Oropharyngeal Candidiasis among Egyptian COVID-19 Patients: Clinical Characteristics, Species Identification, and Antifungal Susceptibility, with Disease Severity and Fungal Coinfection Prediction Models
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design, Participants, Specimens, and Data Collection
2.2. Isolation and Identification of Candida Isolates
2.2.1. Isolation of Candida Species
2.2.2. Phospholipase Assay
2.2.3. Aspartyl Protease Assay
2.2.4. Haemolysin Assay
2.2.5. Biofilm Formation
2.3. Antifungal Susceptibility Test
2.4. Statistical Analysis of the Data
3. Results
3.1. Demographics and Patient Characteristics
3.2. Variables Associated with Oropharyngeal Candidiasis and Severity of Infection
3.3. Prediction Models
3.4. Characterization of Candida Species
3.5. Antifungal Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafarzadeh, A.; Jafarzadeh, S.; Nozari, P.; Mokhtari, P.; Nemati, M. Lymphopenia an important immunological abnormality in patients with COVID-19: Possible mechanisms. Scand. J. Immunol. 2021, 93, e12967. [Google Scholar] [CrossRef] [PubMed]
- Kwamin, F.; Nartey, N.O.; Codjoe, F.S.; Newman, M.J. Distribution of Candida species among HIV-positive patients with oro-pharyngeal candidiasis in Accra, Ghana. J. Infect. Dev. Ctries. 2013, 7, 41–45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. 2022. Available online: https://covid19.who.int (accessed on 1 April 2020).
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Gallelli, L.; Zhang, L.; Wang, T.; Fu, F. Severe Acute Lung Injury Related to COVID-19 Infection: A Review and the Possible Role for Escin. J. Clin. Pharmacol. 2020, 60, 815–825. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). JAMA 2020, 324, 782. [Google Scholar] [CrossRef]
- Wygrecka, M.; Jablonska, E.; Guenther, A.; Preissner, K.T.; Markart, P. Current view on alveolar coagulation and fibrinolysis in acute inflammatory and chronic interstitial lung diseases. Thromb. Haemost. 2008, 99, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Stinson, S.F.; Ryan, D.P.; Hertweck, S.; Hardy, J.D.; Hwang-Kow, S.Y.; Loosli, C.G. Epithelial and surfactant changes in influenzal pulmonary lesions. Arch. Pathol. Lab. Med. 1976, 100, 147–153. [Google Scholar]
- Monedero, P.; Gea, A.; Castro, P.; Candela-Toha, A.M.; Hernández-Sanz, M.L.; Arruti, E.; Villar, J.; Ferrando, C. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: A cohort study. Crit. Care 2021, 25, 2. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19. JAMA 2020, 324, 1330. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.K.G.; Milby, K.M.; Caparroz, A.L.M.A.; Pinto, A.C.P.N.; Santos, R.R.P.; Rocha, A.P.; Ferreira, G.A.; Souza, V.A.; Valadares, L.D.A.; Vieira, R.M.R.A.; et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS ONE 2021, 16, e0253894. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, R.; Malekifar, P.; Shateri, Z.; Zandi, M.; Akhavan Rezayat, S.; Soleymani, M.; Karimi, M.R.; Ahmadi, S.E.; Shahbahrami, R.; Pakzad, I.; et al. Worldwide prevalence of microbial agents’ coinfection among COVID-19 patients: A comprehensive updated systematic review and meta-analysis. J. Clin. Lab. Anal. 2022, 36, e24151. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; McLoughlin, R.M. Staphylococcus aureus and Influenza A Virus: Partners in Coinfection. mBio 2016, 7, e02068-16. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R., 3rd; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T.; et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Gangneux, J.P.; Bougnoux, M.E.; Dannaoui, E.; Cornet, M.; Zahar, J.R. Invasive fungal diseases during COVID-19: We should be prepared. J. Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Trombetta, E.; Cattaneo, A.; Castelli, V.; Palomba, E.; Tirone, M.; Mangioni, D.; Lamorte, G.; Manunta, M.; Prati, D.; et al. Early Phases of COVID-19 Are Characterized by a Reduction in Lymphocyte Populations and the Presence of Atypical Monocytes. Front. Immunol. 2020, 11, 560330. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, J.; Zhu, W.; Feng, R.; Liu, Q.; Chen, X.; Huang, J.; Yang, Z.; Lin, X.; Zhang, Z.; et al. Indicators and prediction models for the severity of Covid-19. Int. J. Clin. Pract. 2021, 75, e14571. [Google Scholar] [CrossRef]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [CrossRef]
- Murray, C.K.; Beckius, M.L.; Green, J.A.; Hospenthal, D.R. Use of chromogenic medium in the isolation of yeasts from clinical specimens. J. Med. Microbiol. 2005, 54, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, L.P.; Raeside, J.M.; MacFarlane, T.W. Factors affecting the phospholipase activity of Candida species in vitro. Sabouraudia 1984, 22, 201–207. [Google Scholar] [CrossRef]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef]
- Staib, F. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia 1965, 4, 187–193. [Google Scholar] [CrossRef]
- Sachin, D.; Santosh, S. Virulence markers and antifungal susceptibility profile of Candida glabrata: An emerging pathogen. Br. Microbiol. Res. J. 2014, 4, 39–49. [Google Scholar]
- Manns, J.M.; Mosser, D.M.; Buckley, H.R. Production of a hemolytic factor by Candida albicans. Infect. Immun. 1994, 62, 5154–5156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Yip, H.K.; Samaranayake, Y.H.; Yau, J.Y.; Samaranayake, L.P. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.H.; Kee, S.J.; Shin, M.G.; Kim, S.H.; Shin, D.H.; Lee, S.K.; Suh, S.P.; Ryang, D.W. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 2002, 40, 1244–1248. [Google Scholar] [CrossRef] [Green Version]
- Noake, T.; Kuriyama, T.; White, P.L.; Potts, A.J.; Lewis, M.A.; Williams, D.W.; Barnes, R.A. Antifungal susceptibility of Candida species using the Clinical and Laboratory Standards Institute disk diffusion and broth microdilution methods. J. Chemother. 2007, 19, 283–287. [Google Scholar] [CrossRef]
- Salehi, M.; Khajavirad, N.; Alavi Darazam, I.; Hashemi, S.J.; Ansari, S.; Ghiasvand, F.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kiyaei, R.S.; Seifi, A.; et al. Risk Factors of Oropharyngeal Candidiasis in COVID-19 Patients: A Case-control Study. Arch. Clin. Infect. Dis. 2021, 16, e114631. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Chiurlo, M.; Mastrangelo, A.; Ripa, M.; Scarpellini, P. Invasive fungal infections in patients with COVID-19: A review on pathogenesis, epidemiology, clinical features, treatment, and outcomes. New Microbiol. 2021, 44, 71–83. [Google Scholar]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Choukér, A.; Woehrle, T. COVID-19 Impairs Immune Response to Candida albicans. Front. Immunol. 2021, 12, 640644. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, B.; Khalili, M.; Amiri, R.; Zartab, H.; Aflatoonian, M. Oral manifestations of COVID-19 disease: A review article. Dermatol. Ther. 2021, 34, e14578. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Gomaa, E.; Hockova, B.; Klugar, M. Oral candidiasis of COVID-19 patients: Case report and review of evidence. J. Cosmet. Dermatol. 2021, 20, 1580–1584. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Van De Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Bleyzac, N.; Goutelle, S.; Bourguignon, L.; Tod, M. Azithromycin for COVID-19: More Than Just an Antimicrobial? Clin. Drug Investig. 2020, 40, 683–686. [Google Scholar] [CrossRef]
- Karampela, I.; Dalamaga, M. Could Respiratory Fluoroquinolones, Levofloxacin and Moxifloxacin, Prove to be Beneficial as an Adjunct Treatment in COVID-19? Arch. Med. Res. 2020, 51, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, V.D.; Momenimovahed, Z.; Ghorbani, M.; Khodadadi, J. Linezolid a potential treatment for COVID-19 coinfections. Braz. J. Anesthesiol. 2021, 71, 198. [Google Scholar] [CrossRef]
- Sieswerda, E.; De Boer, M.G.J.; Bonten, M.M.J.; Boersma, W.G.; Jonkers, R.E.; Aleva, R.M.; Kullberg, B.-J.; Schouten, J.A.; Van De Garde, E.M.W.; Verheij, T.J.; et al. Recommendations for antibacterial therapy in adults with COVID-19—An evidence based guideline. Clin. Microbiol. Infect. 2021, 27, 61–66. [Google Scholar] [CrossRef]
- Yu, S.Y.; Zhang, L.; Chen, S.; Kong, F.; Xiao, M.; Wang, H.; Hou, X.; Zhou, M.L.; Zhang, G.; Zhang, J.J.; et al. Candida isolates causing refractory or recurrent oropharyngeal candidiasis in 11 hospitals in China. Infect. Drug Resist. 2019, 12, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redding, S.W.; Kirkpatrick, W.R.; Coco, B.J.; Sadkowski, L.; Fothergill, A.W.; Rinaldi, M.G.; Eng, T.Y.; Patterson, T.F. Candida glabrata oropharyngeal candidiasis in patients receiving radiation treatment for head and neck cancer. J. Clin. Microbiol. 2002, 40, 1879–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tati, S.; Davidow, P.; McCall, A.; Hwang-Wong, E.; Rojas, I.G.; Cormack, B.; Edgerton, M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathog. 2016, 12, e1005522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
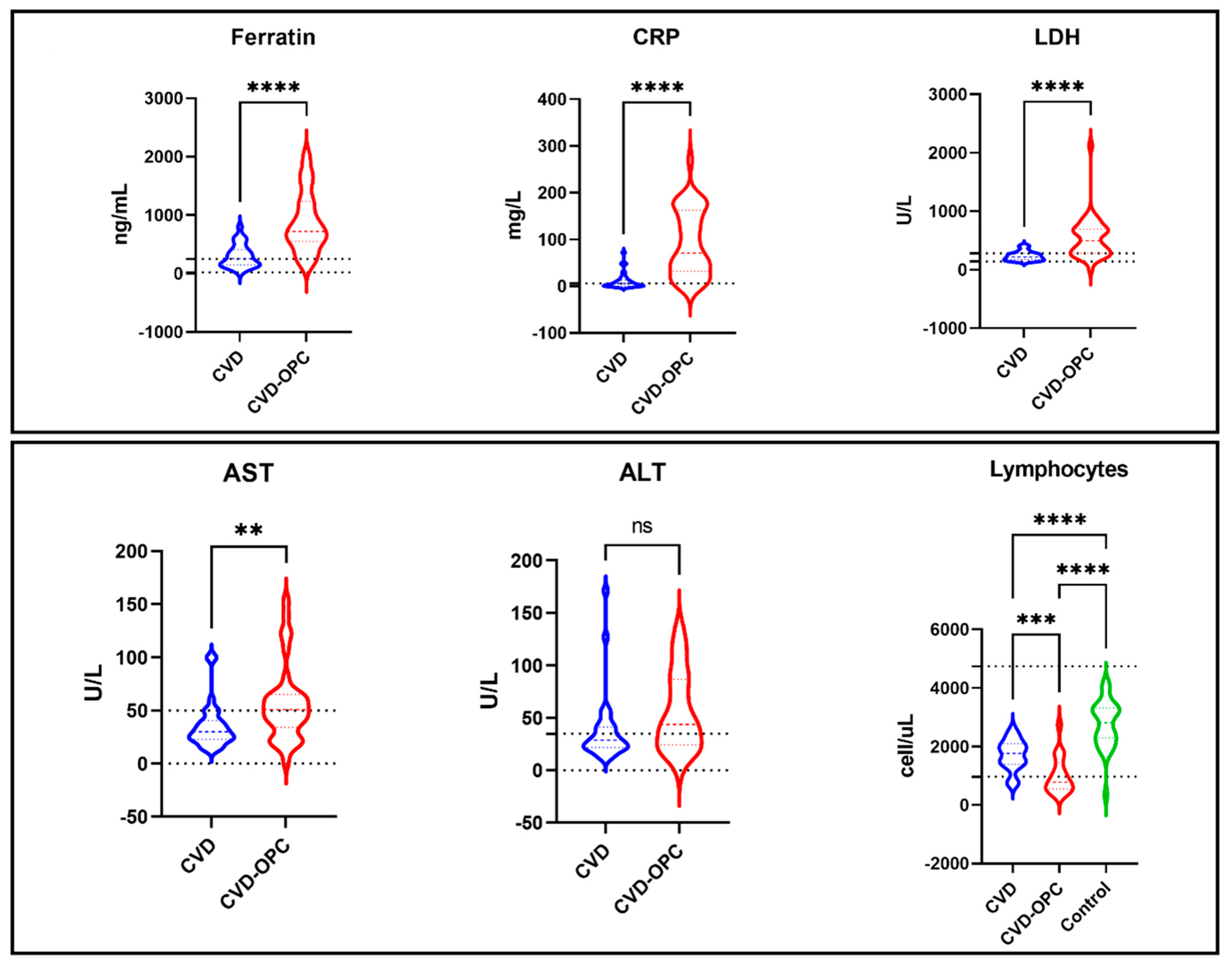
| COVID with OPC “n = 39” | COVID without OPC “n = 30” | Control Group “n = 31” | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 58.36 ± 9.44 | 47.47 ± 14.06 | 41.16 ± 11.10 | 0.062 |
| Gender | ||||
| Male | 25 (64.1%) | 19 (63.3%) | 15 (48.4%) | 0.351 |
| Female | 14 (35.9%) | 11 (36.7%) | 16 (51.6%) | |
| Clinical signs | ||||
| Severity Mderate | 4 (10.3%) | 30 (100.0%) | - | 0.001 * |
| Severe | 8 (20.5%) | 0 (0.0%) | - | |
| Critical | 27 (69.2%) | 0 (0.0%) | - | |
| SBP | 116.85 ± 4.58 | 118.67 ± 5.71 | 119.00 ± 4.56 | 0.146 |
| DBP | 65.85 ± 3.15 | 63.67 ± 7.18 | 65.81 ± 3.09 | 0.116 |
| HR | 82.85 ± 4.48 | 85.40 ± 3.85 | 83.13 ± 4.57 | 0.040 * |
| Temp | 38.36 ± 0.72 | 35.90 ± 6.79 | - | 0.028 |
| RR | 20.46 ± 2.39 | 17.10 ± 1.37 | - | 0.001 * |
| Spo2 | 81.56 ± 6.49 | 96.83 ± 0.83 | - | 0.001 * |
| Laboratory tests | ||||
| Hb | 12.83 ± 2.18 | 12.59 ± 1.37 | 13.32 ± 0.75 | 0.187 |
| Plt | 249.11 ± 128.26 | 239.00 ± 83.78 | 277.06 ± 59.35 | 0.282 |
| Tlc | 8.95 ± 4.54 | 7.90 ± 4.03 | 7.79 ± 1.77 | 0.368 |
| Lymp% | 13.08 ± 8.46 | 25.41 ± 12.01 | 35.55 ± 7.77 | 0.001 * |
| Lymph count | 1015.17 ± 634.52 | 1715.33 ± 522.38 | 2777.16 ± 812.98 | 0.001 * |
| Mono% | 6.75 ± 3.52 | 10.16 ± 4.03 | 5.48 ± 2.32 | 0.001 * |
| Mono count | 476.01 ± 339.45 | 522.67 ± 186.48 | 433.00 ± 221.54 | 0.529 |
| ALT | 91.09 ± 193.26 | 38.36 ± 33.07 | 20.55 ± 4.17 | 0.045 |
| AST | 98.41 ± 255.62 | 35.43 ± 21.01 | 22.94 ± 4.05 | 0.109 |
| Albumin | 3.01 ± 0.60 | 3.89 ± 0.51 | 4.45 ± 0.62 | 0.001 * |
| INR | 1.34 ± 0.49 | 1.26 ± 0.46 | 0.95 ± 0.09 | 0.001 * |
| Urea | 61.65 ± 47.43 | 40.50 ± 24.04 | 16.03 ± 2.36 | 0.001 * |
| Creatinine | 1.83 ± 3.11 | 0.86 ± 0.39 | 0.54 ± 0.22 | 0.018 |
| Na | 132.31 ± 22.93 | 136.83 ± 2.26 | 137.03 ± 2.37 | 0.300 |
| K | 4.06 ± 0.87 | 4.09 ± 0.55 | 4.30 ± 0.55 | 0.306 |
| Ca | 9.08 ± 1.09 | 9.92 ± 0.75 | 9.36 ± 0.53 | 0.001 * |
| Ferretin | 788.73 ± 469.22 | 300.60 ± 196.05 | 159.16 ± 57.26 | 0.001 * |
| CRP | 78.16 ± 69.59 | 11.68 ± 17.22 | 2.55 ± 1.43 | 0.001 * |
| LDH | 539.89 ± 355.94 | 236.90 ± 78.75 | 204.32 ± 39.01 | 0.001 * |
| D-Dimer | ||||
| Negative | 0 (0.0%) | 23 (76.7%) | 31 (100.0%) | |
| Positive | 39 (100.0%) | 7 (23.3%) | 0 (0.0%) | 0.001 * |
| PCR | ||||
| Negative | 0 (0.0%) | 0 (0.0%) | 31 (100.0%) | 0.001 * |
| Positive | 39 (100.0%) | 30 (100.0%) | 0 (0.0%) |
| Medication | COVID with OPC “n = 39” | COVID without OPC “n = 30” |
|---|---|---|
| Paracetamol | 4 (10.26%) | 9 (30%) |
| Vit C | 37 (94.87%) | 29 (96.67%) |
| Zinc | 9 (23.08%) | 29 (96.67%) |
| Plaqunil | 9 (23.08%) | 24 (80%) |
| Steroids | 35 (89.74%) | 0 (0%) |
| Enoxaparin | 6 (15.38%) | 4 (13.33%) |
| Oseltamivir | 35 (89.74%) | 19 (63.33%) |
| Tocilizumab | 4 (10.26%) | 0 (0%) |
| Antibiotics | ||
| Macrolide (AZT: azithromycin) | 38 (97.44%) | 23 (76.67%) |
| Cephalosporin (CFX: Cefotaxime) | 33 (84.62%) | 5 (16.67%) |
| Oxazolidinones (LZ: Linezolid) | 4 (10.26%) | 0 (0%) |
| Fluoroquinolone (LV: levofloxacin) | 3 (7.69%) | 1 (3.33%) |
| Antibiotics combinations | ||
| AZT-CFX | 30 (76.92%) | 5 (16.67%) |
| AZT-CFX-LZ | 2 (5.13%) | 0 (0%) |
| AZT-CFX-LV | 1 (2.56%) | 0 (0%) |
| AZT-LZ | 2 (5.13%) | 0 (0%) |
| AZT-LV | 2 (5.13%) | 0 (0%) |
| Moderate “n = 34” | Severe “n = 8” | Critical “n = 27” | p-Value | |
|---|---|---|---|---|
| Age | 48.97 ± 14.00 | 56.25 ± 12.68 | 58.70 ± 8.87 | 0.001 * |
| SBP | 118.71 ± 5.62 | 117.75 ± 5.37 | 116.26 ± 4.23 | 0.183 |
| DBP | 64.24 ± 6.93 | 65.00 ± 3.59 | 65.70 ± 3.09 | 0.575 |
| HR | 85.03 ± 4.04 | 82.38 ± 3.66 | 83.07 ± 4.75 | 0.123 |
| Temp | 36.15 ± 6.41 | 38.45 ± 0.61 | 38.39 ± 0.71 | 0.129 |
| RR | 17.47 ± 1.81 | 21.38 ± 2.45 | 20.22 ± 2.39 | 0.001 * |
| Spo2 | 95.12 ± 5.03 | 82.50 ± 3.42 | 81.19 ± 7.45 | 0.001 * |
| Hb | 12.66 ± 1.37 | 13.34 ± 2.39 | 12.61 ± 2.23 | 0.639 |
| Plt | 235.52 ± 87.74 | 223.57 ± 118.67 | 262.08 ± 132.32 | 0.577 |
| Tlc | 7.80 ± 3.91 | 6.53 ± 1.99 | 9.89 ± 4.93 | 0.083 |
| Lymp% | 25.13 ± 11.48 | 14.40 ± 9.19 | 11.60 ± 8.15 | 0.001 * |
| Lymph count | 1694.48 ± 519.53 | 860.71 ± 390.05 | 1001.92 ± 687.97 | 0.001 * |
| Neutrophils | 2807.40 ± 1243.65 | 5167.86 ± 2106.56 | 6783.08 ± 4665.86 | 0.002 * |
| Mono% | 10.04 ± 3.95 | 6.73 ± 3.58 | 6.56 ± 3.56 | 0.009 * |
| Mono count | 515.40 ± 177.90 | 410.00 ± 170.98 | 497.43 ± 388.33 | 0.719 |
| ALT | 39.87 ± 33.96 | 62.70 ± 36.59 | 102.25 ± 224.49 | 0.267 |
| AST | 36.67 ± 20.59 | 53.83 ± 9.30 | 115.04 ± 297.87 | 0.290 |
| Albumin | 3.88 ± 0.51 | 2.79 ± 0.64 | 2.94 ± 0.47 | 0.001 * |
| INR | 1.26 ± 0.41 | 1.09 ± 0.13 | 1.40 ± 0.56 | 0.350 |
| Urea | 41.79 ± 24.39 | 41.17 ± 12.22 | 67.64 ± 53.56 | 0.036 * |
| Creatinine | 0.89 ± 0.40 | 1.13 ± 0.48 | 2.07 ± 3.59 | 0.151 |
| Na | 136.41 ± 2.51 | 135.88 ± 3.64 | 131.11 ± 27.55 | 0.484 |
| K | 4.11 ± 0.54 | 4.04 ± 1.16 | 4.03 ± 0.84 | 0.913 |
| Ca | 9.93 ± 0.74 | 9.16 ± 0.82 | 8.91 ± 1.15 | 0.001 * |
| Ferretin | 325.97 ± 223.46 | 762.50 ± 213.86 | 836.87 ± 530.32 | 0.001 * |
| CRP | 18.17 ± 32.92 | 59.13 ± 64.56 | 85.48 ± 71.69 | 0.001 * |
| LDH | 248.26 ± 90.60 | 390.57 ± 211.31 | 609.19 ± 387.57 | 0.001 * |
| Variables | B | Std. Error | O.R. | 95.0% C.I. | t | Sig. |
|---|---|---|---|---|---|---|
| (Constant) | 4.049 | 2.320 | 2.746 | 0.020 * | ||
| Age | 0.002 | 0.009 | 1.00 | 0.65–1.25 | 0.275 | 0.785 |
| RR | 0.032 | 0.051 | 0.98 | 0.38–1.52 | 0.624 | 0.537 |
| Spo2 | −0.033 | 0.016 | 1.32 | 1.52–2.11 | −2.052 | 0.048 * |
| Lymp% | −0.005 | 0.012 | 1.36 | 0.21–0.75 | −2.436 | 0.046 * |
| Lymph count | 0.000 | 0.000 | 1.001 | 0.65–1.65 | −1.431 | 0.162 |
| Neutrophils | 1.537 × 10−5 | 0.000 | 0.91 | 0.62–1.88 | 0.514 | 0.611 |
| Mono% | 0.006 | 0.024 | 1.49 | 1.23–1.85 | 2.234 | 0.036 * |
| Albumin | 0.010 | 0.231 | 1.003 | 0.65–2.11 | 0.042 | 0.966 |
| Urea | 0.0032 | 0.207 | 0.98 | 0.55–1.97 | 0.798 | 0.528 |
| CRP | 0.0001 | 0.002 | 1.77 | 1.42–2.58 | −2.216 | 0.031 * |
| LDH | 0.001 | 0.000 | 1.75 | 1.32–2.11 | 2.234 | 0.033 * |
| D-Dimer | 0.222 | 0.339 | 2.11 | 1.42–1.98 | 2.656 | 0.0216 * |
| Variables | B | Std. Error | O.R. | 95.0% C.I. | t | Sig. |
|---|---|---|---|---|---|---|
| (Constant) | 0.951 | 0.916 | 3.038 | 0.030 * | ||
| Severity | 0.191 | 0.080 | 2.04 | 1.01–2.91 | 2.393 | 0.020 * |
| Respiratory rate (RR) | 0.025 | 0.020 | 2.88 | 0.03–0.81 | 2.232 | 0.023 * |
| Spo2 | −0.014 | 0.008 | 2.62 | 1.31–3.77 | −1.884 | 0.025 * |
| Lymph count | −3.188 × 10−5 | 0.000 | 1.81 | 0.12–0.82 | −2.472 | 0.039 * |
| D-Dimer | 0.193 | 0.125 | 2.41 | 1.71–4.03 | 2.539 | 0.0129 * |
| LDH | 0.00012 | 0.000 | 2.61 | 1.22–3.01 | −2.159 | 0.0451 * |
| Candida | Candida albicans n = 29 | Candida tropicalis n = 6 | Candida glabrata n = 4 |
|---|---|---|---|
| Distribution | 29/39 (74.4%) | 6/39 (15.4%) | 4/39 (10.3%) |
| Hemolysis | |||
| alpha | 27 (93.1%) | 6 (100%) | 4 (100%) |
| beta | 2 (6.9%) | (0%) | (0%) |
| Biofilm | |||
| Non | 12 (41.38%) | 3 (50%) | 3 (75%) |
| Weak | 9 (31.03%) | 1 (16.67%) | 1 (25%) |
| Moderate | 6 (20.69%) | 1 (16.67%) | (0%) |
| Strong | 2 (6.9%) | 1 (16.67%) | (0%) |
| Phospholipase | |||
| NEG | 12 (41.38%) | 5 (83.33%) | 4 (100%) |
| STR | 15 (51.72%) | 1 (16.67%) | (0%) |
| V.STR | 2 (6.9%) | (0%) | (0%) |
| Aspartyl Protease | |||
| NEG | 0 (0%) | 0 (0%) | 1 (25%) |
| STR | 3 (10.34%) | 3 (50%) | 2 (50%) |
| V.STR | 26 (89.66%) | 3 (50%) | 1 (25%) |
| Antifungal Sensitivity | |||
| Fluconazole (FLU) | |||
| SEN | 18 (62.07%) | 3 (50%) | 3 (75%) |
| RES | 8 (27.59%) | 2 (33.33%) | 1 (25%) |
| SDD | 3 (10.34%) | 1 (16.67%) | (0%) |
| Amphotericin B (AMB) | |||
| SEN | 29 (100%) | 6 (100%) | 4 (100%) |
| RES | 0 (0%) | 0 (0%) | 0 (0%) |
| SDD | 0 (0%) | 0 (0%) | 0 (0%) |
| Nystatin (NYS) | |||
| SEN | 27 (93.1%) | 6 (100%) | 4 (100%) |
| RES | (0%) | (0%) | (0%) |
| SDD | 2 (6.9%) | (0%) | (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, M.A.F.; El-Ansary, M.R.M.; Bassyouni, R.H.; Mahmoud, E.E.; Ali, I.A.; Ahmed, T.I.; Hassan, E.A.; Samir, T.M. Oropharyngeal Candidiasis among Egyptian COVID-19 Patients: Clinical Characteristics, Species Identification, and Antifungal Susceptibility, with Disease Severity and Fungal Coinfection Prediction Models. Diagnostics 2022, 12, 1719. https://doi.org/10.3390/diagnostics12071719
Khalil MAF, El-Ansary MRM, Bassyouni RH, Mahmoud EE, Ali IA, Ahmed TI, Hassan EA, Samir TM. Oropharyngeal Candidiasis among Egyptian COVID-19 Patients: Clinical Characteristics, Species Identification, and Antifungal Susceptibility, with Disease Severity and Fungal Coinfection Prediction Models. Diagnostics. 2022; 12(7):1719. https://doi.org/10.3390/diagnostics12071719
Chicago/Turabian StyleKhalil, Mahmoud A. F., Mahmoud R. M. El-Ansary, Rasha H. Bassyouni, Eman E. Mahmoud, Inas A. Ali, Tarek I. Ahmed, Essam A. Hassan, and Tamer M. Samir. 2022. "Oropharyngeal Candidiasis among Egyptian COVID-19 Patients: Clinical Characteristics, Species Identification, and Antifungal Susceptibility, with Disease Severity and Fungal Coinfection Prediction Models" Diagnostics 12, no. 7: 1719. https://doi.org/10.3390/diagnostics12071719
APA StyleKhalil, M. A. F., El-Ansary, M. R. M., Bassyouni, R. H., Mahmoud, E. E., Ali, I. A., Ahmed, T. I., Hassan, E. A., & Samir, T. M. (2022). Oropharyngeal Candidiasis among Egyptian COVID-19 Patients: Clinical Characteristics, Species Identification, and Antifungal Susceptibility, with Disease Severity and Fungal Coinfection Prediction Models. Diagnostics, 12(7), 1719. https://doi.org/10.3390/diagnostics12071719






