Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Ethical Considerations
2.3. Biophysical Characteristics
2.4. Biochemical Blood Analysis
2.5. Magnetic Resonance Imaging for Abdominal Fat Detection
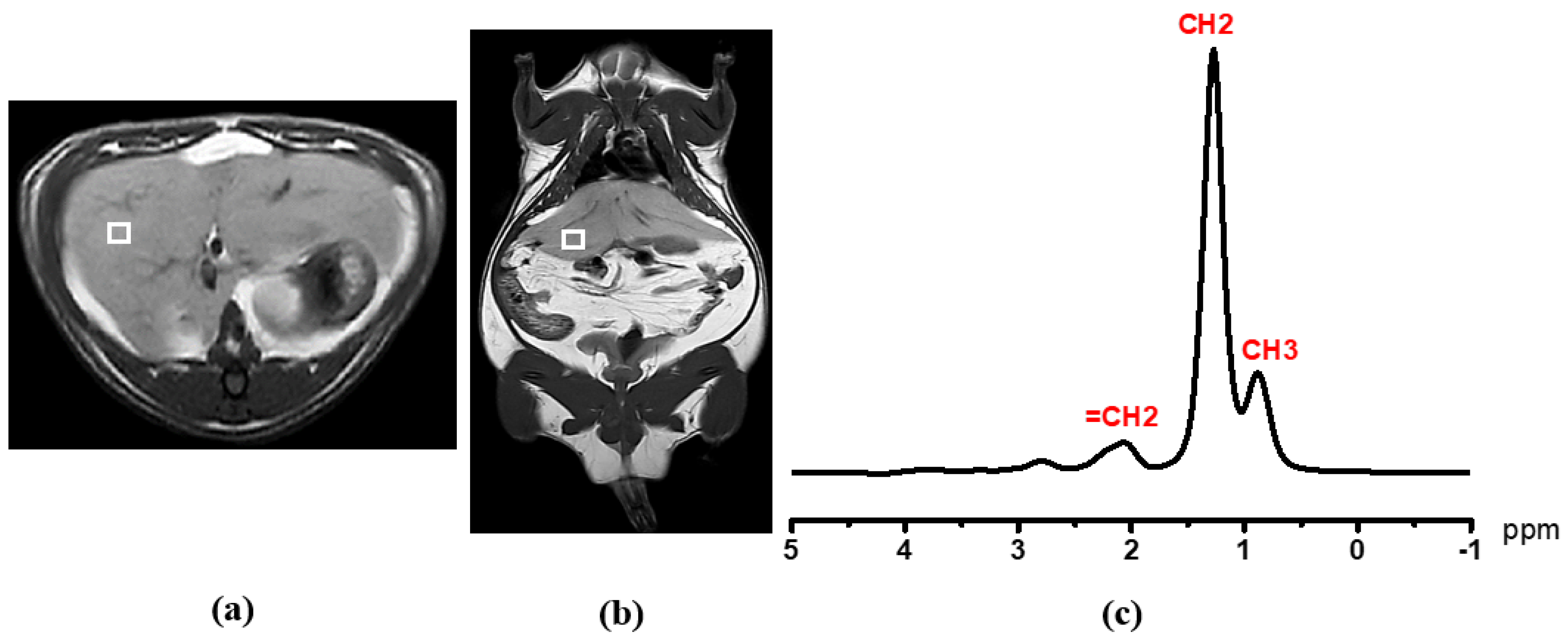
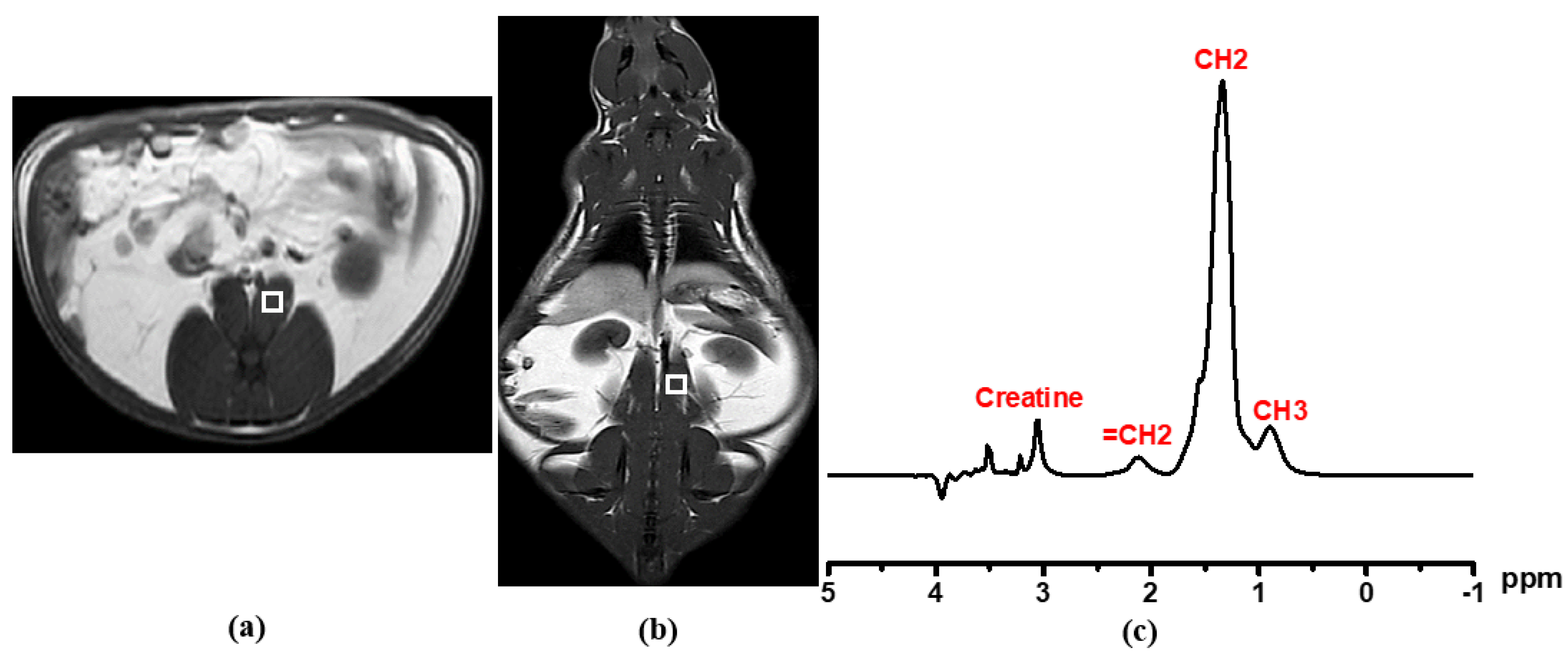
2.6. Single-Voxel Proton Magnetic Resonance Spectroscopy (1H MRS) for Liver and Psoas Muscular Fat Detection
2.7. Proton Nuclear Magnetic Resonance (1H NMR) Acquisition and Analysis
2.8. Statistical Analysis
3. Results
3.1. Biophysical Parameters
3.2. Blood Biochemical Measurements
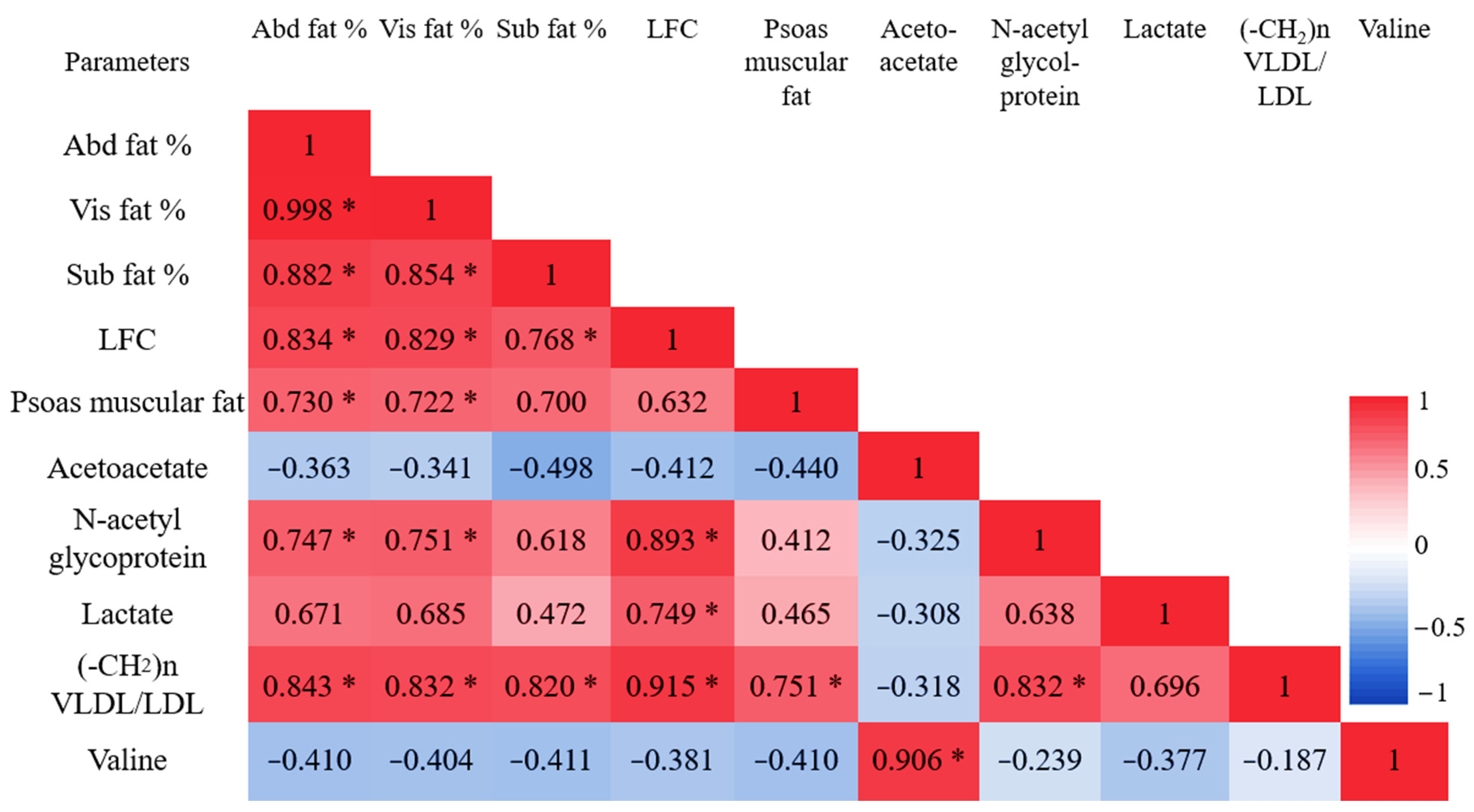
3.3. Abdominal Fat Compartments and Laboratory Characteristics
3.4. Liver Fat Content (LFC) and Laboratory Characteristics
3.5. Psoas Muscular Fat Content and Laboratory Characteristics
3.6. 1H NMR Metabolomic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Córdova Villalobos, J. Obesity: The Real Pandemic of the 21st Century; Masson Doyma Mexico S.A: Ciudad de México, Mexico, 2016; Volume 84, pp. 351–355. [Google Scholar]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Prenner, S.B.; Mulvey, C.K.; Ferguson, J.F.; Rickels, M.R.; Bhatt, A.B.; Reilly, M.P. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis 2014, 236, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmsjö, A.; Rosqvist, F.; Engskog, M.K.R.; Haglöf, J.; Kullberg, J.; Iggman, D.; Johansson, L.; Ahlström, H.; Arvidsson, T.; Risérus, U.; et al. NMR-based metabolic profiling in healthy individuals overfed different types of fat: Links to changes in liver fat accumulation and lean tissue mass. Nutr. Diabetes 2015, 5, e182. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.; Meinders, A.E.; Jazet, I.M. Ectopic fat and insulin resistance: Pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef]
- Mittendorfer, B. Origins of metabolic complications in obesity: Adipose tissue and free fatty acid trafficking. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Fewtrell, M.S. Measuring body composition. Arch. Dis. Child. 2006, 91, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Huber, F.A.; Del Grande, F.; Rizzo, S.; Guglielmi, G.; Guggenberger, R. MRI in the assessment of adipose tissues and muscle composition: How to use it. Quant. Imaging Med. Surg. 2020, 10, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.P.; Heuberger, A.L.; Broeckling, C.D.; Borresen, E.C.; Tillotson, C.; Prenni, J.E. Advances in Nutritional Metabolomics. Current Metabolomics 2013, 1, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Gowda, G.A.N.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larive, C.K.; Barding, G.A.; Dinges, M.M. NMR Spectroscopy for Metabolomics and Metabolic Profiling. Anal. Chem. 2015, 87, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Byun, J.; Pennathur, S. Analytical approaches to metabolomics and applications to systems biology. Semin. Nephrol. 2010, 30, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, T.; Tanaka, S.-I.; Tamura, K.; Isoda, F.; Ukawa, K.; Yamakura, Y.; Takanashi, Y.; Kiuchi, Y.; Umemura, S.; Ishii, M.; et al. Wistar Fatty Rat Is Obese and Spontaneously Hypertensive. Hypertension 1995, 25, 146–150. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Poduri, R. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Akima, H.; Lott, D.; Senesac, C.; Deol, J.; Germain, S.; Arpan, I.; Bendixen, R.; Lee Sweeney, H.; Walter, G.; Vandenborne, K. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2011, 22, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Klopfenstein, B.J.; Kim, M.S.; Krisky, C.M.; Szumowski, J.; Rooney, W.D.; Purnell, J.Q. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol. 2012, 85, e826–e830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karampatos, S.; Papaioannou, A.; Beattie, K.A.; Maly, M.R.; Chan, A.; Adachi, J.D.; Pritchard, J.M. The reliability of a segmentation methodology for assessing intramuscular adipose tissue and other soft-tissue compartments of lower leg MRI images. Magma 2016, 29, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Yokoo, T.; Bydder, M.; Cruite, I.; Schroeder, M.E.; Sirlin, C.B.; Middleton, M.S. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011, 24, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fages, A.; Duarte-Salles, T.; Stepien, M.; Ferrari, P.; Fedirko, V.; Pontoizeau, C.; Trichopoulou, A.; Aleksandrova, K.; Tjønneland, A.; Olsen, A.; et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 2015, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Saccenti, E.; Hoefsloot, H.; Smilde, A.; Westerhuis, J.; Hendriks, M. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2013, 10, 361–374. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Reekmans, G.; Noben, J.-P.; Adriaensens, P. Identification of metabolic phenotypes in childhood obesity by (1)H NMR metabolomics of blood plasma. Future Sci. OA 2018, 4, FSO310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Hu, T.; Zhang, S.; Zhou, L. Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies. Sci. Rep. 2015, 5, 18495. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.E.; Marzullo, P.; Ricotti, R.; Bona, G.; Prodam, F. The pathophysiology of abdominal adipose tissue depots in health and disease. Horm. Mol. Biol. Clin. Investig. 2014, 19, 57–74. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Xu, W.; Li, H.; Lei, H.; Zhang, L.; Hao, F.; Duan, Y.; Yan, X.; Zhao, Y.; Wu, J.; et al. High-fat diet induces dynamic metabolic alterations in multiple biological matrices of rats. J. Proteome. Res. 2013, 12, 3755–3768. [Google Scholar] [CrossRef]
- Van Harmelen, V.; Röhrig, K.; Hauner, H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 2004, 53, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Hernandez-Morante, J.J.; Lujan, J.; Tebar, F.J.; Zamora, S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int. J. Obes. 2006, 30, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.Y.; Kim, I.Y.; Kim, Y.N.; Kim, J.S.; Shin, J.H.; Jang, Z.H.; Lee, H.S.; Hwang, G.S.; Seong, J.K. 1H NMR-based metabolite profiling of diet-induced obesity in a mouse mode. BMB Rep. 2012, 45, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Htun, K.T.; Pan, J.; Pasanta, D.; Tungjai, M.; Udomtanakunchai, C.; Petcharoen, T.; Chamta, N.; Kosicharoen, S.; Chukua, K.; Lai, C.; et al. Advanced Molecular Imaging (MRI/MRS/1H NMR) for Metabolic Information in Young Adults with Health Risk Obesity. Life 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Shelef, I.; Rudich, A.; Gepner, Y.; Shemesh, E.; Chassidim, Y.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Ben Avraham, S.; et al. Abdominal superficial subcutaneous fat: A putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012, 35, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [Green Version]
- Meek, S.E.; Nair, K.S.; Jensen, M.D. Insulin regulation of regional free fatty acid metabolism. Diabetes 1999, 48, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lönnqvist, F.; Thörne, A.; Large, V.; Arner, P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1472–1480. [Google Scholar] [CrossRef]
- Eloi, J.C.; Epifanio, M.; de Goncalves, M.M.; Pellicioli, A.; Vieira, P.F.; Dias, H.B.; Bruscato, N.; Soder, R.B.; Santana, J.C.; Mouzaki, M.; et al. Quantification of Abdominal Fat in Obese and Healthy Adolescents Using 3 Tesla Magnetic Resonance Imaging and Free Software for Image Analysis. PLoS ONE 2017, 12, e0167625. [Google Scholar] [CrossRef]
- Hwang, J.H.; Stein, D.T.; Barzilai, N.; Cui, M.H.; Tonelli, J.; Kishore, P.; Hawkins, M. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: In vivo MR imaging and spectroscopy studies. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1663–E1669. [Google Scholar] [CrossRef] [Green Version]
- Fabbrini, E.; Mohammed, B.S.; Magkos, F.; Korenblat, K.M.; Patterson, B.W.; Klein, S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Després, J.-P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; Larose, E.; Rodés-Cabau, J.; Bertrand Olivier, F.; Poirier, P. Abdominal Obesity and the Metabolic Syndrome: Contribution to Global Cardiometabolic Risk. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Item, F.; Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited. Obes. Rev. 2012, 13 (Suppl. 2), 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Jeyakumar, S.M.; Sheril, A.; Vajreswari, A. Chronic vitamin A-enriched diet feeding regulates hypercholesterolaemia through transcriptional regulation of reverse cholesterol transport pathway genes in obese rat model of WNIN/GR-Ob strain. Indian J. Med. Res. 2016, 144, 238–244. [Google Scholar] [CrossRef]
- Li, H.; Xie, Z.; Lin, J.; Song, H.; Wang, Q.; Wang, K.; Su, M.; Qiu, Y.; Zhao, T.; Song, K.; et al. Transcriptomic and Metabonomic Profiling of Obesity-Prone and Obesity-Resistant Rats under High Fat Diet. J. Proteome Res. 2008, 7, 4775–4783. [Google Scholar] [CrossRef]
- Maltin, C.A. Muscle development and obesity: Is there a relationship? Organogenesis 2008, 4, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Arbanas, J.; Klasan, G.S.; Nikolic, M.; Jerkovic, R.; Miljanovic, I.; Malnar, D. Fibre type composition of the human psoas major muscle with regard to the level of its origin. J. Anat. 2009, 215, 636–641. [Google Scholar] [CrossRef]
- Sinha, R.; Dufour, S.; Petersen, K.; Lebon, V.; Enoksson, S.; Ma, Y.-Z.; Savoye, M.; Rothman, D.; Shulman, G.; Caprio, S. Assessment of Skeletal Muscle Triglyceride Content by 1H Nuclear Magnetic Resonance Spectroscopy in Lean and Obese Adolescents Relationships to Insulin Sensitivity, Total Body Fat, and Central Adiposity. Diabetes 2002, 51, 1022–1027. [Google Scholar] [CrossRef] [Green Version]
- Fonvig, C.E.; Bille, D.S.; Chabanova, E.; Nielsen, T.R.H.; Thomsen, H.S.; Holm, J.-C. Muscle fat content and abdominal adipose tissue distribution investigated by magnetic resonance spectroscopy and imaging in obese children and youths. Pediatr. Rep. 2012, 4, e11. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, N.; Kurihara, T.; Sato, K.; Homma, T.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Intramyocellular and Extramyocellular Lipids Are Associated With Arterial Stiffness. Am. J. Hypertens. 2015, 28, 1473–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Htun, K.T.; Pan, J.; Pasanta, D.; Tungjai, M.; Udomtanakunchai, C.; Chancharunee, S.; Kaewjaeng, S.; Kim, H.J.; Kaewkhao, J.; Kothan, S. Identification of Metabolic Phenotypes in Young Adults with Obesity by 1H NMR Metabolomics of Blood Serum. Life 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- He, M.-Q.; Wang, J.-Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.-Y.; Ren, X.-X.; Shi, B.-Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, M.; Newby, F.D.; Lovejoy, J. Lactate production in adipose tissue: A regulated function with extra-adipose implications. FASEB J. 1992, 6, 2405–2412. [Google Scholar] [CrossRef]
- Rasouli, N. Adipose tissue hypoxia and insulin resistance. J. Investig. Med. 2016, 64, 830–832. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- He, Q.; Ren, P.; Kong, X.; Wu, Y.; Wu, G.; Li, P.; Hao, F.; Tang, H.; Blachier, F.; Yin, Y. Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J. Nutr. Biochem. 2012, 23, 133–139. [Google Scholar] [CrossRef]
- Toledo, F.G.S.; Sniderman, A.D.; Kelley, D.E. Influence of Hepatic Steatosis (Fatty Liver) on Severity and Composition of Dyslipidemia in Type 2 Diabetes. Diabetes Care 2006, 29, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Cali, A.M.; Zern, T.L.; Taksali, S.E.; de Oliveira, A.M.; Dufour, S.; Otvos, J.D.; Caprio, S. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: A perfect proatherogenic state. Diabetes Care 2007, 30, 3093–3098. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Hasegawa, S.; Imai, M.; Takahashi, N.; Fukui, T. High-fat diet-induced obesity stimulates ketone body utilization in osteoclasts of the mouse bone. Biochem. Biophys. Res. Commun. 2016, 473, 654–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, h.h.; Yoon, H.-J.; Shin, H.; Lee, S.-H.; Yoon, H.R. Characterization of Plasma Carnitine Level in Obese Adolescent Korean Women. Biomol. Ther. Biomol. Ther. 2009, 17, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.J.; Santani, A.B. Carnitine Palmitoyltransferase 1A Deficiency. In GeneReviews(®); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington, Seattle Copyright © 2022–2021, GeneReviews Is a Registered Trademark of the University of Washington, Seattle. All Rights Reserved; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Méndez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 53, 1080–1092. [Google Scholar] [CrossRef] [Green Version]
- Sunny, N.E.; Satapati, S.; Fu, X.; He, T.; Mehdibeigi, R.; Spring-Robinson, C.; Duarte, J.; Potthoff, M.J.; Browning, J.D.; Burgess, S.C. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1226–E1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solinas, G.; Borén, J.; Dulloo, A.G. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 2015, 4, 367–377. [Google Scholar] [CrossRef]





| Composition | Normal Chow Diet | ||
|---|---|---|---|
| g (Gram) | kcal (Kilocalorie) | %E (Energy Percentage) | |
| Carbohydrate | 495.30 | 1981.20 | 51.99 |
| Fat | 83.70 | 753.30 | 19.77 |
| Protein | 269.00 | 1076.00 | 28.24 |
| Vitamins | 65.40 | - | - |
| Fiber | 34.30 | - | - |
| Total | 947.70 | 3810.50 | 100 |
| kcal/g | 4.02 kcal/g |
| Composition | High Fat Diet | ||
|---|---|---|---|
| g (Gram) | kcal (Kilocalorie) | %E (Energy Percentage) | |
| Carbohydrate | 190.76 | 763.04 | 14.27 |
| Fat | 342.24 | 3080.16 | 57.60 |
| Protein | 353.60 | 1414.40 | 26.45 |
| Cholesterol | 10 | 90 | 1.68 |
| Vitamins | 85.19 | - | - |
| DL-Methionine | 3 | - | - |
| Fiber | 13.21 | - | - |
| Yeast powder | 1 | - | - |
| Sodium chloride | 1 | - | - |
| Total | 1000 | 5347.60 | 100 |
| kcal/g | 5.35 kcal/g |
| Parameters | ND | HFD | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Food intake (g) | 23.1 ± 0.97 | 22.2 ± 3.02 | 0.50 |
| Energy intake (kcal/day) | 92.96 ± 3.89 | 118.8 ± 16.14 | <0.05 * |
| Body weight (g) | 540 ± 42.43 | 733.33 ± 132.46 | <0.05 * |
| Blood TG (mg/dL) | 95.61 ± 22.08 | 104.05 ± 30.65 | 0.60 |
| Cholesterol (mg/dL) | 92 ± 20.14 | 125.41 ± 14.48 | <0.05 * |
| FG (mg/dL) | 113.09 ± 18.56 | 124.12 ± 23.51 | 0.39 |
| Parameters | ND | HFD | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Abd fat % | 31.31 ± 12.65 | 43.60 ± 7.31 | <0.05 * |
| Vis fat % | 29.50 ± 11.42 | 40.41 ± 7.80 | <0.05 * |
| SC fat % | 1.81 ± 1.78 | 3.19 ± 2.39 | 0.211 |
| LFC | 3.14 ± 1.39 | 60.40 ± 12.90 | <0.001 ** |
| Psoas muscular fat | 3.35 ± 3.01 | 6.25 ± 0.72 | 0.111 |
| No. | Assigned Metabolites | ppm, δ | ND | HFD | p-Value | Change % |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| 1 | Unsaturated lipid (CH=CH) | 5.3 | 0.01 ± 0.003 | 0.011 ± 0.004 | 0.591 | 9.64% |
| 2 | Alpha glucose | 5.23 | 0.019 ± 0.004 | 0.02 ± 0.002 | 0.113 | 10.05% |
| 3 | Beta glucose | 4.63 | 0.022 ± 0.004 | 0.024 ± 0.002 | 0.193 | 7.94% |
| 4 | Lactate | 4.1 | 0.047 ± 0.01 | 0.065 ± 0.011 | <0.05 | 34.96% |
| 5 | Total glucose | 3.35–3.92 | 0.4 ± 0.035 | 0.416 ± 0.033 | 0.261 | 3.83% |
| 6 | TMAO | 3.25 | 0.016 ± 0.003 | 0.018 ± 0.002 | 0.125 | 9.82% |
| 7 | Carnitine | 3.23 | 0.022 ± 0.003 | 0.022 ± 0.002 | 0.717 | 1.71% |
| 8 | Choline | 3.21 | 0.037 ± 0.006 | 0.033 ± 0.005 | 0.097 | −10.05% |
| 9 | Creatine | 3.04 | 0.006 ± 0.001 | 0.008 ± 0.004 | 0.299 | 18.67% |
| 10 | Glutamine | 2.45 | 0.013 ± 0.005 | 0.013 ± 0.003 | 0.7 | −4.30% |
| 11 | Glutamate | 2.34 | 0.012 ± 0.004 | 0.01 ± 0.003 | 0.285 | −13.18% |
| 12 | Acetoacetate | 2.22 | 0.006 ± 0.003 | 0.003 ± 0.001 | <0.05 | −50.94% |
| 13 | N-acetyl glycoprotein | 2.14 | 0.029 ± 0.004 | 0.036 ± 0.007 | <0.05 | 22.49% |
| 14 | Unsaturated lipid (=CH2) | 2.02 | 0.046 ± 0.010 | 0.048 ± 0.011 | 0.696 | 3.63% |
| 15 | Lysine | 1.91 | 0.011 ± 0.005 | 0.012 ± 0.003 | 0.686 | 5.80% |
| 16 | Alanine | 1.48 | 0.016 ± 0.004 | 0.016 ± 0.002 | 0.774 | −2.16% |
| 17 | Lactate | 1.3 | 0.089 ± 0.017 | 0.121 ± 0.049 | <0.05 | 36.13% |
| 18 | (-CH2)n VLDL/LDL | 1.27 | 0.048 ± 0.025 | 0.085 ± 0.03 | <0.05 | 76.02% |
| 19 | 3 hydroxybutyrate | 1.17 | 0.012 ± 0.004 | 0.011 ± 0.004 | 0.596 | −7.05% |
| 20 | Valine | 1.047 | 0.005 ± 0.001 | 0.004 ± 0.001 | <0.05 | −18.03% |
| 21 | Isoleucine | 1.02 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.264 | −11.41% |
| 22 | Valine | 0.996 | 0.01 ± 0.002 | 0.007 ± 0.001 | <0.05 | −25.79% |
| 23 | Leucine | 0.96 | 0.014 ± 0.002 | 0.014 ± 0.007 | 0.881 | 2.16% |
| 24 | (-CH3) VLDL/LDL | 0.87 | 0.041 ± 0.019 | 0.043 ± 0.01 | 0.765 | 4.65% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Htun, K.T.; Jaikumkao, K.; Pan, J.; Moe Moe, A.T.; Intachai, N.; Promsan, S.; Lungkaphin, A.; Tapanya, M.; Pasanta, D.; Tungjai, M.; et al. Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats. Diagnostics 2022, 12, 1621. https://doi.org/10.3390/diagnostics12071621
Htun KT, Jaikumkao K, Pan J, Moe Moe AT, Intachai N, Promsan S, Lungkaphin A, Tapanya M, Pasanta D, Tungjai M, et al. Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats. Diagnostics. 2022; 12(7):1621. https://doi.org/10.3390/diagnostics12071621
Chicago/Turabian StyleHtun, Khin Thandar, Krit Jaikumkao, Jie Pan, Aye Thidar Moe Moe, Nuttawadee Intachai, Sasivimon Promsan, Anusorn Lungkaphin, Monruedee Tapanya, Duanghathai Pasanta, Montree Tungjai, and et al. 2022. "Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats" Diagnostics 12, no. 7: 1621. https://doi.org/10.3390/diagnostics12071621
APA StyleHtun, K. T., Jaikumkao, K., Pan, J., Moe Moe, A. T., Intachai, N., Promsan, S., Lungkaphin, A., Tapanya, M., Pasanta, D., Tungjai, M., Kaewjaeng, S., Kim, H. J., Kaewkhao, J., Lai, C., & Kothan, S. (2022). Noninvasive NMR/MRS Metabolic Parameters to Evaluate Metabolic Syndrome in Rats. Diagnostics, 12(7), 1621. https://doi.org/10.3390/diagnostics12071621











