Adrenal Venous Sampling Could Be Omitted before Surgery in Patients with Conn’s Adenoma Confirmed by Computed Tomography and Higher Normal Aldosterone Concentration after Saline Infusion Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Drugs Management
2.3. Postural Stimulation Test (PST)
2.4. Saline Infusion Test (SIT)
2.5. Adrenal Venous Sampling (AVS)
2.6. Laboratory Methods
2.7. Blood Pressure Measurement
2.8. Statistical Analysis
3. Results
3.1. Development Cohort
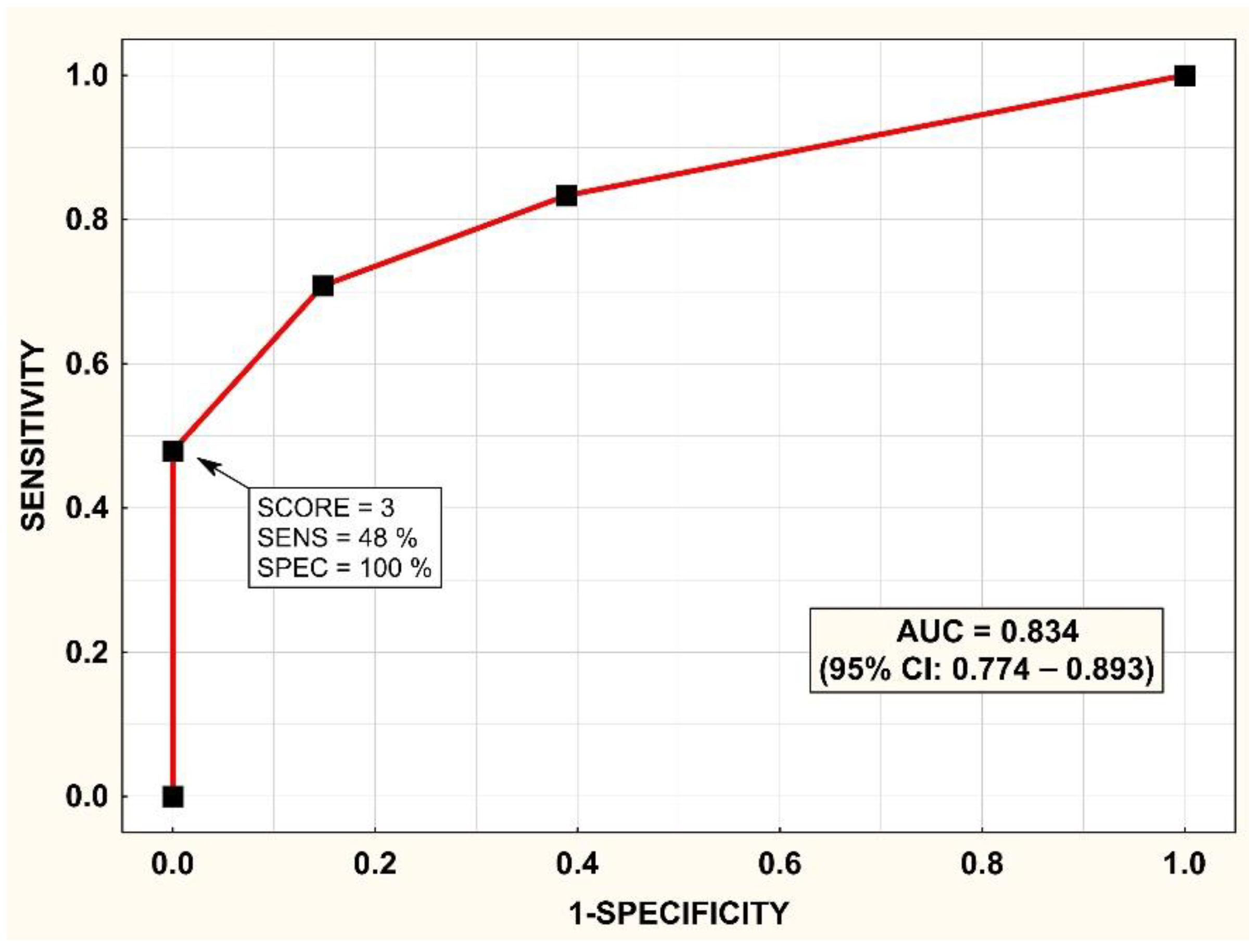
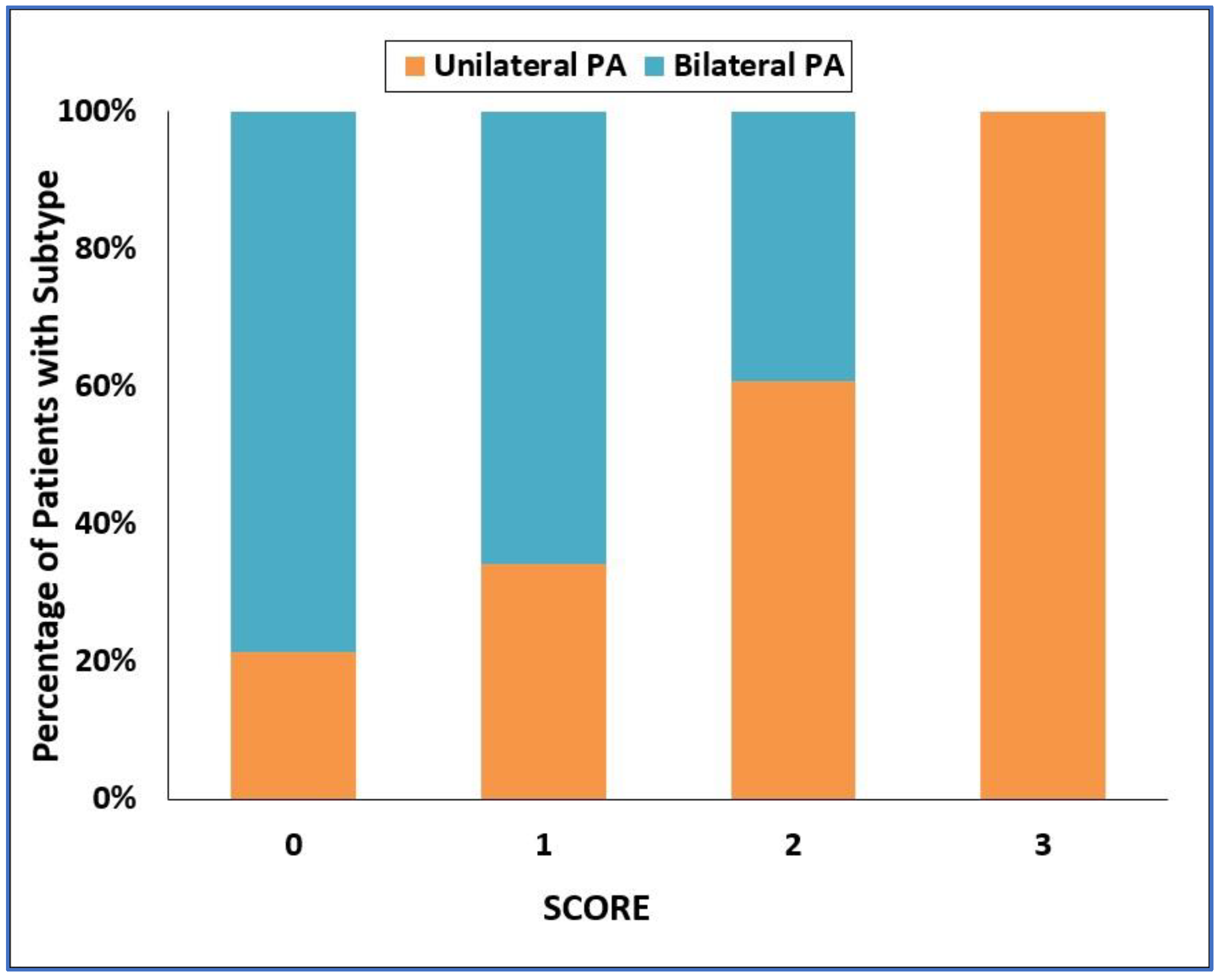
3.2. Development of the SCORE
3.3. Validation Cohort
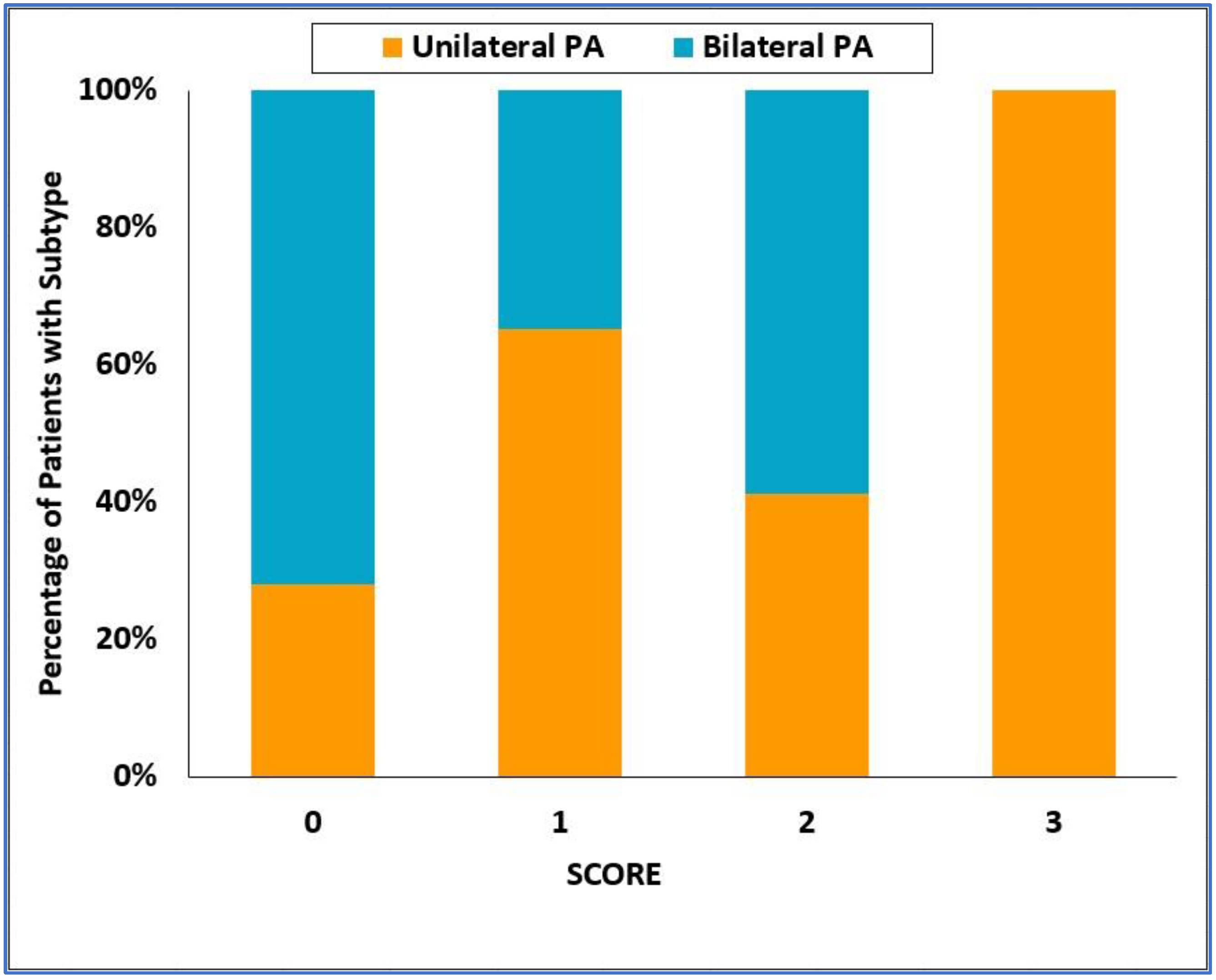
3.4. Validation of the SCORE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaplan, N.M. (Ed.) Primary aldosteronism. In Kaplan’s Clinical Hypertension; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 410–433. [Google Scholar]
- Rizzoni, D.; Agabiti Rosei, E. Small artery remodeling in hypertension and diabetes. Curr. Hypertens. Rep. 2006, 8, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Štrauch, B.; Petrák, O.; Wichterle, D.; Zelinka, T.; Holaj, R.; Widimský, J., Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am. J. Hypertens. 2006, 19, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002, 40, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Holaj, R.; Zelinka, T.; Wichterle, D.; Petrák, O.; Štrauch, B.; Widimský, J., Jr. Increased intima-media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. J. Hypertens. 2007, 25, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.M.; Schmieder, R.E. Aldosterone-induced cardiac damage: Focus on blood pressure independent effects. Am. J. Hypertens. 2003, 16, 80–86. [Google Scholar] [CrossRef]
- Indra, T.; Holaj, R.; Zelinka, T.; Petrák, O.; Štrauch, B.; Rosa, J.; Šomlóová, Z.; Malík, J.; Janota, T.; Hradec, J.; et al. Left ventricle remodeling in men with moderate to severe volume-dependent hypertension. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Verheyen, N.; Pilz, S.; Kraigher-Krainer, E.; Tomaschitz, A.; Sechi, L.A.; Pieske, B. Plasma Aldosterone and Left Ventricular Diastolic Function in Treatment-Naive Patients With Hypertension: Tissue-Doppler Imaging Study. Hypertension 2015, 65, 1231–1237. [Google Scholar] [CrossRef]
- Fardella, C.E.; Mosso, L.; Gomez-Sanchez, C.; Cortes, P.; Soto, J.; Gomez, L.; Pinto, M.; Huete, A.; Oestreicher, E.; Foradori, A.; et al. Primary hyperaldosteronism in essential hypertensives: Prevalence, biochemical profile, and molecular biology. J. Clin. Endocrinol. Metab. 2000, 85, 1863–1867. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bernini, G.; Caliumi, C.; Desideri, G.; Fabris, B.; Ferri, C.; Ganzaroli, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 2006, 48, 2293–2300. [Google Scholar] [CrossRef]
- Kayser, S.C.; Deinum, J.; de Grauw, W.J.; Schalk, B.W.; Bor, H.J.; Lenders, J.W.; Schermer, T.R.; Biermans, M.C. Prevalence of primary aldosteronism in primary care: A cross-sectional study. Br. J. Gen. Pract 2018, 68, e114–e122. [Google Scholar] [CrossRef]
- Štrauch, B.; Zelinka, T.; Hampf, M.; Bernhardt, R.; Widimský, J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J. Hum. Hypertens. 2003, 17, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Siddiqui, M.; Calhoun, D.A.; Carey, R.M.; Hopkins, P.N.; Williams, G.H.; Vaidya, A. The Unrecognized Prevalence of Primary Aldosteronism: A Cross-sectional Study. Ann. Intern. Med. 2020, 173, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Parasiliti-Caprino, M.; Lopez, C.; Prencipe, N.; Lucatello, B.; Settanni, F.; Giraudo, G.; Rossato, D.; Mengozzi, G.; Ghigo, E.; Benso, A.; et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J. Hypertens. 2020, 38, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Conn, J.W. The evolution of primary aldosteronism: 1954–1967. Harvey Lect. 1966, 62, 257–291. [Google Scholar]
- Zelinka, T.; Mašek, M.; Vlková, J.; Kasalický, M.; Michalský, D.; Holaj, R.; Petrák, O.; Štrauch, B.; Rosa, J.; Dvořáková, J.; et al. Discrepant results of adrenal venous sampling in seven patients with primary aldosteronism. Kidney Blood Press. Res. 2012, 35, 205–210. [Google Scholar] [CrossRef]
- Rossi, G.P.; Auchus, R.J.; Brown, M.; Lenders, J.W.; Naruse, M.; Plouin, P.F.; Satoh, F.; Young, W.F., Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension 2014, 63, 151–160. [Google Scholar] [CrossRef]
- Stowasser, M. Adrenal venous sampling for differentiating unilateral from bilateral primary aldosteronism: Still the best, but could be better. Hypertension 2015, 65, 704–706. [Google Scholar] [CrossRef]
- Kupers, E.M.; Amar, L.; Raynaud, A.; Plouin, P.F.; Steichen, O. A clinical prediction score to diagnose unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 2012, 97, 3530–3537. [Google Scholar] [CrossRef]
- Nanba, K.; Tsuiki, M.; Nakao, K.; Nanba, A.; Usui, T.; Tagami, T.; Hirokawa, Y.; Okuno, H.; Suzuki, T.; Shimbo, T.; et al. A subtype prediction score for primary aldosteronism. J. Hum. Hypertens. 2014, 28, 716–720. [Google Scholar] [CrossRef]
- Kocjan, T.; Janez, A.; Stankovic, M.; Vidmar, G.; Jensterle, M. A New Clinical Prediction Criterion Accurately Determines a Subset of Patients with Bilateral Primary Aldosteronism before Adrenal Venous Sampling. Endocr. Pract. 2016, 22, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kamemura, K.; Wada, N.; Ichijo, T.; Matsuda, Y.; Fujii, Y.; Kai, T.; Fukuoka, T.; Sakamoto, R.; Ogo, A.; Suzuki, T.; et al. Significance of adrenal computed tomography in predicting laterality and indicating adrenal vein sampling in primary aldosteronism. J. Hum. Hypertens. 2017, 31, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Soma, M.; Takeda, Y.; Kurihara, I.; Itoh, H.; Umakoshi, H.; Tsuiki, M.; Katabami, T.; Ichijo, T.; et al. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J. Hypertens. 2018, 36, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Puar, T.H.; Loh, W.J.; Lim, D.S.; Loh, L.M.; Zhang, M.; Foo, R.S.; Lee, L.; Swee, D.S.; Khoo, J.; Tay, D.; et al. Aldosterone-potassium ratio predicts primary aldosteronism subtype. J. Hypertens. 2020, 38, 1375–1383. [Google Scholar] [CrossRef]
- Nagano, H.; Kono, T.; Saiga, A.; Kubota, Y.; Fujimoto, M.; Felizola, S.J.A.; Ishiwata, K.; Tamura, A.; Higuchi, S.; Sakuma, I.; et al. Aldosterone Reduction Rate After Saline Infusion Test May Be a Novel Prediction in Patients With Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2020, 105, e319–e327. [Google Scholar] [CrossRef]
- Burrello, J.; Amongero, M.; Buffolo, F.; Sconfienza, E.; Forestiero, V.; Burrello, A.; Adolf, C.; Handgriff, L.; Reincke, M.; Veglio, F.; et al. Development of a Prediction Score to Avoid Confirmatory Testing in Patients With Suspected Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2021, 106, e1708–e1716. [Google Scholar] [CrossRef]
- Holaj, R.; Rosa, J.; Zelinka, T.; Štrauch, B.; Petrák, O.; Indra, T.; Šomlóová, Z.; Michalský, D.; Novák, K.; Wichterle, D.; et al. Long-term effect of specific treatment of primary aldosteronism on carotid intima-media thickness. J. Hypertens. 2015, 33, 874–882. [Google Scholar] [CrossRef]
- Stowasser, M.; Ahmed, A.H.; Pimenta, E.; Taylor, P.J.; Gordon, R.D. Factors affecting the aldosterone/renin ratio. Horm. Metab. Res. 2012, 44, 170–176. [Google Scholar] [CrossRef]
- Rossi, G.P.; Barisa, M.; Allolio, B.; Auchus, R.J.; Amar, L.; Cohen, D.; Degenhart, C.; Deinum, J.; Fischer, E.; Gordon, R.; et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J. Clin. Endocrinol. Metab. 2012, 97, 1606–1614. [Google Scholar] [CrossRef]
- Mulatero, P.; Sechi, L.A.; Williams, T.A.; Lenders, J.W.M.; Reincke, M.; Satoh, F.; Januszewicz, A.; Naruse, M.; Doumas, M.; Veglio, F.; et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1929–1936. [Google Scholar] [CrossRef]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar] [CrossRef]
- Satoh, F.; Abe, T.; Tanemoto, M.; Nakamura, M.; Abe, M.; Uruno, A.; Morimoto, R.; Sato, A.; Takase, K.; Ishidoya, S.; et al. Localization of aldosterone-producing adrenocortical adenomas: Significance of adrenal venous sampling. Hypertens. Res. 2007, 30, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, T.; Vidmar, G.; Popovic, P.; Stankovic, M. Validation of three novel clinical prediction tools for primary aldosteronism subtyping. Endocr. Connect. 2022, 11, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Haketa, A.; Ueno, T.; Ikeda, Y.; Hatanaka, Y.; Tanaka, S.; Otsuka, H.; Abe, M.; Fukuda, N.; Soma, M. Scoring system for the diagnosis of bilateral primary aldosteronism in the outpatient setting before adrenal venous sampling. Clin. Endocrinol. 2017, 86, 467–472. [Google Scholar] [CrossRef]
- Mulatero, P.; Bertello, C.; Rossato, D.; Mengozzi, G.; Milan, A.; Garrone, C.; Giraudo, G.; Passarino, G.; Garabello, D.; Verhovez, A.; et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J. Clin. Endocrinol. Metab. 2008, 93, 1366–1371. [Google Scholar] [CrossRef]
- Lau, J.H.; Sze, W.C.; Reznek, R.H.; Matson, M.; Sahdev, A.; Carpenter, R.; Berney, D.M.; Akker, S.A.; Chew, S.L.; Grossman, A.B.; et al. A prospective evaluation of postural stimulation testing, computed tomography and adrenal vein sampling in the differential diagnosis of primary aldosteronism. Clin. Endocrinol. 2012, 76, 182–188. [Google Scholar] [CrossRef]
- Espiner, E.A.; Ross, D.G.; Yandle, T.G.; Richards, A.M.; Hunt, P.J. Predicting Surgically Remedial Primary Aldosteronism: Role of Adrenal Scanning, Posture Testing, and Adrenal Vein Sampling. J. Clin. Endocrinol. Metab. 2003, 88, 3637–3644. [Google Scholar] [CrossRef]
- Ganguly, A.; Dowdy, A.J.; Luetscher, J.A.; Melada, G.A. Anomalous postural response of plasma aldosterone concentration in patients with aldosterone-producing adrenal adenoma. J. Clin. Endocrinol. Metab. 1973, 36, 401–404. [Google Scholar] [CrossRef]
- Saruta, T.; Okuno, T.; Eguchi, T.; Nakamura, R.; Saito, I.; Kondo, K.; Oka, M.; Matsuki, S. Responses of aldosterone-producing adenomas to ACTH and angiotensins. Acta Endocrinol. 1979, 92, 702–709. [Google Scholar] [CrossRef]
- Wisgerhof, M.; Brown, R.D.; Hogan, M.J.; Carpenter, P.C.; Edis, A.J. The plasma aldosterone response to angiotensin II infusion in aldosterone-producing adenoma and idiopathic hyperaldosteronism. J. Clin. Endocrinol. Metab. 1981, 52, 195–198. [Google Scholar] [CrossRef]
- Feltynowski, T.; Ignatowska-Switalska, H.; Wocial, B.; Lewandowski, J.; Chodakowska, J.; Januszewicz, W. Postural stimulation test in patients with aldosterone producing adenomas. Clin. Endocrinol. 1994, 41, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Fontes, R.G.; Kater, C.E.; Biglieri, E.G.; Irony, I. Reassessment of the predictive value of the postural stimulation test in primary aldosteronism. Am. J. Hypertens. 1991, 4, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Weigel, M.; Riester, A.; Hanslik, G.; Lang, K.; Willenberg, H.S.; Endres, S.; Allolio, B.; Beuschlein, F.; Reincke, M.; Quinkler, M. Post-saline infusion test aldosterone levels indicate severity and outcome in primary aldosteronism. Eur. J. Endocrinol. 2015, 172, 443–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaneko, H.; Umakoshi, H.; Ishihara, Y.; Sugawa, T.; Nanba, K.; Tsuiki, M.; Kusakabe, T.; Satoh-Asahara, N.; Yasoda, A.; Tagami, T. Seated saline infusion test in predicting subtype diagnosis of primary aldosteronism. Clin. Endocrinol. 2019, 91, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, T.; Omura, M.; Suematsu, S.; Saito, J.; Nishikawa, T. KCNJ5 mutation as a predictor for resolution of hypertension after surgical treatment of aldosterone-producing adenoma. J. Hypertens. 2018, 36, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Lenders, J.W.; Burrello, J.; Beuschlein, F.; Reincke, M. KCNJ5 Mutations: Sex, Salt and Selection. Horm. Metab. Res. 2015, 47, 953–958. [Google Scholar] [CrossRef] [PubMed]



| Unilateral PA (n = 96) | Bilateral PA (n = 54) | p-Value | |
|---|---|---|---|
| Age (years) | 51 (44–58) | 52 (47–58) | 0.52 |
| Females | 41 (43%) | 12 (22%) | 0.01 |
| Body mass index (kg/m2) | 29 (25–32) | 30 (28–32) | 0.16 |
| Systolic BP (mm Hg) | 170 (150–180) | 160 (150–170) | 0.17 |
| Diastolic BP (mm Hg) | 100 (90–110) | 97 (88–105) | 0.12 |
| 24 h systolic BP (mm Hg) | 150 (137–161) | 150 (137–163) | 0.87 |
| 24 h diastolic BP (mm Hg) | 93 (85–98) | 91 (84–97) | 0.69 |
| Duration of hypertension (years) | 9 (4–15) | 11 (6–16) | 0.41 |
| Antihypertensive medications (n) | 4 (2–5) | 4 (2–6) | 0.14 |
| Lowest serum potassium recorded (mmol/L) | 3.0 (2.8–3.3) | 3.2 (2.9–3.5) | 0.11 |
| Serum potassium (mmol/L) | 3.4 (3.1–3.7) | 3.7 (3.4–4.0) | 0.0009 |
| Serum potassium < 3.6 mmol/L | 58 (60%) | 18 (33%) | 0.002 |
| eGFR (mL/min/1.73 m2) | 126 (104–153) | 119 (100–133) | 0.11 |
| Baseline PAC (ng/L) | 291 (183–537) | 222 (141–326) | 0.002 |
| Baseline PAC ≥ 280 ng/L | 49 (51%) | 16 (30%) | 0.01 |
| Baseline PRA (ng/mL/h) | 0.32 (0.20–0.43) | 0.35 (0.25–0.53) | 0.046 |
| Baseline ARR [ng/dL/(ng/mL/h)] | 106 (51–192) | 56 (39–91) | <0.0001 |
| Baseline ARR ≥ 100 ng/dL/(ng/mL/h) | 51 (53%) | 8 (15%) | <0.0001 |
| Prevalence of adrenal nodules on CT | 68 (71%) | 8 (15%) | <0.0001 |
| PAC after PST (ng/L) | 493 (313–821) | 378 (310–516) | 0.02 |
| Increase in PAC after PST < 30% | 30 (31%) | 8 (15%) | 0.03 |
| PAC after SIT (ng/L) | 180 (121–348) | 115 (81–163) | <0.0001 |
| PAC after SIT ≥ 165 ng/L | 58 (60%) | 13 (24%) | <0.0001 |
| AUC ROC (95% CI) | Cut-Off Value | SENS | SPEC | PPV | NPV | ACC | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Adrenal nodule on CT | 0.780 (0.714–0.846) | ≥6 mm | 71% | 85% | 90% | 62% | 76% | <0.001 |
| Serum potassium | 0.635 (0.555–0.716) | <3.6 mmol/L | 60% | 67% | 76% | 49% | 63% | 0.005 |
| ARR baseline | 0.692 (0.616–0.768) | ≥100 ng/dL/(ng/mL/h) | 53% | 85% | 86% | 51% | 65% | <0.001 |
| PAC baseline | 0.648 (0.569–0.727) | ≥280 ng/L | 51% | 70% | 75% | 45% | 56% | 0.01 |
| PAC increase after PST | 0.582 (0.501–0.663) | <30% | 31% | 85% | 79% | 41% | 51% | 0.05 |
| PAC after SIT | 0.682 (0.606–0.757) | ≥165 ng/L | 60% | 76% | 82% | 52% | 66% | <0.001 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Factor | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Adrenal nodule | 14.0 (5.9–33.3) | <0.001 | 10.9 (4.3–27.4) | <0.001 |
| Serum potassium < 3.6 mmol/L | 2.9 (1.4–5.6) | 0.003 | 1.9 (0.8–4.5) | 0.13 |
| ARR baseline ≥ 100 ng/dL/(ng/mL/h) | 2.4 (0.6–9.1) | 0.22 | 2.8 (0.4–8.4) | 0.49 |
| PAC after SIT ≥ 165 ng/L | 4.8 (2.3–10.1) | <0.001 | 3.1 (1.3–7.4) | 0.01 |
| Female sex | 3.0 (1.4–6.6) | 0.005 | 1.4 (0.5–3.7) | 0.50 |
| Factor | OR (95% CI) | p-Value | β Coefficient | Points |
|---|---|---|---|---|
| Adrenal nodule | 10.9 (4.3–27.4) | <0.001 | 2.38 | 2 |
| PAC after SIT ≥ 165 ng/L | 3.1 (1.3–7.4) | 0.01 | 1.11 | 1 |
| Unilateral PA (n = 94) | Bilateral PA (n = 44) | p-Value | |
|---|---|---|---|
| Age (years) | 49 (41–57) | 47 (41–56) | 0.55 |
| Females | 23 (24%) | 13 (30%) | 0.53 |
| Body mass index (kg/m2) | 30 (27–33) | 31 (29–34) | 0.16 |
| Systolic BP (mm Hg) | 159 (150–170) | 155 (145–162) | 0.12 |
| Diastolic BP (mm Hg) | 99 (90–105) | 98 (90–103) | 0.68 |
| 24 h systolic BP (mm Hg) | 150 (140–158) | 147 (139–157) | 0.48 |
| 24 h diastolic BP (mm Hg) | 91 (85–96) | 91 (85–98) | 0.96 |
| Duration of hypertension (years) | 8 (5–12) | 7 (3–16) | 0.78 |
| Antihypertensive medications (n) | 4 (2–4) | 3 (2–5) | 0.67 |
| Lowest serum potassium recorded (mmol/L) | 3.2 (2.9–3.5) | 3.6 (3.3–3.9) | <0.0001 |
| Serum potassium (mmol/L) | 3.4 (3.2–3.7) | 3.9 (3.7–4.1) | <0.0001 |
| Serum potassium < 3.6 mmol/L | 56 (60%) | 8 (18%) | <0.0001 |
| eGFR (ml/min/1.73 m2) | 128 (114–165) | 152 (110–186) | 0.24 |
| Baseline serum aldosterone (ng/L) | 265 (187–365) | 168 (131–208) | <0.0001 |
| Baseline serum aldosterone ≥ 280 ng/L | 41 (46%) | 2 (5%) | <0.0001 |
| Baseline DRC (ng/mL) | 1.50 (0.49–2.70) | 1.51 (0.50–3.30) | 0.62 |
| Baseline ARR [ng/dL/(ng/mL)] | 13 (6–35) | 9 (6–15) | 0.04 |
| Prevalence of adrenal nodule on CT | 58 (62%) | 16 (36%) | 0.005 |
| Serum aldosterone after PST (ng/L) | 316 (231–446) | 233 (192–361) | 0.008 |
| Increase in serum aldosterone after PST < 30% | 54 (61%) | 11 (27%) | 0.0003 |
| Serum aldosterone after SIT (ng/L) | 175 (117–232) | 110 (75–139) | <0.0001 |
| Serum aldosterone after SIT ≥165 ng/L | 50 (53%) | 4 (9%) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holaj, R.; Waldauf, P.; Wichterle, D.; Kvasnička, J.; Zelinka, T.; Petrák, O.; Krátká, Z.; Forejtová, L.; Kaván, J.; Widimský, J., Jr. Adrenal Venous Sampling Could Be Omitted before Surgery in Patients with Conn’s Adenoma Confirmed by Computed Tomography and Higher Normal Aldosterone Concentration after Saline Infusion Test. Diagnostics 2022, 12, 1718. https://doi.org/10.3390/diagnostics12071718
Holaj R, Waldauf P, Wichterle D, Kvasnička J, Zelinka T, Petrák O, Krátká Z, Forejtová L, Kaván J, Widimský J Jr. Adrenal Venous Sampling Could Be Omitted before Surgery in Patients with Conn’s Adenoma Confirmed by Computed Tomography and Higher Normal Aldosterone Concentration after Saline Infusion Test. Diagnostics. 2022; 12(7):1718. https://doi.org/10.3390/diagnostics12071718
Chicago/Turabian StyleHolaj, Robert, Petr Waldauf, Dan Wichterle, Jan Kvasnička, Tomáš Zelinka, Ondřej Petrák, Zuzana Krátká, Lubomíra Forejtová, Jan Kaván, and Jiří Widimský, Jr. 2022. "Adrenal Venous Sampling Could Be Omitted before Surgery in Patients with Conn’s Adenoma Confirmed by Computed Tomography and Higher Normal Aldosterone Concentration after Saline Infusion Test" Diagnostics 12, no. 7: 1718. https://doi.org/10.3390/diagnostics12071718
APA StyleHolaj, R., Waldauf, P., Wichterle, D., Kvasnička, J., Zelinka, T., Petrák, O., Krátká, Z., Forejtová, L., Kaván, J., & Widimský, J., Jr. (2022). Adrenal Venous Sampling Could Be Omitted before Surgery in Patients with Conn’s Adenoma Confirmed by Computed Tomography and Higher Normal Aldosterone Concentration after Saline Infusion Test. Diagnostics, 12(7), 1718. https://doi.org/10.3390/diagnostics12071718






