In Vivo Parieto-Occipital White Matter Metabolism Is Correlated with Visuospatial Deficits in Adult DM1 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Evaluation
2.3. Brain MR Protocol
2.4. Neuroradiological MRI Evaluation
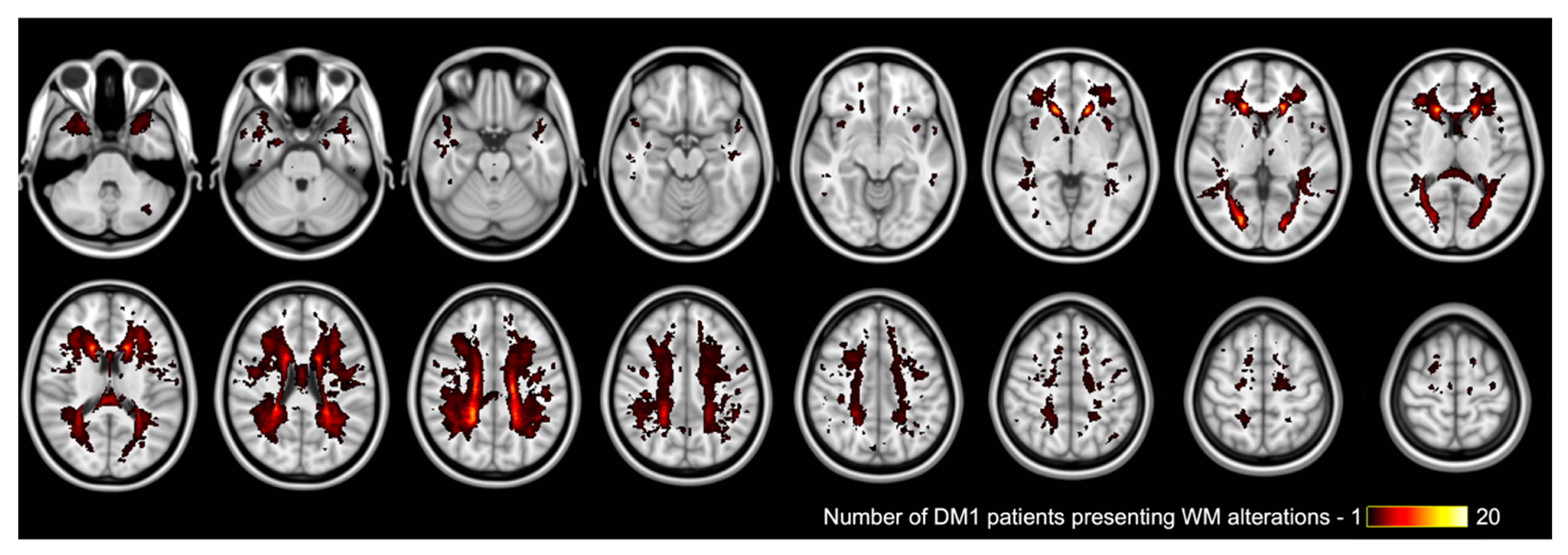
2.5. Whole Brain White Matter Changes Analysis
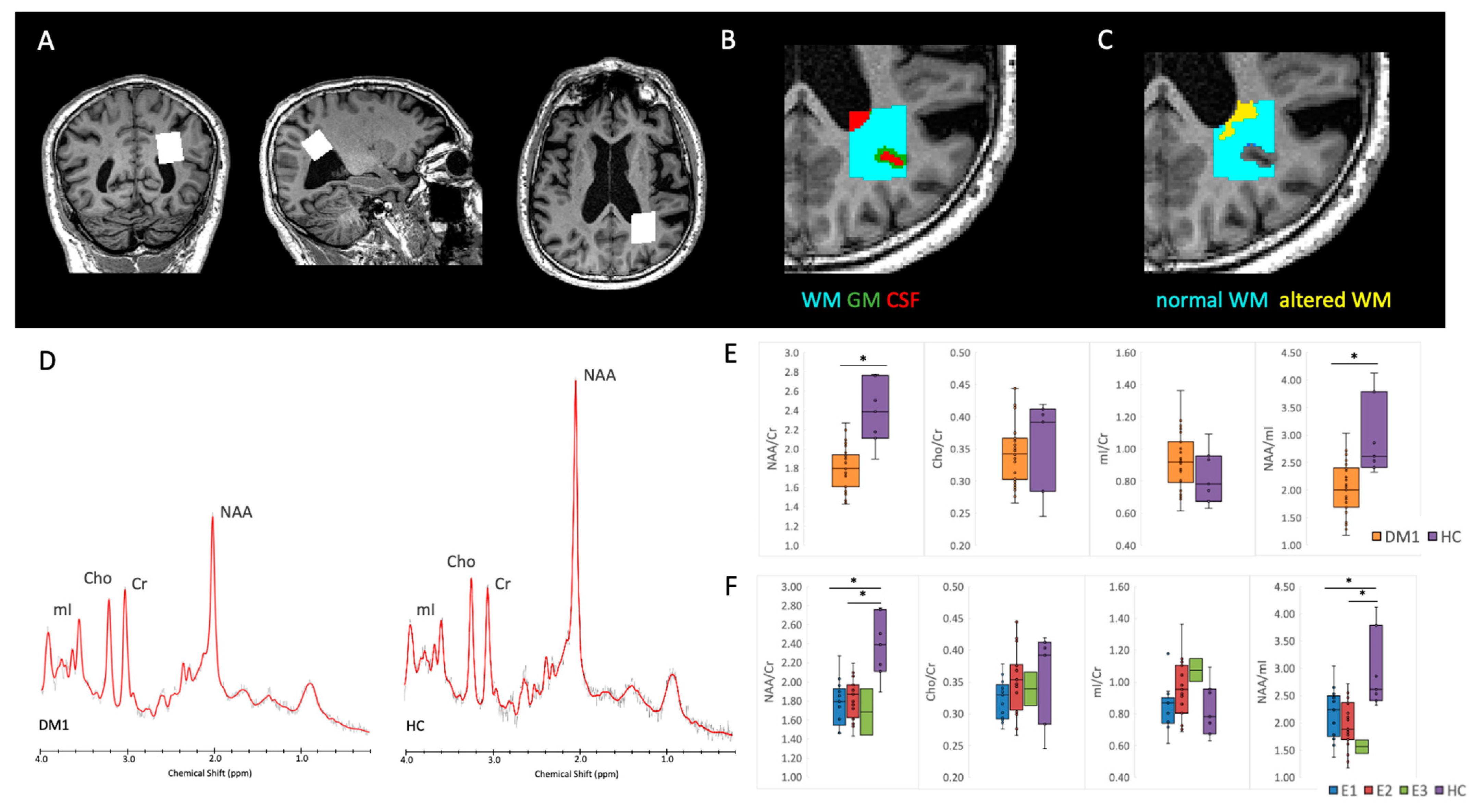
2.6. Proton MRS
2.6.1. Spectra Quality Assessment and Analysis Method
2.6.2. White and Gray Matter Segmentation in the MRS VOI
2.7. Statistical Analysis
3. Results
3.1. DM1 Sample Characteristics
3.2. Neuropsychological Results
3.3. Neuroradiological Results
3.4. Proton MRS
3.5. Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, P.S. Myotonic Dystrophy; 3WB Saunders: London, UK, 2001. [Google Scholar]
- Meola, G.; Cardani, R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 594–606. [Google Scholar] [CrossRef] [PubMed]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G.; French Myotonic Dystrophy Clinical Network. Unravelling the myotonic dystrophy type 1 clinical spectrum: A systematic registry-based study with implications for disease classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Udd, B.; Krahe, R. The myotonic dystrophies: Molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012, 11, 891–905. [Google Scholar] [CrossRef]
- Okkersen, K.; Monckton, D.G.; Le, N.; Tuladhar, A.M.; Raaphorst, J.; van Engelen, B. Brain imaging in myotonic dystrophy type 1: A systematic review. Neurology 2017, 89, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Seino, H.; Nagahata, F.; Tatsuo, S.; Maruyama, S.; Kon, S.; Takada, H.; Matsuzaka, M.; Sugimoto, K.; Kakeda, S. Cerebral ventriculomegaly in myotonic dystrophy type 1: Normal pressure hydrocephalus-like appearances on magnetic resonance imaging. BMC Neurosci. 2021, 22, 62. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Di Salle, F.; Santoro, L.; Bonavita, V.; Tedeschi, G. Brain MRI features of congenital- and adult-form myotonic dystrophy type 1: Case-control study. Neuromuscul. Disord. 2002, 12, 476–483. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tayama, M.; Yoshimoto, T.; Miyazaki, M.; Harada, M.; Miyoshi, H.; Tanouchi, M.; Kuroda, Y. Proton magnetic resonance spectroscopy of brain in congenital myotonic dystrophy. Pediatr. Neurol. 1995, 12, 335–340. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; Osborn, D.; Seltzer, W.; Leonido-Yee, M.; Poland, R.E. Proton spectroscopy in myotonicdystrophy: Correlationswith CTG repeats. Arch. Neurol. 1998, 55, 305–311. [Google Scholar] [CrossRef][Green Version]
- Akiguchi, I.; Nakano, S.; Shiino, A.; Kimura, R.; Inubushi, T.; Handa, J.; Nakamura, M.; Tanaka, M.; Oka, N.; Kimura, J. Brain proton magnetic resonance spectroscopy and brain atrophy in myotonic dystrophy. Arch. Neurol. 1999, 56, 325–330. [Google Scholar] [CrossRef][Green Version]
- Vielhaber, S.; Jakubiczka, S.; Gaul, C.; Schoenfeld, M.A.; Debska-Vielhaber, G.; Zierz, S.; Heinze, H.J.; Niessen, H.G.; Kaufmann, J. Brain 1H magnetic resonance spectroscopic differences in myotonic dystrophy type 2 and type 1. Muscle Nerve 2006, 34, 145–152. [Google Scholar] [CrossRef]
- Takado, Y.; Terajima, K.; Ohkubo, M.; Okamoto, K.; Shimohata, T.; Nishizawa, M.; Igarashi, H.; Nakada, T. Diffuse brain abnormalities in myotonic dystrophy type 1 detected by 3.0 T proton magnetic resonance spectroscopy. Eur. Neurol. 2015, 73, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, L.L.; Giannoccaro, M.P.; Manners, D.N.; Testa, C.; Zanigni, S.; Evangelisti, S.; Bianchini, C.; Oppi, F.; Poda, R.; Avoni, P.; et al. Mitochondrial dysfunction in myotonic dystrophy type 1. Neuromuscul. Disord. 2018, 28, 144–149. [Google Scholar] [CrossRef]
- Meola, G.; Sansone, V. Cerebral involvement in myotonic dystrophies. Muscle Nerve 2007, 36, 294–306. [Google Scholar] [CrossRef]
- Baldanzi, S.; Cecchi, P.; Fabbri, S.; Pesaresi, I.; Simoncini, C.; Angelini, C.; Bonuccelli, U.; Cosottini, M.; Siciliano, G. Relationship between neuropsychological impairment and grey and white matter changes in adult-onset myotonic dystrophy type 1. NeuroImage Clin. 2016, 12, 190–197. [Google Scholar] [CrossRef]
- Baldanzi, S.; Bevilacqua, F.; Lorio, R.; Volpi, L.; Simoncini, C.; Petrucci, A.; Cosottini, M.; Massimetti, G.; Tognoni, G.; Ricci, G.; et al. Disease awareness in myotonic dystrophy type 1: An observational cross-sectional study. Orphanet J. Rare Dis. 2016, 11, 34. [Google Scholar] [CrossRef]
- Cabada, T.; Iridoy, M.; Jericó, I.; Lecumberri, P.; Seijas, R.; Gargallo, A.; Gomez, M. Brain involvement in myotonic dystrophy type 1: A morphometric and diffusion tensor imaging study with neuropsychological correlation. Arch. Clin. Neuropsychol. 2017, 32, 401–412. [Google Scholar] [CrossRef]
- Cabada, T.; Díaz, J.; Iridoy, M.; López, P.; Jericó, I.; Lecumberri, P.; Remirez, B.; Seijas, R.; Gomez, M. Longitudinal study in patients with myotonic dystrophy type 1: Correlation of brain MRI abnormalities with cognitive performances. Neuroradiology 2021, 63, 1019–1029. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Atri, A. (Eds.) Dementia: Comprehensive Principles and Practice; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Trojano, L.; Conson, M. Visuospatial and visuoconstructive deficits. Handb. Clin. Neurol. 2008, 88, 373–391. [Google Scholar]
- Gliem, C.; Minnerop, M.; Roeske, S.; Gärtner, H.; Schoene-Bake, J.-C.; Adler, S.; Witt, J.-A.; Hoffstaedter, F.; Schneider-Gold, C.; Betz, R.C.; et al. Tracking the brain in myotonic dystrophies: A 5-year longitudinal follow-up study. PLoS ONE 2019, 14, e0213381. [Google Scholar] [CrossRef] [PubMed]
- Okkersen, K.; Buskes, M.; Groenewoud, J.; Kessels, R.P.; Knoop, H.; van Engelen, B.; Raaphorst, J. The cognitive profile of myotonic dystrophy type 1: A systematic review and meta-analysis. Cortex 2017, 95, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Labayru, G.; Jimenez-Marin, A.; Fernández, E.; Villanua, J.; Zulaica, M.; Cortes, J.M.; Díez, I.; Sepulcre, J.; López de Munain, A.; Sistiaga, A. Neurodegeneration trajectory in pediatric and adult/late DM1: A follow-up MRI study across a decade. Ann. Clin. Transl. Neurol. 2020, 7, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Labayru, G.; Camino, B.; Jimenez-Marin, A.; Garmendia, J.; Villanua, J.; Zulaica, M.; Cortes, J.M.; López de Munain, A.; Sistiaga, A. White matter integrity changes and neurocognitive functioning in adult-late onset DM1: A follow-up DTI study. Sci. Rep. 2022, 12, 3988. [Google Scholar] [CrossRef] [PubMed]
- Gallais, B.; Montreuil, M.; Gargiulo, M.; Eymard, B.; Gagnon, C.; Laberge, L. Prevalence and correlates of apathy in myotonic dystrophy type 1. BMC Neurol. 2015, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, M.; Tsuruya, N.; Kawamura, M. Theory of mind impairment in adult-onset myotonic dystrophy type 1. Neurosci. Res. 2012, 72, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Pinzana, E.; Bevilacqua, F.; Lorio, R.; Siciliano, G. Are white matters changes in dm1 brain related to anosognosia? J. Neurol. Sci. 2015, 357, e235–e254. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; Mchugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Caltagirone, C.; Gainotti, G.; Nocentini, U. Batteria per la valutazione del deterioramento mentale: II. Standardizzazione e affidabilitàdiagnosticanell’identificazione di pazientiaffetti da sindromedemenziale [The Mental Deterioration Battery: II. Standardization and diagnostic reliability in the identification of demented patients]. Arch. Psicol. Neurol. Psichiatr. 1995, 56, 471–488. [Google Scholar]
- Benton, A.L.; Varney, N.R.; Hamsher, K.D. Visuospatial judgment. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Street, R.F. A Gestalt completion test. Teach. Coll. Contrib. Educ. 1931, 481, vii + 65. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Spinnler, H.; Tognoni, G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987, 6, 47–50. [Google Scholar]
- Stewart, G.; McGeown, W.J.; Shanks, M.F.; Venneri, A. Anosognosia for memory impairment in Alzheimer’s disease. Acta Neuropsychiatr. 2010, 22, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Valera-Bermejo, J.M.; De Marco, M.; Mitolo, M.; McGeown, W.J.; Venneri, A. Neuroanatomical and cognitive correlates of domain-specific anosognosia in early Alzheimer’s disease. Cortex 2020, 129, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Valera-Bermejo, J.M.; De Marco, M.; Venneri, A. Altered Interplay Among Large-Scale Brain Functional Networks Modulates Multi-Domain Anosognosia in Early Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 781465. [Google Scholar] [CrossRef]
- Gramegna, L.L.; Evangelisti, S.; Di Vito, L.; La Morgia, C.; Maresca, A.; Caporali, L.; Amore, G.; Talozzi, L.; Bianchini, C.; Testa, C.; et al. Brain MRS correlates with mitochondrial dysfunction biomarkers in MELAS-associated mtDNA mutations. Ann. Clin. Transl. Neurol. 2021, 8, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Abraira, L.; Gramegna, L.L.; Quintana, M.; Santamarina, E.; Salas-Puig, J.; Sarria, S.; Rovira, A.; Toledo, M. Cerebrovascular disease burden in late-onset non-lesional focal epilepsy. Seizure 2019, 66, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ishikawa, M.; Yamamoto, K. Optimal diagnostic indices for idiopathic normal pressure hydrocephalus based on the 3D quantitative volumetric analysis for the cerebral ventricle and subarachnoid space. Am. J. Neuroradiol. 2015, 36, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ishikawa, M.; Yamamoto, K. Comparison of CSF distribution between idiopathic normal pressure hydrocephalus and Alzheimer disease. Am. J. Neuroradiol. 2016, 37, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, M.; Jenkinson, M.; De Stefano, N. Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum. Brain Mapp. 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Zanigni, S.; Evangelisti, S.; Giannoccaro, M.P.; Oppi, F.; Poda, R.; Giorgio, A.; Testa, C.; Manners, D.N.; Avoni, P.; Gramegna, L.L.; et al. Relationship of white and gray matter abnormalities to clinical and genetic features in myotonic dystrophy type 1. Neuroimage Clin. 2016, 11, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Near, J.; Harris, A.D.; Juchem, C.; Kreis, R.; Marjańska, M.; Öz, G.; Slotboom, J.; Wilson, M.; Gasparovic, C. Preprocessing, analysis and quantification in single-voxel magnetic resonance spectroscopy: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4257. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.T.; Stagg, C.J.; Jbabdi, S. FSL-MRS: An end-to-end spectroscopy analysis package. Magn. Reson. Med. 2021, 85, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- Tal, A.; Kirov, I.I.; Grossman, R.I.; Gonen, O. The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. 2012, 25, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ishikawa, M.; Mori, E.; Kuwana, N. Study of INPH on neurological improvement (SINPHONI). Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: A prospective cohort study. Cereb. Fluid Res. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Weijs, R.; Okkersen, K.; van Engelen, B.; Küsters, B.; Lammens, M.; Aronica, E.; Raaphorst, J.; van Cappellen van Walsum, A.M. Human brain pathology in myotonic dystrophy type 1: A systematic review. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2021, 41, 3–20. [Google Scholar] [CrossRef]
- Itoh, K.; Mitani, M.; Kawamoto, K.; Futamura, N.; Funakawa, I.; Jinnai, K.; Fushiki, S. Neuropathology does not Correlate with Regional Differences in the Extent of Expansion of CTG Repeats in the Brain with Myotonic Dystrophy Type 1. Acta Histochem. Cytochem. 2010, 43, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Abe K., H. Fujimura K. Toyooka S. Yorifuji Y. Nishikawa T. Hazama, T. Yanagihara. Involvement of the central nervous system in myotonic dystrophy. J. Neurol. Sci. 1994, 127, 179–185. [Google Scholar] [CrossRef]
- Mizukami, K.; Sasaki, M.; Baba, A.; Suzuki, T.; Shiraishi, H. An autopsy case of myotonic dystrophy with mental disorders and various neuropathologic features. Psychiatry Clin. Neurosci. 1999, 53, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Rosman, N.P. The cerebral defect and myopathy in Duchenne muscular dystrophy. A comparative clinicopathological study. Neurology 1970, 20, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alix, A.; Cabañas, F.; Morales, C.; Pellicer, A.; Echevarria, J.; Paisan, L.; Quero, J. Cerebral abnormalities in congenital myotonic dystrophy. Pediatric Neurol. 1991, 7, 28–32. [Google Scholar] [CrossRef]
- Watanabe, C.; Katayama, S.; Noda, K.; Kaneko, M.; Inai, K.; Nakamura, S. Heterotopic neurons in congenital myotonic dystrophy with mental retardation. Neuropathology 1997, 17, 243–247. [Google Scholar] [CrossRef]
- Jiang, H.; Mankodi, A.; Swanson, M.S.; Moxley, R.T.; Thornton, C.A. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum. Mol. Genet. 2004, 13, 3079–3088. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Huguet-Lachon, A.; Gourdon, G. Sense and Antisense DMPK RNA Foci Accumulate in DM1 Tissues during Development. PLoS ONE 2015, 10, e0137620. [Google Scholar] [CrossRef]
- Renard, D.; Menjot de Champfleur, N. MRI hydrographic 3D sequences: Myotonic dystrophy type 1 meets CADASIL. Acta Neurol. Belg. 2018, 118, 307–308. [Google Scholar] [CrossRef]
- De Carolis, A.; Cipollini, V.; Corigliano, V.; Comparelli, A.; Sepe-Monti, M.; Orzi, F.; Ferracuti, S.; Giubilei, F. Anosognosia in people with cognitive impairment: Association with cognitive deficits and behavioral disturbances. Dement. Geriatr. Cogn. Disord. Extra 2015, 5, 42–50. [Google Scholar] [CrossRef]
- Prigatano, G.P. Anosognosia, denial, and other disorders of phenomenological experience. Acta Neuropsychol. 2012, 10, 371–384. [Google Scholar] [CrossRef][Green Version]
- Prigatano, G.P. Anosognosia and patterns of impaired self-awareness observed in clinical practice. Cortex 2014, 61, 81–92. [Google Scholar] [CrossRef]
- Geldmacher, D.S. Visuospatial dysfunction in the neurodegenerative diseases. Front. Biosci.-Landmark 2003, 8, e428–e436. [Google Scholar] [CrossRef]
- Stiles, J.; Akshoomoff, N.; Haist, F. Neural Circuit Development and Function in the Brain: Comprehensive Developmental Neuroscience; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Salimi, S.; Irish, M.; Foxe, D.; Hodges, J.R.; Piguet, O.; Burrell, J.R. Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 10, 66–74. [Google Scholar] [CrossRef]
- Van Dorst, M.; Okkersen, K.; Kessels, R.; Meijer, F.; Monckton, D.G.; van Engelen, B.; Tuladhar, A.M.; Raaphorst, J.; OPTIMISTIC consortium. Structural white matter networks in myotonic dystrophy type 1. NeuroImage Clin. 2019, 21, 101615. [Google Scholar] [CrossRef] [PubMed]
- Sistiaga, A.; Urreta, I.; Jodar, M.; Cobo, A.M.; Emparanza, J.; Otaegui, D.; Poza, J.J.; Merino, J.J.; Imaz, H.; Martí-Massó, J.F.; et al. Cognitive/personality pattern and triplet expansion size in adult myotonic dystrophy type 1 (DM1): CTG repeats, cognition and personality in DM1. Psychol. Med. 2010, 40, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Labayru, G.; Diez, I.; Sepulcre, J.; Fernández, E.; Zulaica, M.; Cortés, J.M.; López de Munain, A.; Sistiaga, A. Regional brain atrophy in gray and white matter is associated with cognitive impairment in Myotonic Dystrophy type 1. NeuroImage Clin. 2019, 24, 102078. [Google Scholar] [CrossRef] [PubMed]
- Labayru, G.; Aliri, J.; Zulaica, M.; López de Munain, A.; Sistiaga, A. Age-related cognitive decline in myotonic dystrophy type 1: An 11-year longitudinal follow-up study. J. Neuropsychol. 2020, 14, 121–134. [Google Scholar] [CrossRef] [PubMed]
| DM1 | HC | p-Value | |||
| N | 34 | 10 | - | ||
| Sex F/M | 19/15 | 2/8 | 0.07 | ||
| Age, years | 46.4 ± 12.1 (22.3–71.0) | 41.5 ± 19.4 (19.8–75.4) | 0.48 | ||
| Disease duration, years | 18.7 ± 11.6 (0.6–47.9) | - | - | ||
| E1 | E2 | E3 | p-Value | ||
| (50–150 CTG Repeats) | (150–1000 CTG Repeats) | (More than 1000 CTG Repeats) | |||
| N | 13 | 19 | 2 | - | |
| Sex F/M | 6/7 | 12/7 | 1/1 | 0.14 | |
| Age, years | 50.2 ± 10.4 (28.1–71.0) | 43.8 ± 13.0 (22.9–67.8) | 46.3 ± 13.3 (36.8–55.7) | 0.49 | |
| Disease duration, years | 14.2 ± 9.5 (0.6–29.3) | 17.9 ± 9.2 (6.4–35.5) | 43.5 ± 9.2 (36.8–47.9) | 0.29 | |
| Congenital/Childhood | Juvenile/Adult | Late | p-Value | ||
| (from Birth to 10 Years of Age) | (from 11 to 40 Years of Age) | (More than 40 Years of Age) | |||
| N | 4 | 22 | 8 | - | |
| Sex F/M | 2/2 | 11/11 | 6/2 | 0.59 | |
| Age, years | 37.5 ± 13.0 (25.3–55.7) | 43.2 ± 10.0 (22.9–57.4) | 59.5 ± 6.8 (52.9–71.0) | 0.0004 * | |
| Disease duration, years | 34.0 ± 12.2 (21.3–49.7) | 19.2 ± 8.3 (7.2–35.5) | 6.5 ± 4.6 (0.6–13.1) | <0.0001 * | |
| Cognitive Domain | Tests | All DM1 | ||||||
| Corrected Score Mean ± sd (Range) | Cut Offs | Pathological Scores (%) | ||||||
| Cognitive screening | MMSE | 26.5 ± 2.7 (18.7–30.0) | ≤23.8 | 11.8 | ||||
| Non-verbal Intelligence | CPM-47 | 26.4 ± 5.7 (10.8–36.0) | ≤18.9 | 14.7 | ||||
| Visuoperception | BJLOT | 22.5 ± 7.2 (6.0–30.0) | ≤18 | 26.5 | ||||
| SCT | 6.4 ± 2.3 (1.3–10.2) | ≤2.3 | 5.9 | |||||
| Visuoconstructional abilities | ROCF-copy | 27.3 ± 7.8 (8.0–36.0) | ≤28.9 | 47.1 | ||||
| Anosognosia | Measurement of Anosognosia Instrument | −1.3 ± 2.1 (−5.0–3.0) | - | 8.8 | ||||
| Cognitive Domain | Tests | E1 | E2 | E3 | p-Value | |||
| Corrected Score Mean ± sd (Range) | Pathological Scores (%) | Corrected Score Mean ± sd (Range) | Pathological Scores (%) | Corrected Score Mean ± sd (Range) | Pathological Scores (%) | |||
| Cognitive screening | MMSE | 27.1 ± 1.6 (24.8–30.0) | 0 | 26.6 ± 2.8 (18.7–30.0) | 15.8 | 21.9 ± 4.2 (18.9–24.9) | 50.0 | 0.19 |
| Non-verbal Intelligence | CPM-47 | 28.8 ± 3.6 (23.7–36.0) | 0 | 25.9 ± 5.3 (14.8–33.3) | 15.8 | 14.1 ± 4.7 (10.8–17.4) | 100 | 0.14 |
| Visuoperception | BJLOT | 24.9 ± 6.1 (10.0–30.0) | 15.4 | 22.5 ± 6.3 (8.0–30.0) | 23.3 | 7.5 ± 2.1 (6.0–9.0) | 100 | 0.33 |
| SCT | 5.4 ± 2.2 (1.3–8.6) | 7.7 | 7.4 ± 1.9 (3.1–10.3) | 5.3 | 3.6 ± 0.8 (3.0–4.1) | 0 | 0.017 * | |
| Visuoconstructional abilities | ROCF-copy | 29.8 ± 7.4 (8.0–36.0) | 23.1 | 27.3 ± 6.2 (8.0–34.6) | 57.9 | 10.8 ± 2.5 (9.1–12.5) | 100 | 0.29 |
| Anosognosia | Measurement of Anosognosia Instrument | −1.0 ± 2.0 (−4.0–3.0) | 7.7 | −1.3 ± 2.3 (-5.0–2.0) | 10.5 | −2.0 | 0 | 0.73 |
| Patient Number | Sex | Age | Onset | Genetic Class | Fazekas Scale Score | Temporo-Polar Fazekas Scale Score | Hyperostosis | Hyperostosis Distribution | Normal Pressure Hydrocephalus-like Appearance | Incidental Brain Findings | 1H-MRS | |||||||
| Cerebral Ventriculomegaly | Maximum Axial Length of the Frontal Horns (mm) | Maximum Cranial Axial Length (mm) | z-Score EVANS | DESH | Callosal Angle (°) | Enlarged Perivascular Spaces | NAA/Cr | Altered | ||||||||||
| 1 | M | 28 | Juvenile/adult | E1 | 1 | 0 | 0 | 0 | 22.2 | 95.0 | 0.23 | 0 | 107 | 1 | 0 | 1.961 | 1 | |
| 2 | F | 39 | Juvenile/adult | E1 | 1 | 0 | 0 | 1 | 24.0 | 80.6 | 0.29 | 0 | 99 | 1 | Periventricular gray matter heterotopy | 1.804 | 1 | |
| 3 | M | 40 | Juvenile/adult | E1 | 1 | 0 | 0 | 1 | 28.5 | 92.7 | 0.30 | 0 | 111 | 1 | Retrovermian arachnoid cyst | 1.793 | 1 | |
| 4 | M | 46 | Juvenile/adult | E1 | 1 | 1 | 0 | 0 | 20.8 | 83.3 | 0.25 | 0 | 122 | 1 | 0 | 1.608 | 1 | |
| 5 | F | 50 | Juvenile/adult | E1 | 1 | 0 | 1 | Frontal | 0 | 20.0 | 80.7 | 0.25 | 0 | 118 | 1 | 0 | 1.855 | 1 |
| 6 | M | 52 | Juvenile/adult | E1 | 2 | 1 | 0 | 1 | 35.5 | 82.2 | 0.43 | 1 | 63.5 | 1 | 0 | 1.751 | 1 | |
| 7 | M | 52 | Juvenile/adult | E1 | 1 | 1 | 1 | Diffuse | 1 | 30.0 | 92.3 | 0.32 | 0 | 102 | 1 | 0 | 1.455 | 1 |
| 8 | F | 53 | Juvenile/adult | E1 | 2 | 1 | 0 | 0 | 21.5 | 84.3 | 0.25 | 0 | 121 | 1 | 0 | 1.465 | 1 | |
| 9 | F | 53 | Late | E1 | 1 | 1 | 0 | 1 | 24.1 | 92.7 | 0.26 | 0 | 126 | 1 | 0 | 2.033 | 1 | |
| 10 | F | 55 | Late | E1 | 0 | 0 | 0 | 0 | 21.0 | 90.0 | 0.23 | 0 | 130 | 1 | Left parietal microbleed | 1.899 | 1 | |
| 11 | F | 56 | Late | E1 | 1 | 0 | 0 | 1 | 26.4 | 82.1 | 0.32 | 0 | 124 | 1 | 0 | 1.739 | 1 | |
| 12 | M | 57 | Juvenile/adult | E1 | 1 | 1 | 0 | 1 | 24.8 | 89.0 | 0.28 | 0 | 116 | 1 | 0 | 1.479 | 1 | |
| 13 | M | 71 | Late | E1 | 2 | 0 | 0 | 1 | 30.3 | 84.9 | 0.36 | 1 | 81 | 1 | Left lacunar chronic infarct | 2.272 | 0 | |
| 14 | F | 23 | Juvenile/adult | E2 | 0 | 0 | 0 | 1 (asymmetrical) | 19.5 | 83.8 | 0.23 | 0 | 109 | 1 | 0 | 2.095 | 1 | |
| 15 | M | 25 | Congenital/childhood | E2 | 1 | 0 | 1 | Diffuse | 1 | 27.1 | 89.1 | 0.30 | 0 | 129 | 1 | 0 | 1.868 | 1 |
| 16 | F | 30 | Juvenile/adult | E2 | 1 | 1 | 1 | Frontal | 0 | 25.4 | 86.1 | 0.29 | 0 | 106 | 1 | 0 | 2.195 | 0 |
| 17 | F | 32 | Congenital/childhood | E2 | 1 | 0 | 1 | Diffuse | 1 | 21.9 | 84.0 | 0.26 | 0 | 130 | 1 | Ectopic pituitary adenoma | 1.624 | 1 |
| 18 | F | 34 | Juvenile/adult | E2 | 1 | 1 | 0 | 0 | 20.2 | 84.3 | 0.24 | 0 | 120 | 1 | 0 | 1.914 | 1 | |
| 19 | F | 34 | Juvenile/adult | E2 | 1 | 1 | 0 | 0 | 20.6 | 88.4 | 0.23 | 0 | 118 | 1 | 0 | 1.912 | 1 | |
| 20 | M | 35 | Juvenile/adult | E2 | 0 | 0 | 0 | 1 | 24.6 | 80.5 | 0.30 | 0 | 110 | 1 | 0 | 1.873 | 1 | |
| 21 | M | 36 | Juvenile/adult | E2 | 1 | 0 | 1 | Frontal | 0 | 20.0 | 81.6 | 0.24 | 0 | 122 | 1 | Tumor-like lesion | 1.532 | 1 |
| 22 | F | 43 | Juvenile/adult | E2 | 1 | 1 | 1 | Frontal | 0 | 20.3 | 81.6 | 0.25 | 0 | 100 | 1 | 0 | 1.931 | 1 |
| 23 | M | 43 | Juvenile/adult | E2 | 2 | 1 | 0 | 1 (asymmetrical) | 23.8 | 89.8 | 0.26 | 0 | 112 | 1 | 0 | 1.740 | 1 | |
| 24 | M | 45 | Juvenile/adult | E2 | 1 | 0 | 0 | 0 | 21.3 | 79.0 | 0.27 | 0 | 122 | 1 | 0 | 1.967 | 1 | |
| 25 | F | 47 | Juvenile/adult | E2 | 2 | 1 | 0 | 0 | 20.1 | 77.8 | 0.26 | 0 | 119 | 1 | 0 | 1.719 | 1 | |
| 26 | F | 51 | Juvenile/adult | E2 | 1 | 1 | 1 | Frontal | 1 | 24.2 | 80.5 | 0.30 | 0 | 135 | 1 | 0 | 1.429 | 1 |
| 27 | M | 53 | Late | E2 | 1 | 0 | 0 | 1 | 27.5 | 88.6 | 0.31 | 1 | 130 | 1 | 0 | 1.796 | 1 | |
| 28 | F | 55 | Juvenile/adult | E2 | 0 | 0 | 1 | Frontal | 0 | 23.3 | 80.0 | 0.29 | 0 | 116 | 1 | 0 | 1.606 | 1 |
| 29 | M | 57 | Juvenile/adult | E2 | 3 | 1 | 0 | 1 | 25.0 | 85.0 | 0.29 | 0 | 97 | 1 | 0 | 1.760 | 1 | |
| 30 | F | 59 | Late | E2 | 1 | 1 | 0 | 0 | 21.5 | 83.2 | 0.26 | 0 | 125 | 1 | 0 | 1.975 | 1 | |
| 31 | F | 61 | Late | E2 | 1 | 0 | 0 | 0 | 23.2 | 86.8 | 0.27 | 0 | 139 | 1 | 0 | 2.059 | 1 | |
| 32 | F | 68 | Late | 2 | 1 | 1 | Frontal | 1 | 30.2 | 80.0 | 0.37 | 1 | 80 | 1 | 0 | 1.559 | 1 | |
| 33 | F | 37 | Congenital/childhood | E3 | 1 | 1 | 1 | Diffuse | 1 | 25.7 | 83.5 | 0.30 | 1 | 93 | 1 | 0 | 1.928 | 1 |
| 34 | M | 56 | Congenital/childhood | E3 | 3 | 1 | 0 | 1 | 28.7 | 85.3 | 0.34 | 1 | 103 | 1 | Cerebellar malacia, talamic hyperintensity | 1.442 | 1 | |
| DM1 | HC | p-Value | |||||
| NAA/Cr | 1.80 ± 0.22 (1.43–2.27) | 2.32 ± 0.29 (1.89–2.78) | <0.0001 * | ||||
| Cho/Cr | 0.34 ± 0.05 (0.27–0.44) | 0.34 ± 0.07 (0.25–0.42) | 0.86 | ||||
| mI/Cr | 0.92 ± 0.17 (0.61–1.36) | 0.83 ± 0.14 (0.63–1.09) | 0.15 | ||||
| NAA/mI | 2.02 ± 0.46 (1.18–3.04) | 2.86 ± 0.61 (2.33–4.12) | <0.0001 * | ||||
| E1 | E2 | E3 | p-Value | Post-Hoc | |||
| NAA/Cr | 1.78 ± 0.24 (1.46–2.27) | 1.82 ± 0.21 (1.24–2.20) | 1.68 ± 0.34 (1.44–1.93) | < 0.0001 * | E1 vs. HC E2 vs. HC E1 vs. E2 | <0.0001 * <0.0001 * 0.88 | |
| Cho/Cr | 0.32 ± 0.03 (0.28–0.38) | 0.35 ± 0.05 (0.27–0.44) | 0.34 ± 0.04 (0.31-0.37) | 0.3663 | |||
| mI/Cr | 0.84 ± 0.14 (0.61–1.18) | 0.95 ± 0.18 (0.69–1.36) | 1.07 ± 0.10 (1.00-1.15) | 0.0737 | |||
| NAA/mI | 2.17 ± 0.48 (1.37–3.04) | 1.97 ± 0.44 (1.18–2.72) | 1.56 ± 0.17 (1.44–1.69) | 0.0003 * | E1 vs. HC E2 vs. HC E1 vs. E2 | 0.0044 * 0.0001 * 0.51 | |
| VOI | DM1 | HC | p-Value | ||
| % WM | 70 ± 9 (55–89) | 77 ± 10 (62–86) | 0.07 | ||
| % altered WM (§) | 5.4 ± 9.1 (0–45.6) 1.7 [7.5] | NA | - | ||
| % GM | 20 ± 6 (4–32) | 15 ± 9 (4–28) | 0.11 | ||
| % CSF | 9 ± 6 (2–28) | 8 ± 4 (2–15) | 0.50 | ||
| VOI | E1 | E2 | E3 | p-Value | |
| % WM | 68 ± 9 (55–83) | 72 ± 8 (56–89) | 66 ± 12 (58–75) | 0.23 | |
| % altered WM (§) | 2.6 ± 5.5 (0–19.2) 0 [1.0] | 7.4 ± 10.7 (0–45.6) 3.2 [9.6] | 3.2 | 0.027 * (#) | |
| % GM | 21 ± 5 (15–30) | 20 ± 7 (4–32) | 18 ± 1 (17–19) | 0.67 | |
| % CSF | 11 ± 8 (2–28) | 8 ± 4 (3–21) | 16 ± 11 (8–24) | 0.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelisti, S.; Gramegna, L.L.; De Pasqua, S.; Rochat, M.J.; Morandi, L.; Mitolo, M.; Bianchini, C.; Vornetti, G.; Testa, C.; Avoni, P.; et al. In Vivo Parieto-Occipital White Matter Metabolism Is Correlated with Visuospatial Deficits in Adult DM1 Patients. Diagnostics 2022, 12, 2305. https://doi.org/10.3390/diagnostics12102305
Evangelisti S, Gramegna LL, De Pasqua S, Rochat MJ, Morandi L, Mitolo M, Bianchini C, Vornetti G, Testa C, Avoni P, et al. In Vivo Parieto-Occipital White Matter Metabolism Is Correlated with Visuospatial Deficits in Adult DM1 Patients. Diagnostics. 2022; 12(10):2305. https://doi.org/10.3390/diagnostics12102305
Chicago/Turabian StyleEvangelisti, Stefania, Laura Ludovica Gramegna, Silvia De Pasqua, Magali Jane Rochat, Luca Morandi, Micaela Mitolo, Claudio Bianchini, Gianfranco Vornetti, Claudia Testa, Patrizia Avoni, and et al. 2022. "In Vivo Parieto-Occipital White Matter Metabolism Is Correlated with Visuospatial Deficits in Adult DM1 Patients" Diagnostics 12, no. 10: 2305. https://doi.org/10.3390/diagnostics12102305
APA StyleEvangelisti, S., Gramegna, L. L., De Pasqua, S., Rochat, M. J., Morandi, L., Mitolo, M., Bianchini, C., Vornetti, G., Testa, C., Avoni, P., Liguori, R., Lodi, R., & Tonon, C. (2022). In Vivo Parieto-Occipital White Matter Metabolism Is Correlated with Visuospatial Deficits in Adult DM1 Patients. Diagnostics, 12(10), 2305. https://doi.org/10.3390/diagnostics12102305





