Practical Echocardiographic Approach of the Regurgitant Mitral Valve Assessment
Abstract
1. Introduction
2. Etiology and Regurgitant Mechanisms
2.1. Primary Mitral Regurgitation
2.2. Secondary Mitral Regurgitation
2.3. Carpentier Classification
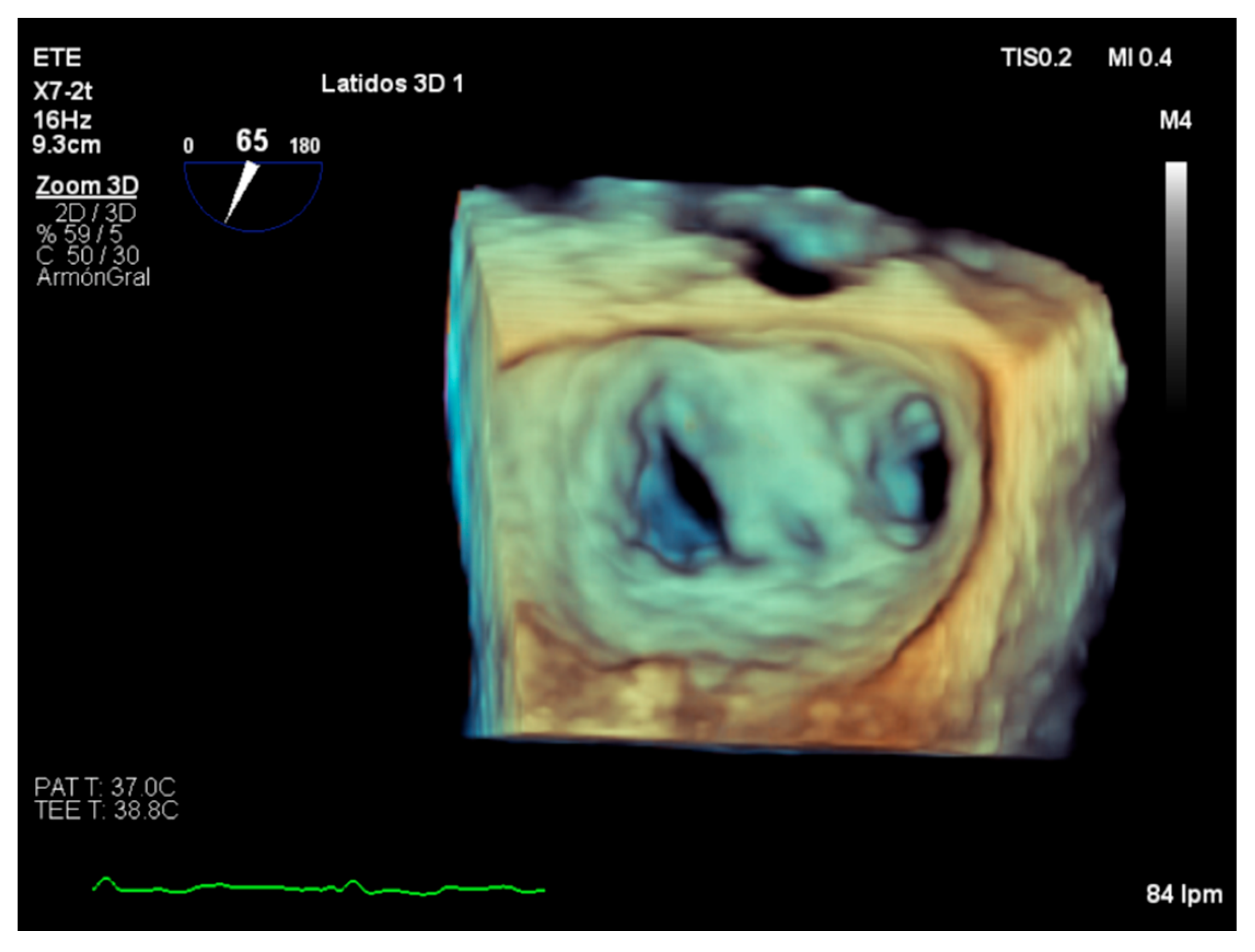
3. Severity Assessment in Practice
3.1. Quantitative Parameters
3.2. Semiquantitative and Qualitative Parameters
3.3. Role of Stress Echocardiography
4. Assessment of Suitability of Valvular Repair
5. Role of Intraoperative Echocardiography
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enriquez-Sarano, M.; Akins, C.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Oliveira, D.; Srinivasan, J.; Espino, D.; Buchan, K.; Dawson, D.; Shepherd, D. Geometric description for the anatomy of the mitral valve: A review. J. Anat. 2020, 237, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Ring, L.; Augustine, D.; Rekhraj, S.; Oxborough, D.; Harkness, A.; Lancellotti, P.; Rana, B. The assessment of mitral valve disease: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G87–G136. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bianco, J.; Beaudoin, J.; Handschumacher, M.; Levine, R. Basic Mechanisms of Mitral Regurgitation. Can. J. Cardiol. 2014, 30, 971–981. [Google Scholar] [CrossRef]
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.A.; Satpathy, R.; et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation. J. Am. Coll. Cardiol. 2020, 75, 2236–2270. [Google Scholar] [CrossRef]
- Dulgheru, R.; Bruls, S.; Lancellotti, P. How I look at the regurgitant mitral valve—A stepwise echocardiographic assessment. Eur. Heart J.-Cardiovasc. Imaging 2020, 22, 491–493. [Google Scholar] [CrossRef]
- El Sabbagh, A.; Reddy, Y.; Nishimura, R. Mitral Valve Regurgitation in the Contemporary Era. JACC Cardiovasc. Imaging 2018, 11, 628–643. [Google Scholar] [CrossRef]
- Zeng, X.; Tan, T.; Dudzinski, D.; Hung, J. Echocardiography of the Mitral Valve. Prog. Cardiovasc. Dis. 2014, 57, 55–73. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Ingraham, B.; Chareonthaitawee, P.; Reddy, Y. Exercise-Induced Left Bundle Branch Block Resulting in Severe Mitral Regurgitation. JACC Case Rep. 2021, 3, 1287–1290. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.; Verbrugge, F.; Verhaert, D.; Rega, F.; Thomas, J.; Vandervoort, P. Atrial Functional Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef]
- El Oakley, R.; Shah, A. Management-Oriented Classification of Mitral Valve Regurgitation. ISRN Cardiol. 2011, 2011, 858714. [Google Scholar] [CrossRef][Green Version]
- Grayburn, P.; Thomas, J. Basic Principles of the Echocardiographic Evaluation of Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 843–853. [Google Scholar] [CrossRef]
- Furukawa, A.; Abe, Y.; Ito, K.; Hosogi, S.; Yamamoto, K.; Ito, H. Mechanisms of changes in functional mitral regurgitation by preload alterations. J. Cardiol. 2018, 71, 570–576. [Google Scholar] [CrossRef]
- Stevenson, L.; Brunken, R.; Belil, D.; Grover-McKay, M.; Schwaiger, M.; Schelbert, H.; Tillisch, J. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure. J. Am. Coll. Cardiol. 1990, 15, 174–180. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.; Edvardsen, T.; Pierard, L.; Badano, L.; Zamorano, J. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Quéré, J.; Tribouilloy, C.; Enriquez-Sarano, M. Vena contracta width measurement: Theoretic basis and usefulness in the assessment of valvular regurgitation severity. Curr. Cardiol. Rep. 2003, 5, 110–115. [Google Scholar] [CrossRef]
- Grayburn, P.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef]
- Báez-Ferrer, N.; Izquierdo-Gómez, M.; Marí-López, B.; Montoto-López, J.; Duque-Gómez, A.; García-Niebla, J.; Miranda-Bacallado, J.; de la Rosa Hernández, A.; Laynez-Cerdeña, I.; Lacalzada-Almeida, J. Clinical manifestations, diagnosis, and treatment of ischemic mitral regurgitation: A review. J. Thorac. Dis. 2018, 10, 6969–6986. [Google Scholar] [CrossRef]
- Little, S.H.; Pirat, B.; Kumar, R.; Igo, S.R.; McCulloch, M.; Hartley, C.J.; Xu, J.; Zoghbi, W.A. Three-Dimensional Color Doppler Echocardiography for Direct Measurement of Vena Contracta Area in Mitral Regurgitation: In Vitro Validation and Clinical Experience. JACC Cardiovasc. Imaging 2008, 1, 695–704. [Google Scholar] [CrossRef]
- Buck, T.; Plicht, B. Real-Time Three-Dimensional Echocardiographic Assessment of Severity of Mitral Regurgitation Using Proximal Isovelocity Surface Area and Vena Contracta Area Method. Lessons We Learned and Clinical Implications. Curr. Cardiovasc. Imaging Rep. 2015, 8, 38. [Google Scholar] [CrossRef]
- Zoghbi, W.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Buck, T.; Plicht, B.; Kahlert, P.; Schenk, I.; Hunold, P.; Erbel, R. Effect of Dynamic Flow Rate and Orifice Area on Mitral Regurgitant Stroke Volume Quantification Using the Proximal Isovelocity Surface Area Method. J. Am. Coll. Cardiol. 2008, 52, 767–778. [Google Scholar] [CrossRef]
- Uretsky, S.; Gillam, L.; Lang, R.; Chaudhry, F.A.; Argulian, E.; Supariwala, A.; Gurram, S.; Jain, K.; Subero, M.; Jang, J.J.; et al. Discordance Between Echocardiography and MRI in the Assessment of Mitral Regurgitation Severity. J. Am. Coll. Cardiol. 2015, 65, 1078–1088. [Google Scholar] [CrossRef]
- NNamazi, F.; van der Bijl, P.; Fortuni, F.; Mertens, B.J.; Kamperidis, V.; van Wijngaarden, S.E.; Stone, G.W.; Narula, J.; Marsan, N.A.; Vahanian, A.; et al. Regurgitant Volume/Left Ventricular End-Diastolic Volume Ratio. JACC Cardiovasc. Imaging 2021, 14, 730–739. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Kawano, S.; Okamura, A.; Kurotobi, T.; Date, M.; Inoue, K.; Fujii, K. Comparison of orifice area by transthoracic three-dimensional Doppler echocardiography versus proximal isovelocity surface area (PISA) method for assessment of mitral regurgitation. Am. J. Cardiol. 2006, 97, 1630–1637. [Google Scholar] [CrossRef]
- Igata, S.; Cotter, B.R.; Hang, C.T.; Morikawa, N.; Strachan, M.; Raisinghani, A.; Blanchard, D.G.; DeMaria, A.N. Optimal Quantification of Functional Mitral Regurgitation: Comparison of Volumetric and Proximal Isovelocity Surface Area Methods to Predict Outcome. J. Am. Heart Assoc. 2021, 10, e018553. [Google Scholar] [CrossRef]
- Xie, G.; Berk, M.; Smith, M.; DeMaria, A. A simplified method for determining regurgitant fraction by Doppler echocardiography in patients with aortic regurtitation. J. Am. Coll. Cardiol. 1994, 24, 1041–1045. [Google Scholar] [CrossRef][Green Version]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Asch, F.M.; Grayburn, P.A.; Siegel, R.J.; Kar, S.; Lim, D.S.; Zaroff, J.G.; Mishell, J.M.; Whisenant, B.; Mack, M.J.; Lindenfeld, J.; et al. Echocardiographic Outcomes After Transcatheter Leaflet Approximation in Patients with Secondary Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Shen, W.F.; Rey, J.L.; Adam, M.C.; Lesbre, J.P. Mitral to aortic velocity-time integral ratio. A non-geometric pulsed-Doppler regurgitant index in isolated pure mitral regurgitation. Eur. Heart J. 1994, 15, 1335–1339. [Google Scholar] [CrossRef]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Dujardin, K.S.; Tribouilloy, C.M.; Seward, J.B.; Yoganathan, A.P.; Bailey, K.R.; Tajik, A. Determinants of pulmonary venous flow reversal in mitral regurgitation and its usefulness in determining the severity of regurgitation. Am. J. Cardiol. 1999, 83, 535–541. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010, 122, 33–41. [Google Scholar] [CrossRef]
- Pierard, L.; Dulgheru, R.; Magne, J.; Lancellotti, P. Dynamic ischaemic mitral regurgitation and the role of stress echocardiography. J. Cardiovasc. Echogr. 2013, 23, 10–17. [Google Scholar] [CrossRef]
- Suzuki, T.; Izumo, M.; Suzuki, K.; Koto, D.; Tsukahara, M.; Teramoto, K.; Sato, Y.; Watanabe, M.; Mizukoshi, K.; Kamijima, R.; et al. Prognostic value of exercise stress echocardiography in patients with secondary mitral regurgitation: A long-term follow-up study. J. Echocardiogr. 2018, 17, 147–156. [Google Scholar] [CrossRef]
- Jung, J.C.; Jang, M.J.; Hwang, H.Y. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am. J. Cardiol. 2019, 123, 446–453. [Google Scholar] [CrossRef]
- Omran, A.; Woo, A.; David, T.; Feindel, C.; Rakowski, H.; Siu, S. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J. Am. Soc. Echocardiogr. 2002, 15, 950–957. [Google Scholar] [CrossRef]
- Thaden, J.J.; Malouf, J.F.; Rehfeldt, K.H.; Ashikhmina, E.; Bagameri, G.; Enriquez-Sarano, M.; Stulak, J.M.; Schaff, H.V.; Michelena, H.I. Adult Intraoperative Echocardiography: A Comprehensive Review of Current Practice. J. Am. Soc. Echocardiogr. 2020, 33, 735–755.e11. [Google Scholar] [CrossRef]
- Wunderlich, N.; Siegel, R. Peri-interventional echo assessment for the MitraClip procedure. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 935–949. [Google Scholar] [CrossRef]
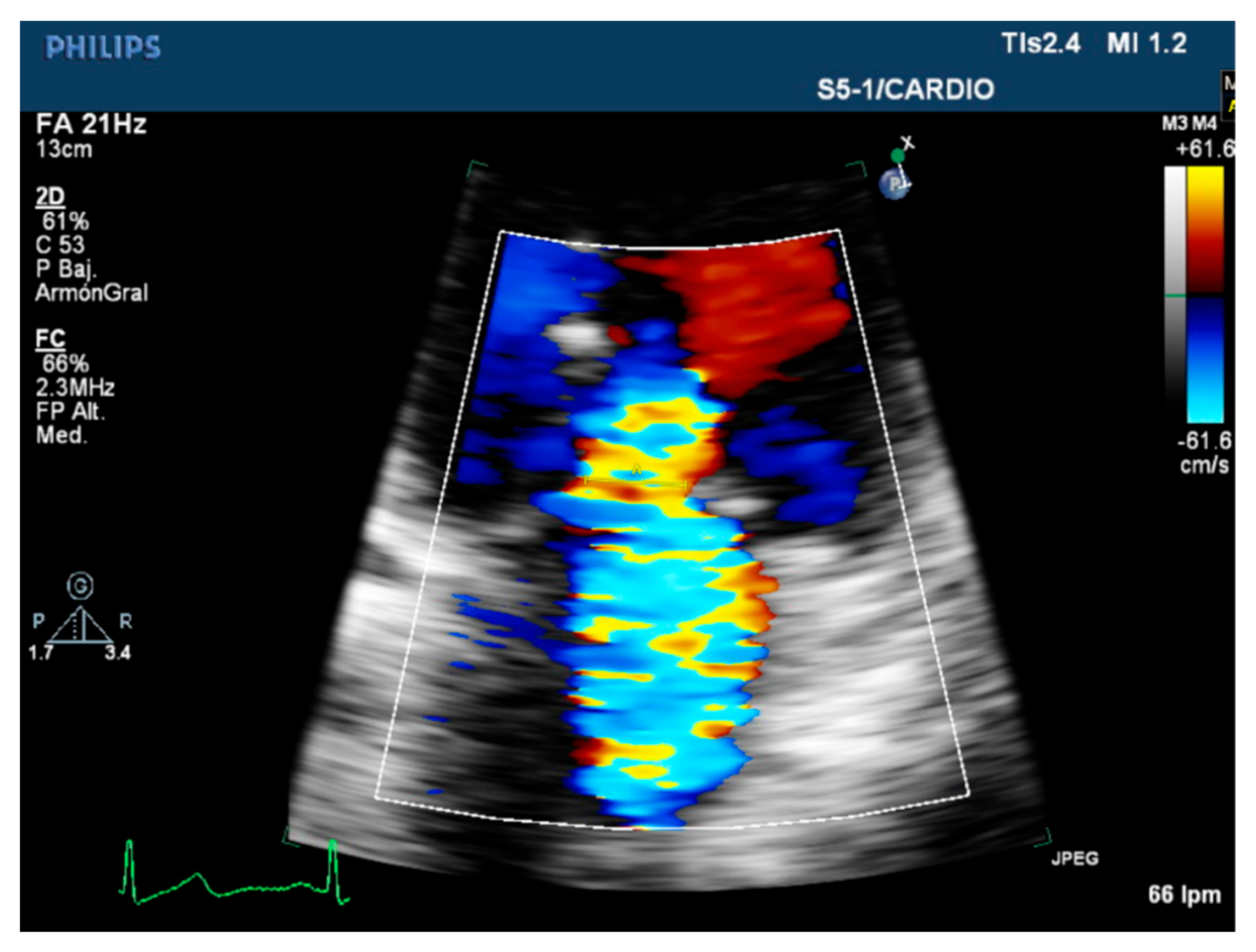
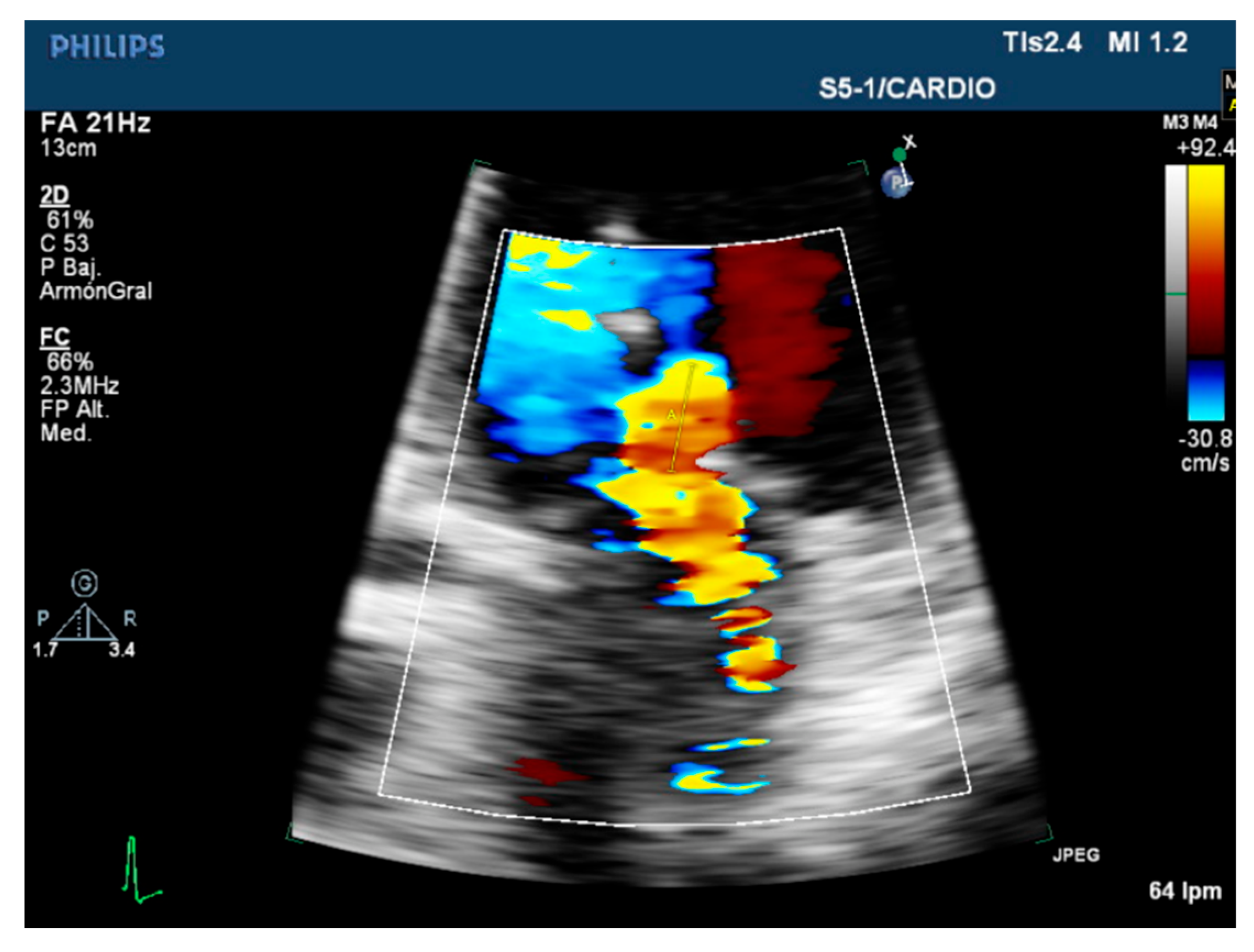
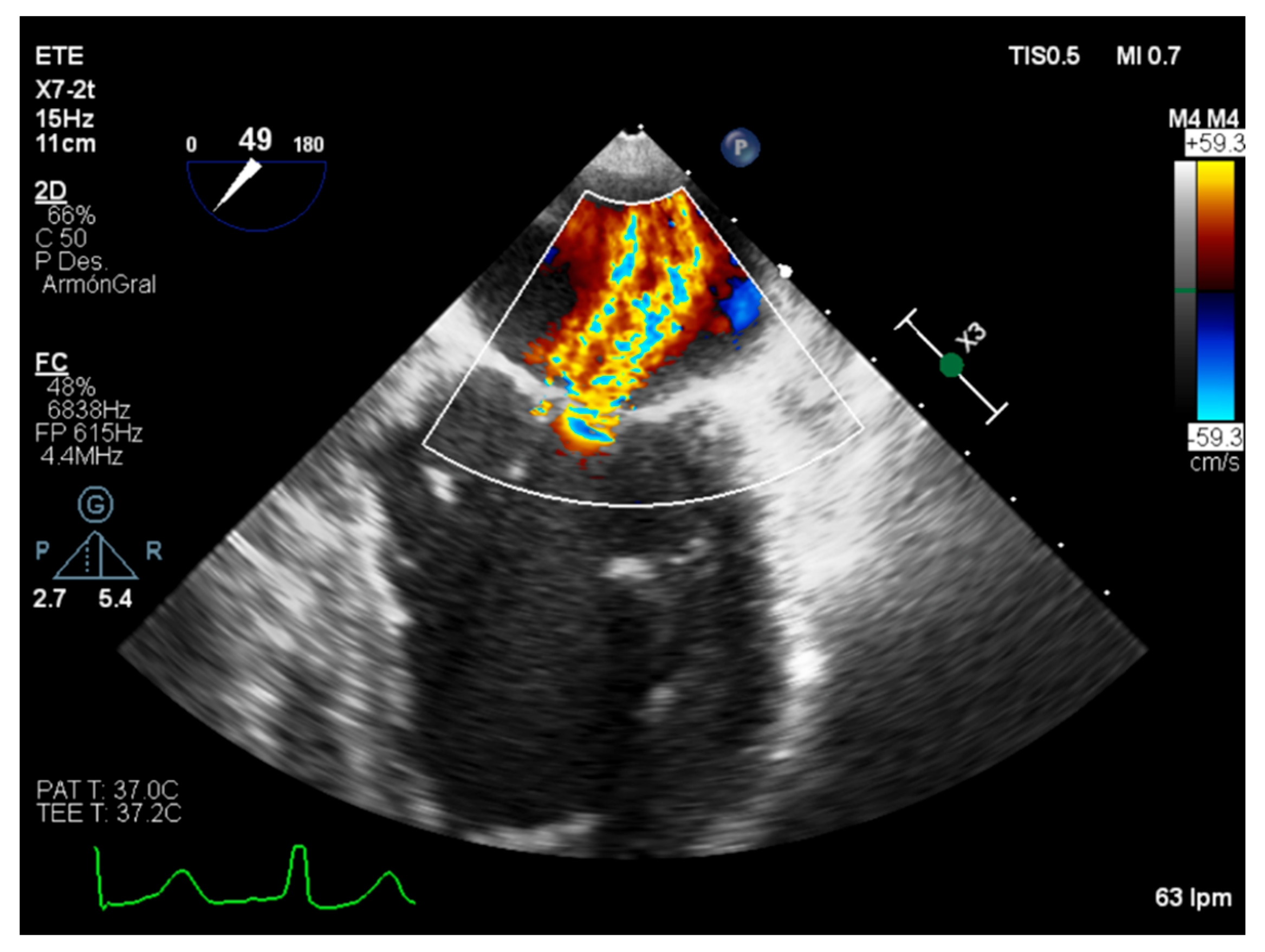
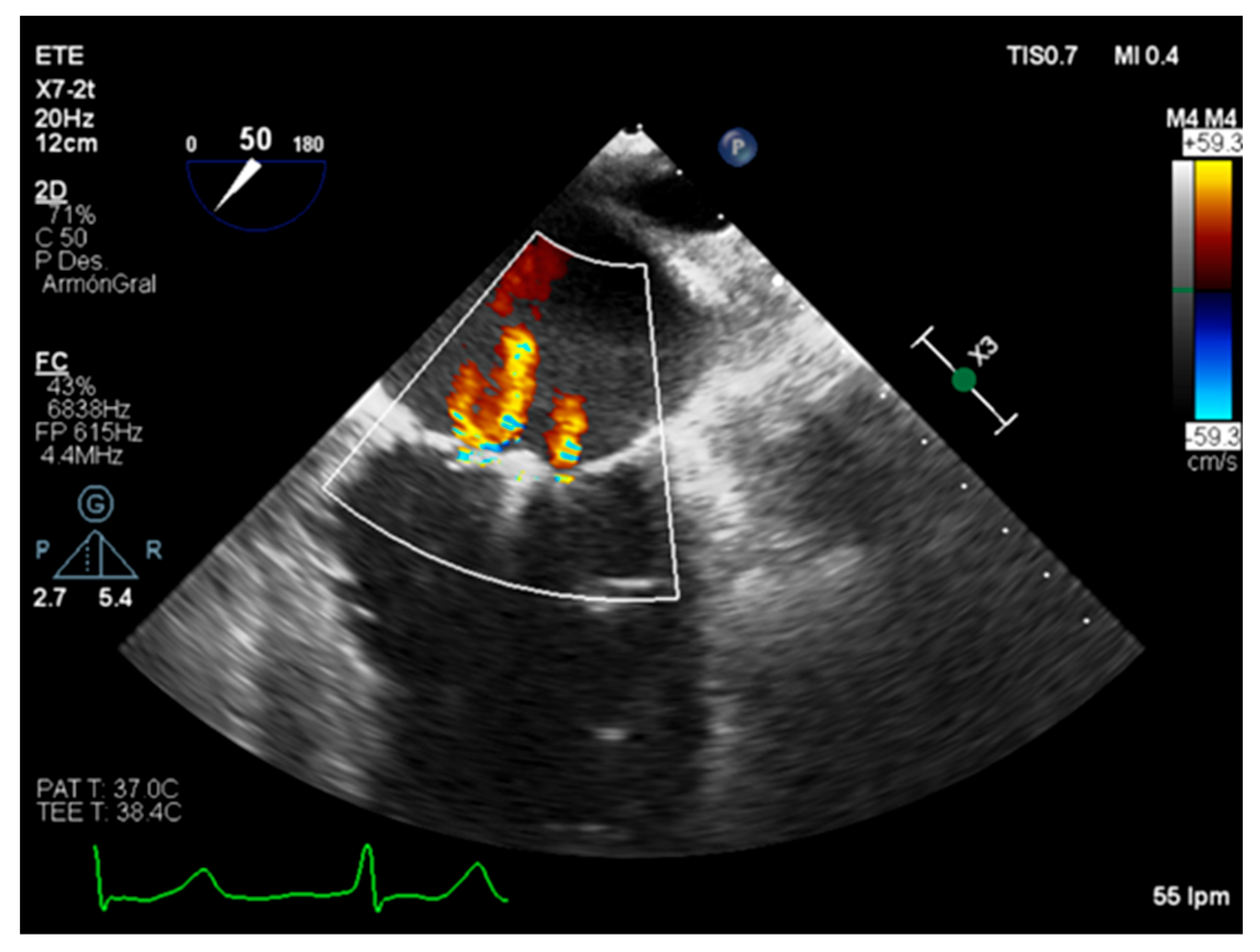
| Carpentier’s Classification | Leaflets Motion | Anatomical Session | Etiologies |
|---|---|---|---|
| Type I | Normal. | Leaflet perforation. Annular dilatation. | Degenerative (annular calcification), infectious endocarditis, inflammatory, congenital cleft defect. |
| Type II | Excesive. | Chordal rupture. Chordal elongation. Papillary muscle rupture. | Degenerative (Barlow’s disease), congenital, infectious, ischemic. |
| Type IIIa | Restricted in both systole and diastole. | Commisural or chordal fussion. Leaflet thickening. Leaflet calcification. | Rheumatic, inflammatory, radiation, drugs. |
| Type IIIb | Restricted in systole. | Ventricular dilatation. Chordal thickening or shortening. | Ischemic and non-ischemic. |
| Parameters | Type | Indicatives of Mild MR | Indicatives of Severe MR |
|---|---|---|---|
| EROA (mm2) | Quantitative. | <20 | ≥40 |
| RV | Quantitative. | <30 | ≥60 |
| Vena contracta width (mm) | Semi-quantitative. Quantitative in 3D performance. | <3 | ≥7 (≥8 for biplane) |
| Mitral inflow | Semi-quantitative. | A wave dominance. | E wave dominance. |
| TVI mitral/TVI aortic | Semi-quantitative. | <1 | <1.4 |
| Pulmonary vein inflow | Semi-quantitative. | Systolic dominance. | Systolic flow reversal. |
| MV morphology | Qualitative. | Normal/abnormal. | Flail leaflet or ruptured papillary muscle. |
| Color flow jet | Qualitative. | Small, central. | Large central jet or eccentric reaching the posterior LA wall. |
| Continuous doppler wave morphology. | Qualitative. | Faint/parabolic. | Dense/triangular. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Rodríguez, R.; Duque-González, M.A.; Igareta-Herraiz, A.T.; Di Silvestre, M.; Izquierdo-Gómez, M.M.; Baeza-Garzón, F.; Barragán-Acea, A.; Bosa-Ojeda, F.; Lacalzada-Almeida, J. Practical Echocardiographic Approach of the Regurgitant Mitral Valve Assessment. Diagnostics 2022, 12, 1717. https://doi.org/10.3390/diagnostics12071717
Muñoz-Rodríguez R, Duque-González MA, Igareta-Herraiz AT, Di Silvestre M, Izquierdo-Gómez MM, Baeza-Garzón F, Barragán-Acea A, Bosa-Ojeda F, Lacalzada-Almeida J. Practical Echocardiographic Approach of the Regurgitant Mitral Valve Assessment. Diagnostics. 2022; 12(7):1717. https://doi.org/10.3390/diagnostics12071717
Chicago/Turabian StyleMuñoz-Rodríguez, Rebeca, María Amelia Duque-González, Aida Tindaya Igareta-Herraiz, Mauro Di Silvestre, María Manuela Izquierdo-Gómez, Flor Baeza-Garzón, Antonio Barragán-Acea, Francisco Bosa-Ojeda, and Juan Lacalzada-Almeida. 2022. "Practical Echocardiographic Approach of the Regurgitant Mitral Valve Assessment" Diagnostics 12, no. 7: 1717. https://doi.org/10.3390/diagnostics12071717
APA StyleMuñoz-Rodríguez, R., Duque-González, M. A., Igareta-Herraiz, A. T., Di Silvestre, M., Izquierdo-Gómez, M. M., Baeza-Garzón, F., Barragán-Acea, A., Bosa-Ojeda, F., & Lacalzada-Almeida, J. (2022). Practical Echocardiographic Approach of the Regurgitant Mitral Valve Assessment. Diagnostics, 12(7), 1717. https://doi.org/10.3390/diagnostics12071717






