Arteriovenous Hemodialysis Access Stenosis Diagnosed by Duplex Doppler Ultrasonography: A Review
Abstract
:1. Introduction
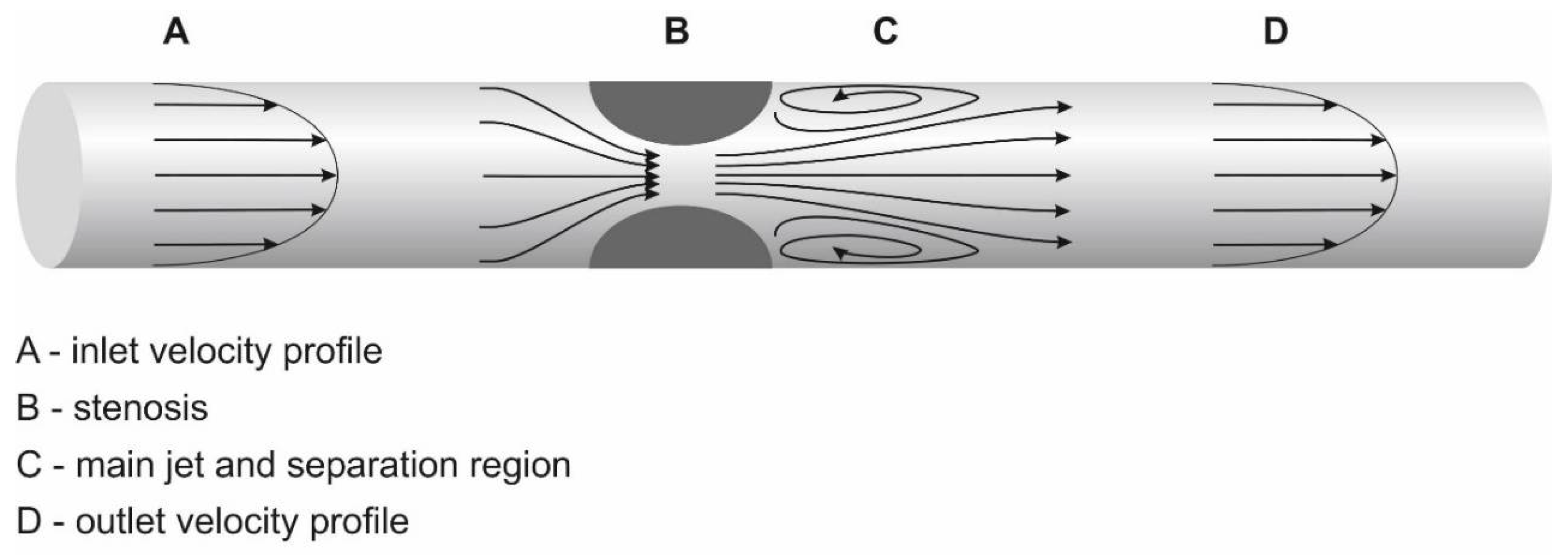
2. General Definition of Stenoses
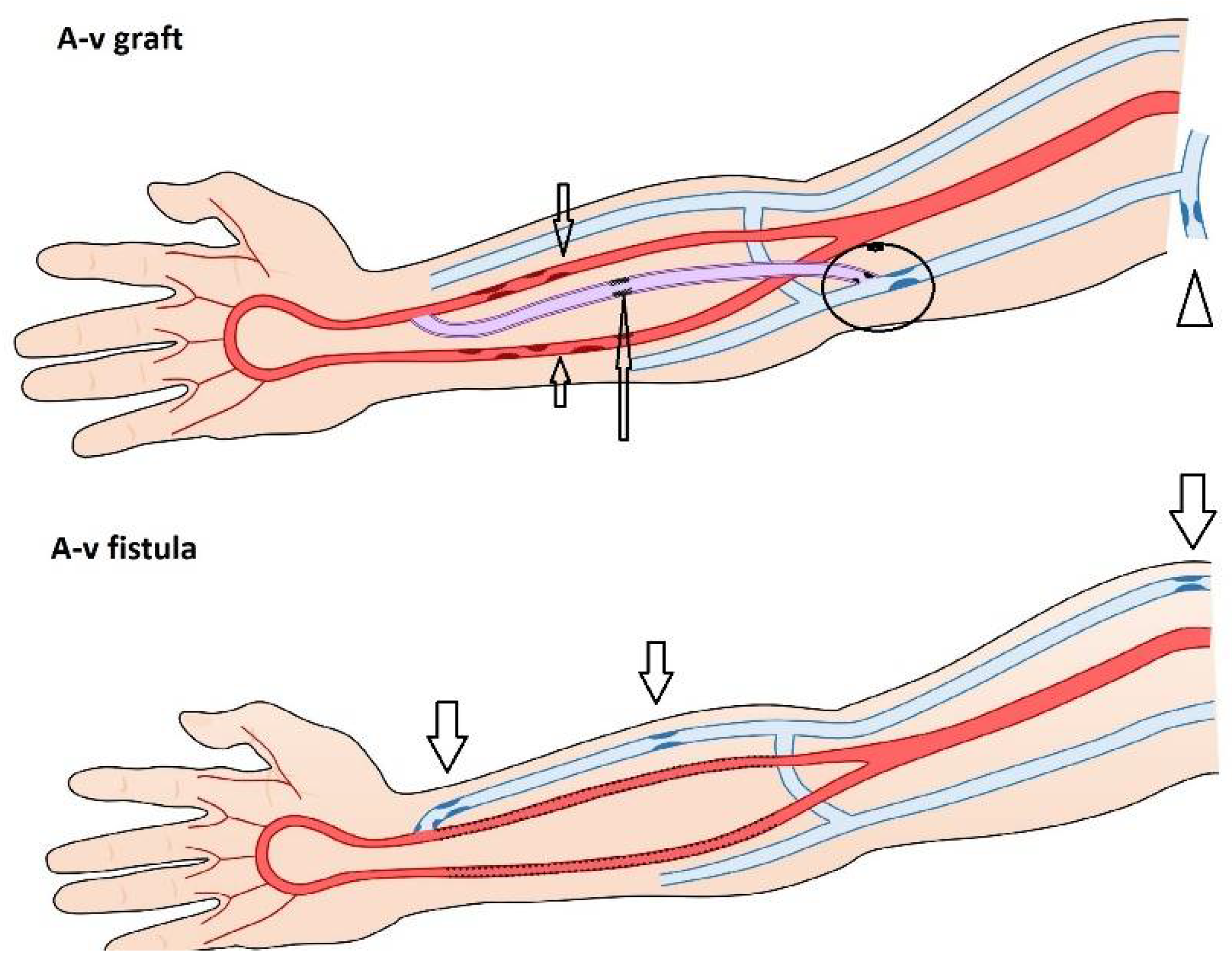
3. Etiology and Types of Stenoses
4. Predilectional Sites of Stenoses
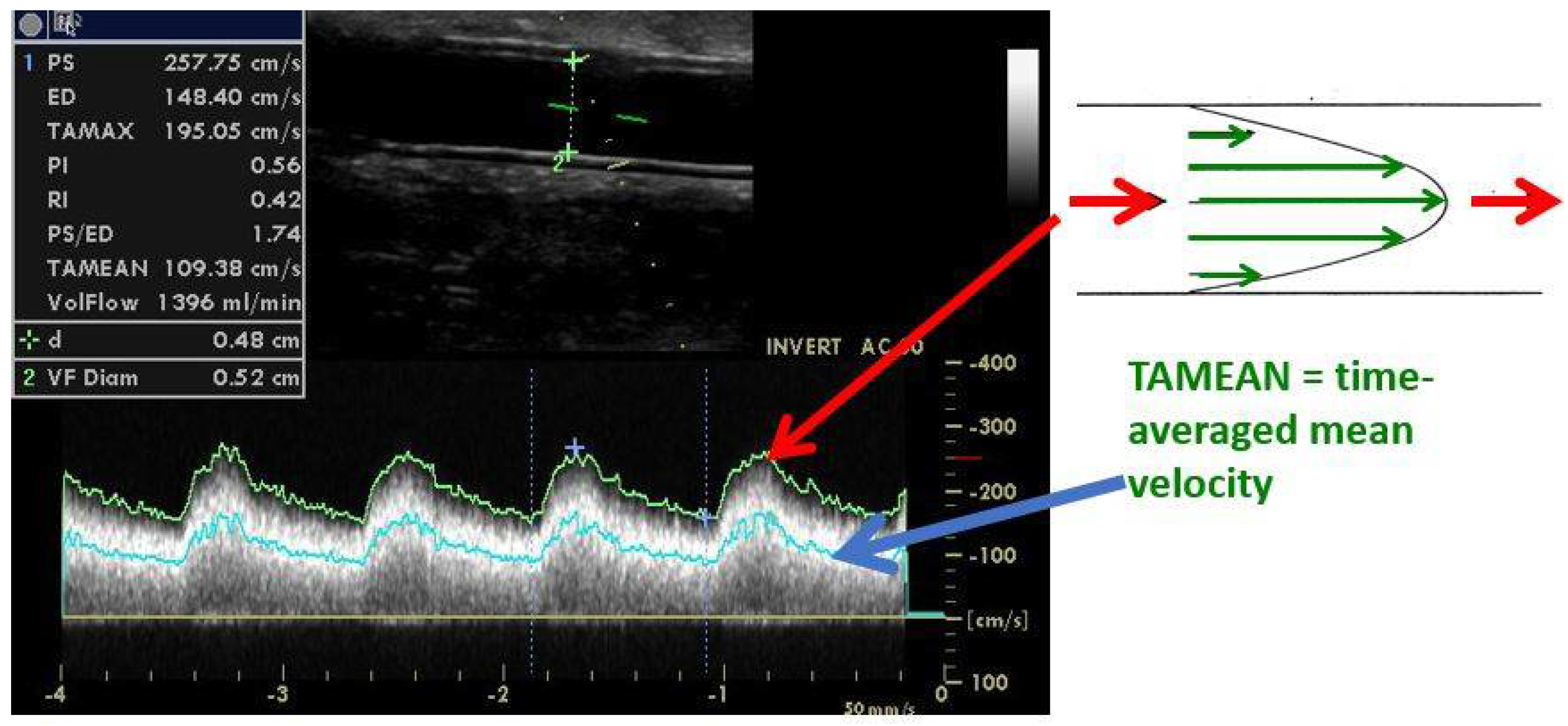
5. Significant Stenosis Definition by Ultrasonography
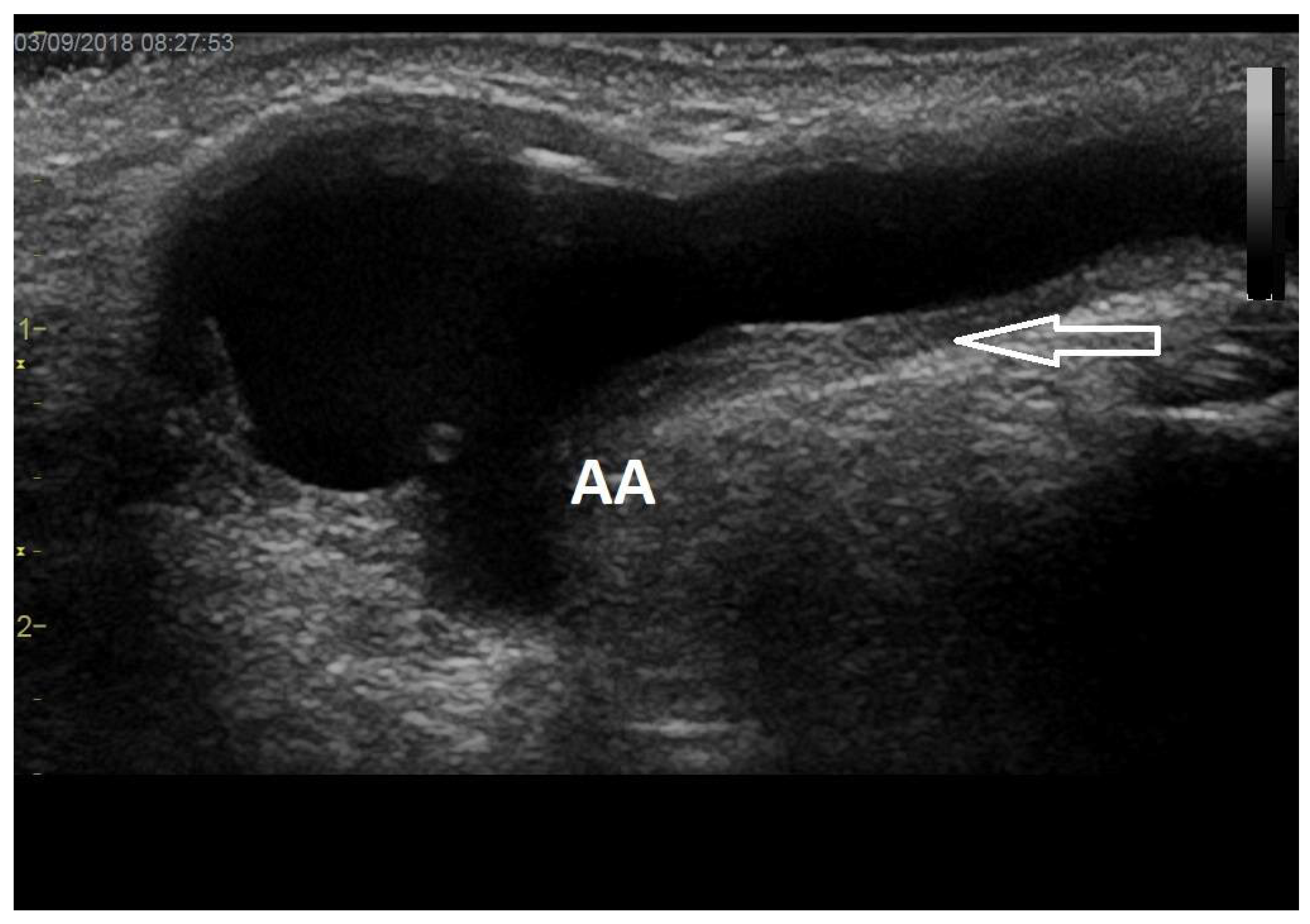
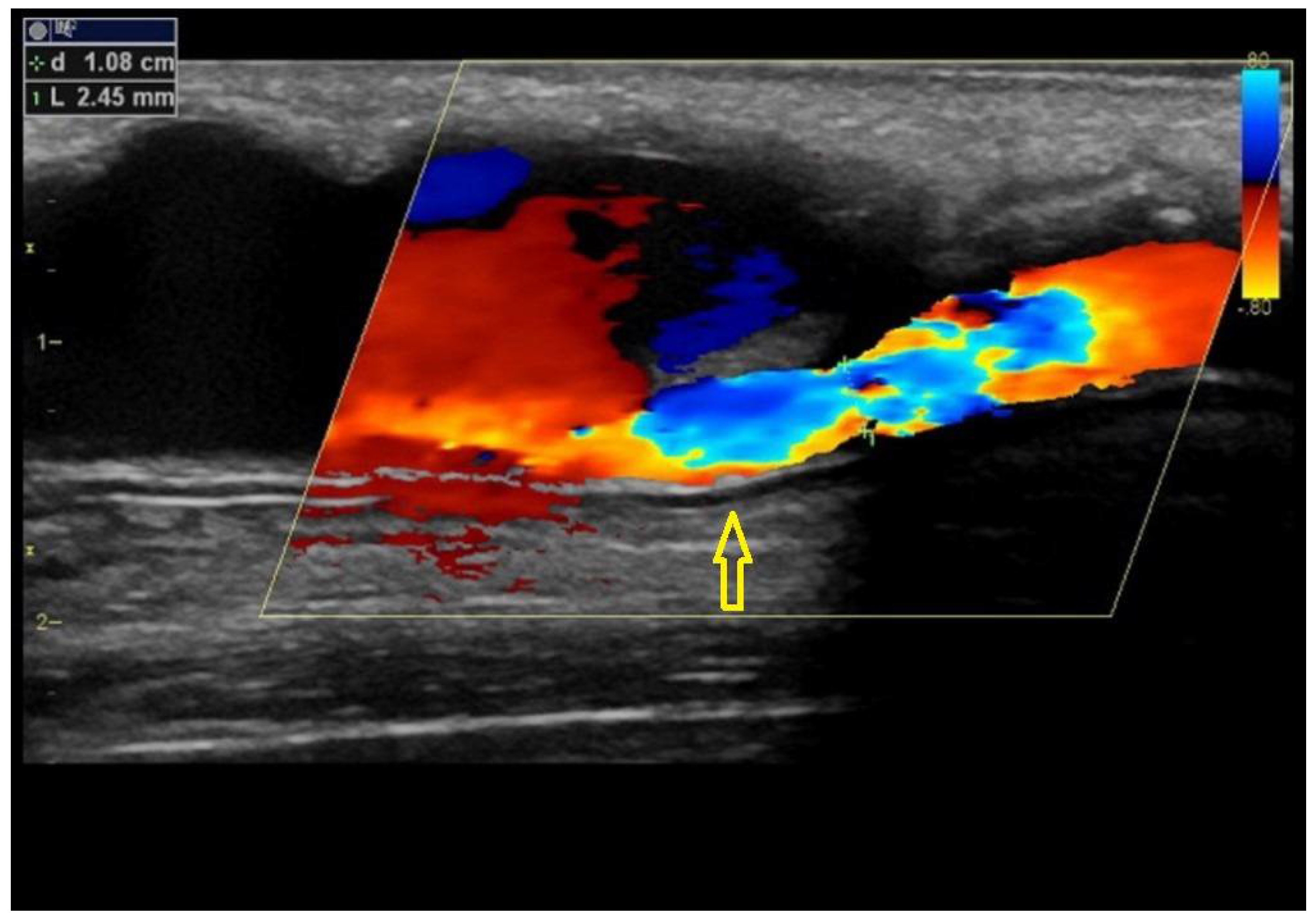
6. Arteriovenous Access Surveillance by Ultrasonography
7. Symptoms of AVF/AVG Stenosis and When to Intervene a Stenosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soleymanian, T.; Sheikh, V.; Tareh, F.; Argani, H.; Ossareh, S. Hemodialysis vascular access and clinical outcomes: An observational multicenter study. J. Vasc. Access 2017, 18, 35–42. [Google Scholar] [CrossRef]
- Haddad, D.J.; Jasty, V.S.; Mohan, B.; Hsu, C.-H.; Chong, C.C.; Zhou, W.; Tan, T.-W. Comparing outcomes of upper extremity brachiobasilic arteriovenous fistulas and arteriovenous grafts: A systematic review and meta-analysis. J. Vasc. Access 2022, 23, 32–41. [Google Scholar] [CrossRef]
- Chytilova, E.; Jemcov, T.A.-O.; Malik, J.A.-O.; Pajek, J.A.-O.; Fila, B.; Kavan, J. Role of Doppler ultrasonography in the evaluation of hemodialysis arteriovenous access maturation and influencing factors. J. Vasc. Access 2021, 22 (Suppl. S1), 42–55. [Google Scholar] [CrossRef]
- Strauch, B.S.; O’Connell, R.S.; Geoly, K.L.; Grundlehner, M.; Yakub, Y.N.; Tietjen, D.P. Forecasting thrombosis of vascular access with Doppler color flow imaging. Am. J. Kidney Dis. 1992, 19, 554–557. [Google Scholar] [CrossRef]
- Gadallah, M.F.; Paulson, W.D.; Vickers, B.; Work, J. Accuracy of Doppler ultrasound in diagnosing anatomic stenosis of hemodialysis arteriovenous access as compared with fistulography. Am. J. Kidney Dis. 1998, 32, 273–277. [Google Scholar] [CrossRef]
- Kudlicka, J.; Kavan, J.; Tuka, V.; Malik, J. More precise diagnosis of access stenosis: Ultrasonography versus angiography. J. Vasc. Access 2012, 13, 310–314. [Google Scholar] [CrossRef]
- Sullivan, K.L.; Besarab, A.; Bonn, J.; Shapiro, M.J.; Gardiner, G.A.; Moritz, M.J. Hemodynamics of failing dialysis grafts. Radiology 1993, 186, 867–872. [Google Scholar] [CrossRef]
- Quencer, K.B.; Arici, M. Arteriovenous Fistulas and Their Characteristic Sites of Stenosis. Am. J. Roentgenol. 2015, 205, 726–734. [Google Scholar] [CrossRef]
- Badero, O.J.; Salifu, M.O.; Wasse, H.; Work, J. Frequency of swing-segment stenosis in referred dialysis patients with angiographically documented lesions. Am. J. Kidney Dis. 2008, 51, 93–98. [Google Scholar] [CrossRef]
- Ene-Iordache, B.; Cattaneo, L.; Dubini, G.; Remuzzi, A. Effect of anastomosis angle on the localization of disturbed flow in ‘side-to-end’ fistulae for haemodialysis access. Nephrol. Dial. Transplant. 2013, 28, 997–1005. [Google Scholar] [CrossRef]
- Sivananthan, G.; Menashe, L.; Halin, N.J. Cephalic arch stenosis in dialysis patients: Review of clinical relevance, anatomy, current theories on etiology and management. J. Vasc. Access 2014, 15, 157–162. [Google Scholar] [CrossRef]
- Shemesh, D.; Goldin, I.; Zaghal, I.; Berlowitz, D.; Raveh, D.; Olsha, O. Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: A prospective randomized clinical trial. J. Vasc. Surg. 2008, 48, 1524–1531. [Google Scholar] [CrossRef]
- Bozof, R.; Kats, M.; Barker, J.; Allon, M. Time to symptomatic vascular stenosis at different locations in patients with arteriovenous grafts. Semin. Dial. 2008, 21, 285–288. [Google Scholar] [CrossRef]
- De Nisco, G.; Gallo, D.; Siciliano, K.; Tasso, P.; Rizzini, M.L.; Mazzi, V.; Calò, K.; Antonucci, M.; Morbiducci, U. Hemodialysis arterio-venous graft design reducing the hemodynamic risk of vascular access dysfunction. J. Biomech. 2020, 100, 109591. [Google Scholar] [CrossRef]
- Agarwal, A.K. Central vein stenosis. Am. J. Kidney Dis. 2013, 61, 1001–1015. [Google Scholar] [CrossRef]
- Asif, A.; Gadalean, F.N.; Merrill, D.; Cherla, G.; Cipleu, C.D.; Epstein, D.L.; Roth, D. Inflow stenosis in arteriovenous fistulas and grafts: A multicenter, prospective study. Kidney Int. 2005, 67, 1986–1992. [Google Scholar] [CrossRef]
- Pirozzi, N.; De Alexandris, L.; Scrivano, J.; Fazzari, L.; Malik, J. Ultrasound evaluation of dialysis access-related distal ischaemia. J. Vasc. Access 2021, 22 (Suppl. S1), 84–90. [Google Scholar] [CrossRef]
- Wo, K.; Morrison, B.J.; Harada, R.N. Developing Duplex Ultrasound Criteria for Diagnosis of Arteriovenous Fistula Stenosis. Ann. Vasc. Surg. 2017, 38, 99–104. [Google Scholar] [CrossRef]
- Plato, S.A., II; Kudlaty, E.A.; Allemang, M.T.; Kendrick, D.E.; Wong, V.L.; Wang, J.C.; Kashyap, V.S. Elevated Peak Systolic Velocity and Velocity Ratio from Duplex Ultrasound are Associated with Hemodynamically Significant Lesions in Arteriovenous Access. Ann. Vasc. Surg. 2016, 35, 68–74. [Google Scholar] [CrossRef]
- Doelman, C.; Duijm, L.E.; Liem, Y.S.; Froger, C.L.; Tielbeek, A.V.; Rossum, A.B.D.-V.; Cuypers, P.W.; Douwes-Draaijer, P.; Buth, J.; Bosch, H.C.V.D. Stenosis detection in failing hemodialysis access fistulas and grafts: Comparison of color Doppler ultrasonography, contrast-enhanced magnetic resonance angiography, and digital subtraction angiography. J. Vasc. Surg. 2005, 42, 739–746. [Google Scholar] [CrossRef]
- Robbin, M.L.; Oser, R.F.; Allon, M.; Clements, M.W.; Dockery, J.; Weber, T.M.; Hamrick-Waller, K.M.; Smith, J.K.; Jones, B.C.; E Morgan, D.; et al. Hemodialysis access graft stenosis: US detection. Radiology 1998, 208, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Kudlicka, J.; Novakova, L.; Adamec, J.; Malikova, H.; Kavan, J. Surveillance of arteriovenous accesses with the use of duplex Doppler ultrasonography. J. Vasc. Access 2014, 15 (Suppl. S7), S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J. Duplex ultrasound scanning of the autogenous arterio venous hemodialysis fistula: A vascular surgeon’s perspective. Australas. J. Ultrasound Med. 2011, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nassar, G.M.; Beathard, G. Exploring correlations between anatomic characteristics of dialysis arteriovenous fistula stenosis and arteriovenous fistula blood flow rate (Qa). J. Vasc. Access. 2020, 21, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Manov, J.J.; Mohan, P.P.; Vazquez-Padron, R. Arteriovenous fistulas for hemodialysis: Brief review and current problems. J. Vasc. Access 2021. [Google Scholar] [CrossRef] [PubMed]
- Fahrtash, F.; Kairaitis, L.; Gruenewald, S.; Spicer, T.; Sidrak, H.; Fletcher, J.; Allen, R.; Swinnen, J. Defining a significant stenosis in an autologous radio-cephalic arteriovenous fistula for hemodialysis. Semin. Dial. 2011, 24, 231–238. [Google Scholar] [CrossRef]
- Kairaitis, L.K.; Collett, J.P.; Swinnen, J. Diameter of inflow as a predictor of radiocephalic fistula flow. J. Vasc. Access. 2018, 19, 548–554. [Google Scholar] [CrossRef]
- Ishii, T.; Suzuki, Y.; Nakayama, T.; Ohmori, M.; Masai, S.; Sasagawa, N.; Ohyama, K. Duplex ultrasound for the prediction of vascular events associated with arteriovenous fistulas in hemodialysis patients. J. Vasc. Access 2016, 17, 499–505. [Google Scholar] [CrossRef]
- Tirinescu, D.C.; Bondor, C.I.; Vladutiu, D.S.; Patiu, I.M.; Moldovan, D.; Orasan, R.; Kacso, I.M. Ultrasonographic diagnosis of stenosis of native arteriovenous fistulas in haemodialysis patients. Med. Ultrason. 2016, 18, 332–338. [Google Scholar] [CrossRef]
- Bae, M.; Jeon, C.H.; Han, M.; Jin, M.; Kim, H.J. Analysis of access flow using duplex ultrasonography and the ultrasound dilutional method. J. Vasc. Access 2022, 23, 286–294. [Google Scholar] [CrossRef]
- Malik, J.; Lomonte, C.; Rotmans, J.; Chytilova, E.; Roca-Tey, R.; Kusztal, M.; Grus, T.; Gallieni, M. Hemodialysis vascular access affects heart function and outcomes: Tips for choosing the right access for the individual patient. J. Vasc. Access 2021, 22 (Suppl. S1), 32–41. [Google Scholar] [CrossRef] [PubMed]
- Valek, M.; Lopot, F.; Dusilova-Sulkova, S.; Polakovic, V. Physiologic variability of vascular access blood flow for hemodialysis. Blood Purif. 2008, 26, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Tuka, V.; Slavikova, M.; Krupickova, Z.; Mokrejsova, M.; Chytilova, E.; Malik, J. Short-term outcomes of borderline stenoses in vascular accesses with PTFE grafts. Nephrol. Dial. Transplant. 2009, 24, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Moreira, C.; Almeida, P.; De Matos, N.; Loureiro, L.; Teixeira, G.; Rego, D.; Teixeira, S.; Pinheiro, J.; Carvalho, T.; et al. The Role of Doppler Ultrassonography in Significant and Borderline Stenosis Definition. Blood Purif. 2018, 46, 94–102. [Google Scholar] [CrossRef]
- Berman, S.S.; Mendoza, B.; Westerband, A.; Quick, R.C. Predicting arteriovenous fistula maturation with intraoperative blood flow measurements. J. Vasc. Access 2008, 9, 241–247. [Google Scholar] [CrossRef]
- Chawla, A.; DiRaimo, R.; Panetta, T.F. Balloon angioplasty to facilitate autogenous arteriovenous access maturation: A new paradigm for upgrading small-caliber veins, improved function, and surveillance. Semin. Vasc. Surg. 2011, 24, 82–88. [Google Scholar] [CrossRef]
- Gameiro, J.; Ibeas, J. Factors affecting arteriovenous fistula dysfunction: A narrative review. J. Vasc. Access 2020, 21, 134–147. [Google Scholar] [CrossRef]
- Mayer, D.A.; Zingale, R.G.; Tsapogas, M.J. Duplex Scanning of Expanded Polytetrafluoroethylene Dialysis Shunts: Impact on Patient Management and Graft Survival. Vascular and Endovascular. Surgery 1993, 27, 647–658. [Google Scholar] [CrossRef]
- Lumsden, A.B.; MacDonald, M.J.; Kikeri, D.; Cotsonis, G.A.; Harker, L.A.; Martin, L.G. Prophylactic balloon angioplasty fails to prolong the patency of expanded polytetrafluoroethylene arteriovenous grafts: Results of a prospective randomized study. J. Vasc. Surg. 1997, 26, 382–390. [Google Scholar]
- Ram, S.J.; Work, J.; Caldito, G.C.; Eason, J.M.; Pervez, A.; Paulson, W.D. A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney Int. 2003, 64, 272–280. [Google Scholar] [CrossRef]
- Robbin, M.L.; Oser, R.F.; Lee, J.Y.; Heudebert, G.R.; Mennemeyer, S.T.; Allon, M. Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney Int. 2006, 69, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Slavikova, M.; Svobodova, J.; Tuka, V. Regular ultrasonographic screening significantly prolongs patency of PTFE grafts. Kidney Int. 2005, 67, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; James, M.; Wiebe, N.; Jindal, K.; Hemmelgarn, B. Ultrasound monitoring to detect access stenosis in hemodialysis patients: A systematic review. Am. J. Kidney Dis. 2008, 51, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Quinn, R.R.; Oliver, M.J.; Karsanji, D.J.; James, M.T.; MacRae, J.M.; Palmer, S.C.; Strippoli, G.F. Preemptive Correction of Arteriovenous Access Stenosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2016, 67, 446–460. [Google Scholar] [CrossRef]
- Schmidli, J.; Widmer, M.K.; Basile, C.; de Donato, G.; Gallieni, M.; Gibbons, C.P.; Haage, P.; Hamilton, G.; Hedin, U.; Kamper, L. Editor’s Choice—Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 757–818. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef]
- Renaud, C.J.; Francois, M.; Nony, A.; Fodil-Cherif, M.; Turmel-Rodrigues, L. Comparative outcomes of treated symptomatic versus non-treated asymptomatic high-grade central vein stenoses in the outflow of predominantly dialysis fistulas. Nephrol. Dial. Transplant. 2012, 27, 1631–1638. [Google Scholar] [CrossRef]
- Maldonado-Carceles, A.B.; Garcia-Medina, J.; Torres-Cantero, A.M. Performance of physical examination versus ultrasonography to detect stenosis in haemodialysis arteriovenous fistula. J. Vasc. Access 2017, 18, 30–34. [Google Scholar] [CrossRef]
- Malik, J.; Slavikova, M.; Malikova, H.; Maskova, J. Many clinically silent access stenoses can be identified by ultrasonography. J. Nephrol. 2002, 15, 661–665. [Google Scholar]
- Caputo, B.C.; Leong, B.; Sibona, A.; Jhajj, S.; Kohne, C.; Gabel, J.; Shih, W.; Zamzam, A.A.; Bianchi, C.; Teruya, T. Arteriovenous fistula maturation: Physical exam versus flow study. Ann. Vasc. Surg. 2021, 77, 16–24. [Google Scholar] [CrossRef]
- Asif, A.; Leon, C.; Orozco-Vargas, L.C.; Krishnamurthy, G.; Choi, K.L.; Mercado, C.; Merrill, D.; Thomas, I.; Salman, L.; Artikov, S.; et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin. J. Am. Soc. Nephrol. 2007, 2, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, N.; Bedogna, V.; Melilli, E.; Millardi, D.; Mansueto, G.; Lipari, G.; Mantovani, W.; Baggio, E.; Poli, A.; Lupo, A. In search of an optimal bedside screening program for arteriovenous fistula stenosis. Clin. J. Am. Soc. Nephrol. 2011, 6, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.P.; Chula, D.C.; Perreto, S.; Riella, M.C.; Mazza do Nascimento, M. Accuracy of physical examination and intra-access pressure in the detection of stenosis in hemodialysis arteriovenous fistula. Semin. Dial. 2008, 21, 269–273. [Google Scholar] [CrossRef] [PubMed]




| SIGNIFICANT | BORDERLINE |
|---|---|
| Main criteria | |
| Diameter reduction by >50% | |
| Peak systolic velocity increase > 2–3x | |
| +Additional criteria (≥1) | |
| Residual diameter < 1.9–2.0 mm | No additional criterion |
| Flow volume decrease by >25% * | |
| Flow volume < 600 mL/min for AVGs, <500 mL/min for AVFs | |
| Physical examination | Ipsilateral extremity edema |
| Pulse alterations (weak or resistant pulse), difficult to compress in the area of stenosis | |
| Abnormal thrill (weak, discontinuous) with only the systolic component in the stenotic area | |
| Abnormal bruit (high pitched with a systolic component in the area of stenosis) | |
| Failure of outflow vein collapse during arm elevation | |
| Lack of pulse augmentation during arm elevation | |
| Excessive collapse of the outflow veins during arm elevation | |
| During hemodialysis | New difficulty with cannulation |
| Aspiration of blood clots | |
| Inability to achieve the target dialysis blood flow | |
| Prolonged bleeding after needle withdrawal for 3 consecutive dialysis sessions | |
| Unexplained decrease in the target dialysis dose (Kt/V) on a constant dialysis prescription and without dialysis prolongation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, J.; de Bont, C.; Valerianova, A.; Krupickova, Z.; Novakova, L. Arteriovenous Hemodialysis Access Stenosis Diagnosed by Duplex Doppler Ultrasonography: A Review. Diagnostics 2022, 12, 1979. https://doi.org/10.3390/diagnostics12081979
Malik J, de Bont C, Valerianova A, Krupickova Z, Novakova L. Arteriovenous Hemodialysis Access Stenosis Diagnosed by Duplex Doppler Ultrasonography: A Review. Diagnostics. 2022; 12(8):1979. https://doi.org/10.3390/diagnostics12081979
Chicago/Turabian StyleMalik, Jan, Cora de Bont, Anna Valerianova, Zdislava Krupickova, and Ludmila Novakova. 2022. "Arteriovenous Hemodialysis Access Stenosis Diagnosed by Duplex Doppler Ultrasonography: A Review" Diagnostics 12, no. 8: 1979. https://doi.org/10.3390/diagnostics12081979
APA StyleMalik, J., de Bont, C., Valerianova, A., Krupickova, Z., & Novakova, L. (2022). Arteriovenous Hemodialysis Access Stenosis Diagnosed by Duplex Doppler Ultrasonography: A Review. Diagnostics, 12(8), 1979. https://doi.org/10.3390/diagnostics12081979







