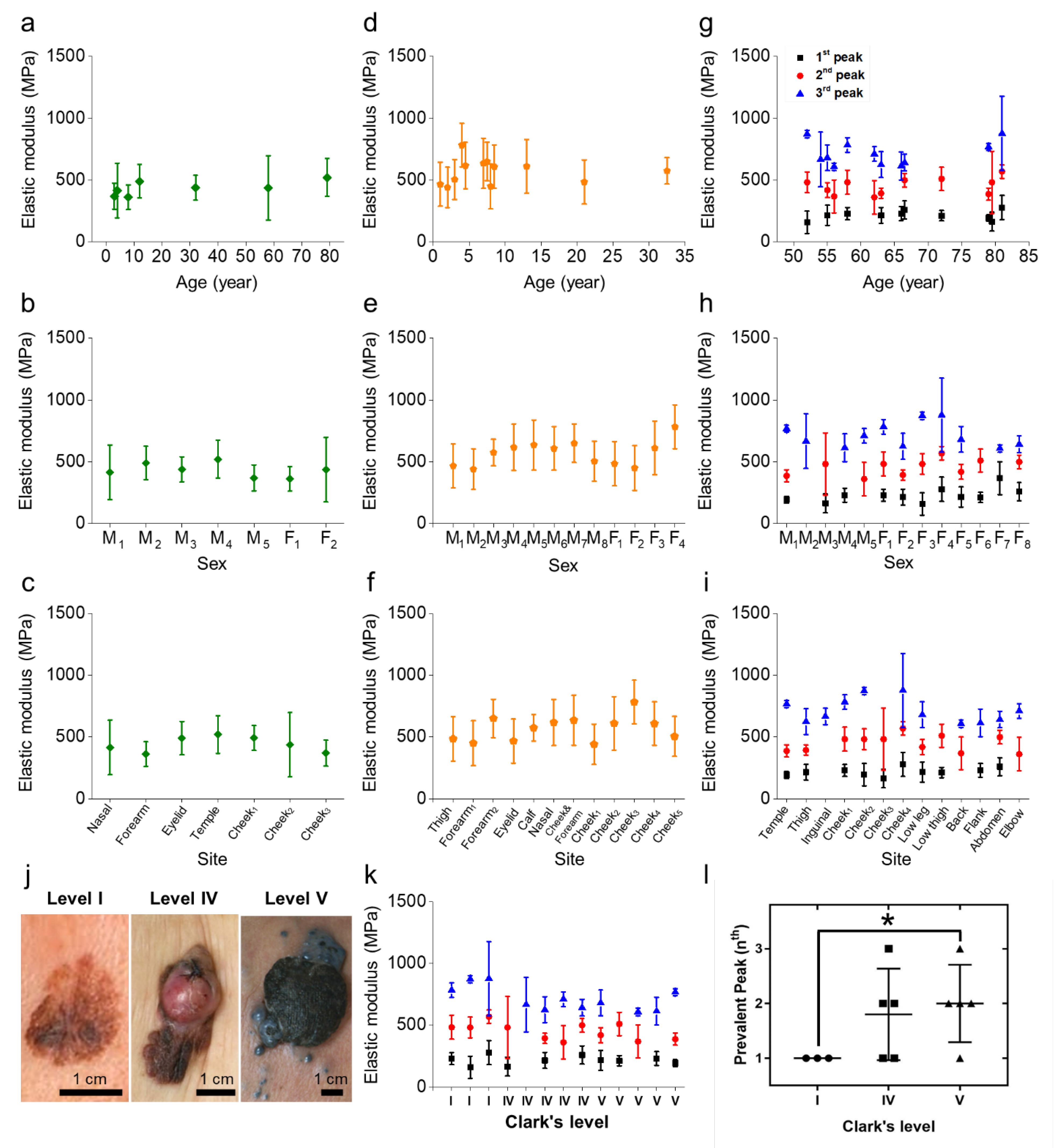
2.2. Mechanical Properties of Histological Specimens That Can Discriminate between Benign Nevus and Melanoma
2.3. Versatility of the Mechanical Specimen Signatures Regardless of Age, Sex, or Site
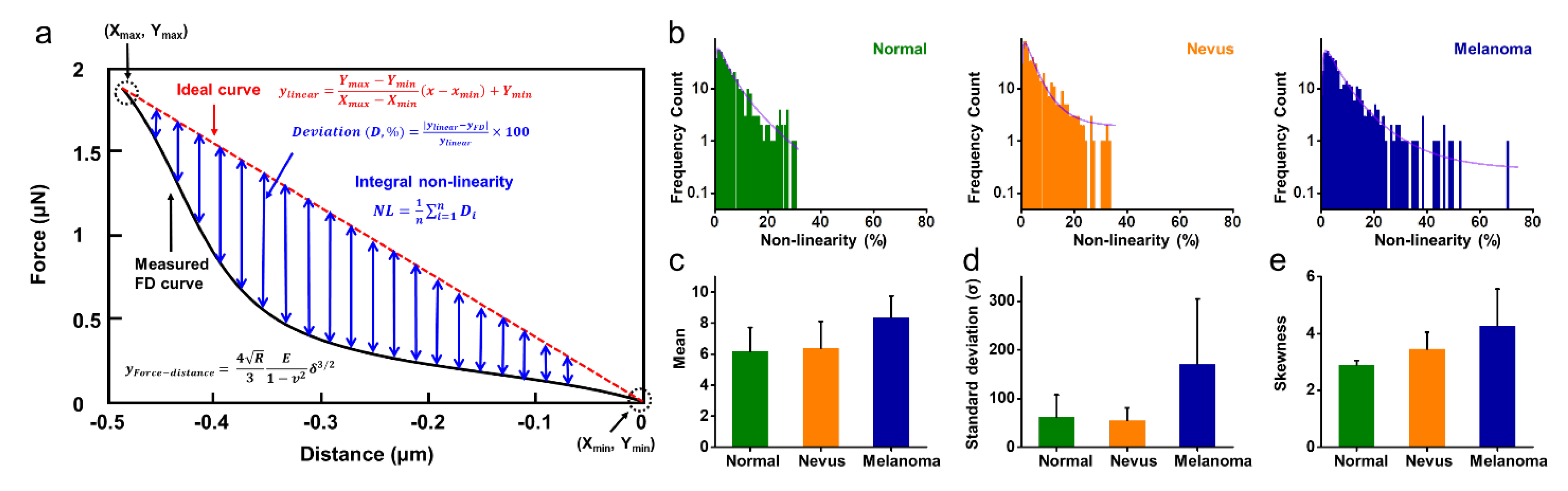
2.4. Non-Linearity of FD Curves for Normal, Benign Nevus, and Melanoma
3. Discussion
4. Methods
4.1. Biopsy from Patients
4.2. Preparation of Histological Specimens
4.3. Histological Analysis
4.4. AFM Topography of Histological Specimens
4.5. AFM Indentation of Histological Specimens
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, A.J.; Mihm, M.C.J. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Zhuang, L.; Palmer, A.A.; Thompson, J.F.; McCarthy, S.W. Combined naevus: A benign lesion frequently misdiagnosed both clinically and pathologically as melanoma. Pathology 2004, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.E.; Bourke, J.F.; Graham-Brown, R.A.C.; Hutchinson, P.E. False negative clinical diagnoses of malignant melanoma. Br. J. Dermatol. 1999, 140, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Tannous, Z.S.; Mihma, M.C.; Flottea, T.J.; Gonzálezb, S. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: Comparison of in vivo confocal images with histologic sections. J. Am. Acad. Dermatol. 2002, 46, 260–263. [Google Scholar] [CrossRef]
- Puig, S.; Argenziano, G.; Zalaudek, I.; Ferrara, G.; Palou, J.; Massi, D.; Hofmann-Wellenhof, R.; Soyer, H.P.; Malvehy, J. Melanomas that failed dermoscopic detection: A combined clinicodermoscopic approach for not missing melanoma. Dermatol. Surg. 2007, 33, 1262–1273. [Google Scholar] [CrossRef]
- Carli, P.; Mannone, F.; de Giorgi, V.; Nardini, P.; Chiarugi, A.; Giannotti, B. The problem of false-positive diagnosis in melanoma screening: The impact of dermoscopy. Melanoma Res. 2003, 13, 179–182. [Google Scholar] [CrossRef]
- Gambichler, T.; Moussa, G.; Bahrenberg, K.; Vogt, M.; Ermert, H.; Weyhe, D.; Altmeyer, P.; Hoffmann, K. Preoperative Ultrasonic Assessment of Thin Melanocytic Skin Lesions Using a 100-MHz Ultrasound Transducer: A Comparative Study. Dermatol. Surg. 2007, 33, 818–824. [Google Scholar]
- Uren, R.F.; Sanki, A.; Thompson, J.F. The utility of ultrasound in patients with melanoma. Expert Rev. Anticancer Ther. 2007, 7, 1633–1642. [Google Scholar] [CrossRef]
- Celebi, M.E.; Iyatomi, H.; Schaefer, G.; Stoecker, W.V. Lesion border detection in dermoscopy images. Comput. Med. Imaging Graph. 2009, 33, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, M.; Gass, R.; Rehg, J.M.; Ferris, L.; Ho, J.; Drogowski, L. Feature-preserving artifact removal from dermoscopy images. Proc. SPIE 2008, 6914, 439–447. [Google Scholar]
- Ulrik, B.; Erik, B.; Peter, Å.; Ingrid, N.; Stig, O. Non-invasive bioimpedance of intact skin: Mathematical modeling and experiments. Physiol. Meas. 2011, 32, 1–18. [Google Scholar]
- Mohr, P.; Birgersson, U.; Berking, C.; Henderson, C.; Trefzer, U.; Kemeny, L.; Sunderkötter, C.; Dirschka, T.; Motley, R.; Frohm-Nilsson, M.; et al. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res. Technol. 2013, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Glickman, Y.A.; Filo, O.; David, M.; Yayon, A.; Topaz, M.; Zamir, B.; Ginzburg, A.; Rozenman, D.; Kenan, G. Electrical impedance scanning: A new approach to skin cancer diagnosis. Skin Res. Technol. 2003, 9, 262–268. [Google Scholar] [CrossRef]
- Åberg, P.; Birgersson, U.; Elsner, P.; Mohr, P.; Ollmar, S. Electrical impedance spectroscopy and the diagnostic accuracy for malignant melanoma. Exp. Dermatol. 2011, 20, 648–652. [Google Scholar] [CrossRef]
- Wolf, I.H.; Smolle, J.; Soyer, H.P.; Kerl, H. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998, 8, 425–429. [Google Scholar] [CrossRef]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the skin: Cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Watt, F.M.; Fujiwara, H. Cell-Extracellular Matrix Interactions in Normal and Diseased Skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, M.J.; Sood, A.; Sevinsky, C.; Pris, A.D.; Zavodszky, M.I.; Ginty, F. Emerging Understanding of Multiscale Tumor Heterogeneity. Front. Oncol. 2014, 4, 366. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, P.P.; Eliceiri, K.W.; Keely, P.J. Shining new light on 3D cell motility and the metastatic process. Trends Cell Biol. 2009, 19, 638–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues that Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giussani, M.; Merlino, G.; Cappelletti, V.; Tagliabue, E.; Daidone, M.G. Tumor-extracellular matrix interactions: Identification of tools associated with breast cancer progression. Semin. Cancer Biol. 2015, 35, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveh Tilleman, T.; Tilleman, M.; Neumann, H. The elastic properties of cancerous skin: Poisson’s ratio and Young’s modulus. In Optimization of Incisions in Cutaneous Surgery Including Mohs’ Micrographic Surgery; Erasmus University Rotterdam: Rotterdam, The Netherlands, 2004; pp. 105–116. Available online: https://www.researchgate.net/publication/254757833_Optimization_of_Incisions_in_Cutaneous_Surgery_including_Mohs%27_Micrographic_Surgery (accessed on 14 May 2022).
- Taloni, A.; Alemi, A.A.; Ciusani, E.; Sethna, J.P.; Zapperi, S.; La Porta, C.A.M. Mechanical Properties of Growing Melanocytic Nevi and the Progression to Melanoma. PLoS ONE 2014, 9, e94229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarna, M.; Zadlo, A.; Pilat, A.; Olchawa, M.; Gkogkolou, P.; Burda, K.; Böhm, M.; Sarna, T. Nanomechanical analysis of pigmented human melanoma cells. Pigment Cell Melanoma Res. 2013, 26, 727–730. [Google Scholar] [CrossRef]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef] [Green Version]
- Seiberg, M. Keratinocyte–Melanocyte Interactions During Melanosome Transfer. Pigment Cell Res. 2001, 14, 236–242. [Google Scholar] [CrossRef]
- Delevoye, C. Melanin Transfer: The Keratinocytes Are More than Gluttons. J. Investig. Dermatol. 2014, 134, 877–879. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wang, N.; Tsurui, H.; Kato, M.; Iida, M.; Kobayashi, T. Noninvasive, label-free, three-dimensional imaging of melanoma with confocal photothermal microscopy: Differentiate malignant melanoma from benign tumor tissue. Sci. Rep. 2016, 6, 30209. [Google Scholar] [CrossRef] [Green Version]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; LimRoderick, Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Tian, M.; Li, Y.; Liu, W.; Jin, L.; Jiang, X.; Wang, X.; Ding, Z.; Peng, Y.; Zhou, J.; Fan, J.; et al. The nanomechanical signature of liver cancer tissues and its molecular origin. Nanoscale 2015, 7, 12998–13010. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.J.; Roy, R.; Chen, W.; Chekmareva, M.A.; Foran, D.J.; Desai, J.P. Accurate characterization of benign and cancerous breast tissues: Aspecific patient studies using piezoresistive microcantilevers. Biosens. Bioelectron. 2015, 63, 414–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Lee, S.W.; Lee, G.; Yoon, D.S. Atomic force microscopy analysis of EPPS-Driven degradation and reformation of amyloid-β aggregates. J. Alzheimer’s Dis. Rep. 2018, 2, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.G.; Lee, D.; Lee, S.W.; Kim, I.; Kim, Y.; Jang, J.W.; Lee, J.H.; Lee, G.; Yoon, D.S. Nanoindentation for Monitoring the Time-Variant Mechanical Strength of Drug-Loaded Collagen Hydrogel Regulated by Hydroxyapatite Nanoparticles. ACS Omega 2021, 6, 9269–9278. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Lee, G.; Kim, I.; Lee, S.W.; Kim, W.; Lee, S.W.; Lee, J.H.; Park, J.; Yoon, D.S. Extremely sensitive and wide-range silver ion detection via assessing the integrated surface potential of a DNA-capped gold nanoparticle. Nanotechnology 2019, 30, 085501. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, D.; Kim, I.; Lee, S.W.; Lee, W.; Hwang, K.S.; Lee, J.H.; Lee, G.; Yoon, D.S. Plasmonic nanoparticle amyloid corona for screening Aβ oligomeric aggregate-degrading drugs. Nat. Commun. 2021, 12, 639. [Google Scholar] [CrossRef]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012, 41, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.; Schwarzer, N.; Sherratt, M.J.; Watson, R.E.B.; Graham, H.K.; Trafford, A.W.; Mummery, P.M.; Derby, B. Nanoindentation of histological specimens: Mapping the elastic properties of soft tissues. J. Mater. Res. 2009, 24, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Longo, C.; Casari, A.; Beretti, F.; Cesinaro, A.M.; Pellacani, G. Skin aging: In vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J. Am. Acad. Dermatol. 2013, 68, e73–e82. [Google Scholar] [CrossRef]
- Cua, A.B.; Wilhelm, K.-P.; Maibach, H.I. Elastic properties of human skin: Relation to age, sex, and anatomical region. Arch. Dermatol. Res. 1990, 282, 283–288. [Google Scholar] [CrossRef]
- Chen, K.G.; Leapman, R.D.; Zhang, G.; Lai, B.; Valencia, J.C.; Cardarelli, C.O.; Vieira, W.D.; Hearing, V.J.; Gottesman, M.M. Influence of melanosome dynamics on melanoma drug sensitivity. J. Natl. Cancer Inst. 2009, 101, 1259–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, Y.; Lee, M.; An, S.; Cho, S.J. Quantitative Visualization of the Nanomechanical Young’s Modulus of Soft Materials by Atomic Force Microscopy. Nanomaterials 2021, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lin, L.; Tang, B.; Du, C. Structure and nanomechanics of collagen fibrils in articular cartilage at different stages of osteoarthritis. RSC Adv. 2014, 4, 51165–51170. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver&Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar]

| Case No. | Age/Sex | Biomechanical Property | Histopathological Diagnosis | ||
|---|---|---|---|---|---|
| 1st Peak (0~300 MPa) | 2nd Peak (300~600 MPa) | 3rd Peak (600~900 MPa) | |||
| 1 | 4/Male | 415 ± 221 MPa | Normal | ||
| 2 | 8/Female | 401 ± 148 MPa | Normal | ||
| 3 | 12/Male | 491 ± 135 MPa | Normal | ||
| 4 | 32/Male | 439 ± 101 MPa | Normal | ||
| 5 | 79/Male | 521 ± 153 MPa | Normal | ||
| 6 | 58/Female | 437 ± 261 MPa | Normal | ||
| 7 | 3/Male | 370 ± 105 MPa | Normal | ||
| 8 | 21/Female | 485 ± 179 MPa | Intradermal nevus (rt. Thigh) | ||
| 9 | 8/Female | 450 ± 182 MPa | Congenital melanocytic nevus (lt. Forearm) | ||
| 10 | 1/Male | 467 ± 178 MPa | Congenital melanocytic nevus (rt. Eyelid) | ||
| 11 | 2/Male | 441 ± 164 MPa | Congenital melanocytic nevus (rt. Cheek) | ||
| 12 | 32/Male | 575 ± 107 MPa | Compound nevus (rt. Calf) | ||
| 13 | 3/Male | 505 ± 162 MPa | Congenital melanocytic nevus (rt. Cheek) | ||
| 14 | 13/Female | 611 ± 217 MPa | Compound nevus (lt. Cheek) | ||
| 15 | 4/Female | 848 ± 299 MPa | Congenital melanocytic nevus (rt. Cheek) | ||
| 16 | 4/Male | 618 ± 187 MPa | Congenital melanocytic nevus (rt. Nasal area) | ||
| 17 | 7/Male | 636 ± 203 MPa | Congenital melanocytic nevus (rt. Cheek & forearm) | ||
| 18 | 8/Male | 609 ± 176 MPa | Compound nevus (rt. Cheek) | ||
| 19 | 7/Male | 650 ± 156 MPa | Congenital melanocytic nevus (rt. Forearm) | ||
| 20 | 12/Female | 783 ± 177 MPa | Congenital melanocytic nevus (lt. cheek) | ||
| 21 | 58/Female | 229 ± 49 MPa | 483 ± 97 MPa | 784 ± 59 MPa | Malignant melanoma, Clark’s level I (rt. Cheek) |
| 22 | 52/Female | 159 ± 90 MPa | 482 ± 84 MPa | 873 ± 30 MPa | Malignant melanoma, Clark’s level I (lt. Cheek) |
| 23 | 81/Female | 278 ± 98 MPa | 569 ± 55 MPa | 878 ± 300 MPa | Malignant melanoma, Clark’s level I (lt. Cheek) |
| 24 | 79/Male | 164 ± 76 MPa | 482 ± 255 MPa | Malignant melanoma, Clark’s level IV (lt. Cheek) | |
| 25 | 54/Male | 667 ± 221 MPa | Metastatic malignant melanoma, Clark’s level IV (lt. Inguinal area) | ||
| 26 | 63/Female | 215 ± 64 MPa | 394 ± 41 MPa | 625 ± 105 MPa | Malignant melanoma, Clark’s level IV (rt. Thigh) |
| 27 | 62/Male | 361 ± 136 MPa | 711 ± 59 MPa | Malignant melanoma, Clark’s level IV (lt. Elbow) | |
| 28 | 66/Female | 259 ± 73 MPa | 499 ± 56 MPa | 642 ± 67 MPa | Malignant melanoma, Clark’s level IV (rt. Abdomen) |
| 29 | 55/Female | 216 ± 81 MPa | 419 ± 61 MPa | 681 ± 104 MPa | Malignant melanoma, Clark’s level V (lt. Low leg) |
| 30 | 72/Female | 212 ± 42 MPa | 510 ± 94 MPa | Malignant melanoma, Clark’s level V (lt. Low thigh) | |
| 31 | 56/Female | 368 ± 133 MPa | 608 ± 30 MPa | Malignant melanoma, Clark’s level V (lt. Back) | |
| 32 | 66/Male | 230 ± 56 MPa | 614 ± 112 MPa | Malignant melanoma, Clark’s level V (rt. Flank) | |
| 33 | 79/Male | 193 ± 27 MPa | 387 ± 49 MPa | 768 ± 28 MPa | Malignant melanoma, Clark’s level V (rt. Temple) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, B.; Jung, H.G.; Lee, S.W.; Lee, G.; Shim, J.H.; Kim, M.O.; Kim, B.J.; Kim, S.-H.; Lee, H.; Lee, S.W.; et al. Melanoma Detection by AFM Indentation of Histological Specimens. Diagnostics 2022, 12, 1736. https://doi.org/10.3390/diagnostics12071736
Jeon B, Jung HG, Lee SW, Lee G, Shim JH, Kim MO, Kim BJ, Kim S-H, Lee H, Lee SW, et al. Melanoma Detection by AFM Indentation of Histological Specimens. Diagnostics. 2022; 12(7):1736. https://doi.org/10.3390/diagnostics12071736
Chicago/Turabian StyleJeon, Byoungjun, Hyo Gi Jung, Sang Won Lee, Gyudo Lee, Jung Hee Shim, Mi Ok Kim, Byung Jun Kim, Sang-Hyon Kim, Hyungbeen Lee, Sang Woo Lee, and et al. 2022. "Melanoma Detection by AFM Indentation of Histological Specimens" Diagnostics 12, no. 7: 1736. https://doi.org/10.3390/diagnostics12071736
APA StyleJeon, B., Jung, H. G., Lee, S. W., Lee, G., Shim, J. H., Kim, M. O., Kim, B. J., Kim, S.-H., Lee, H., Lee, S. W., Yoon, D. S., Jo, S. J., Choi, T. H., & Lee, W. (2022). Melanoma Detection by AFM Indentation of Histological Specimens. Diagnostics, 12(7), 1736. https://doi.org/10.3390/diagnostics12071736







