Radiomics Profiling Identifies the Value of CT Features for the Preoperative Evaluation of Lymph Node Metastasis in Papillary Thyroid Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
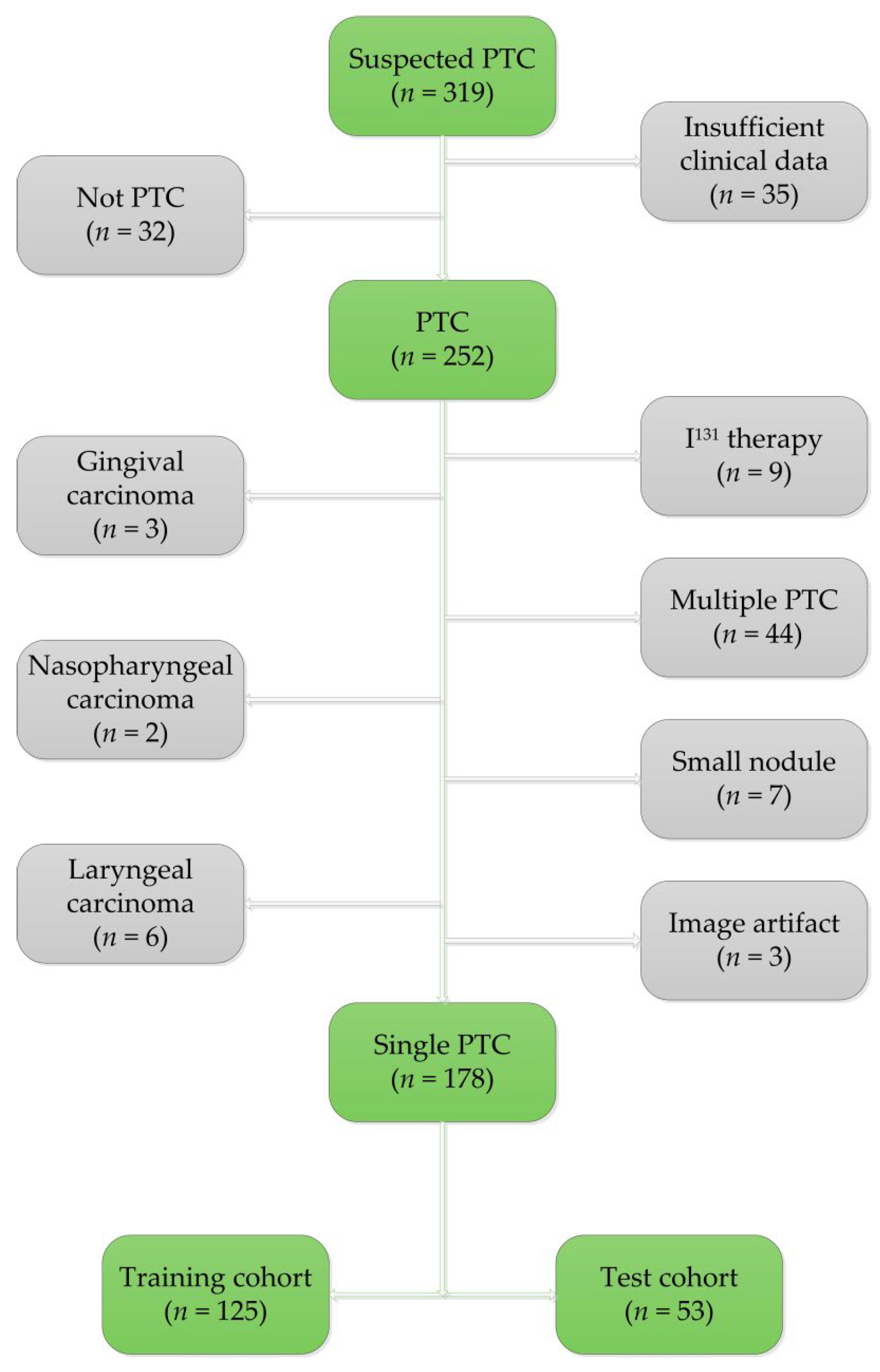
2.1. Patients
2.2. CT Acquisition
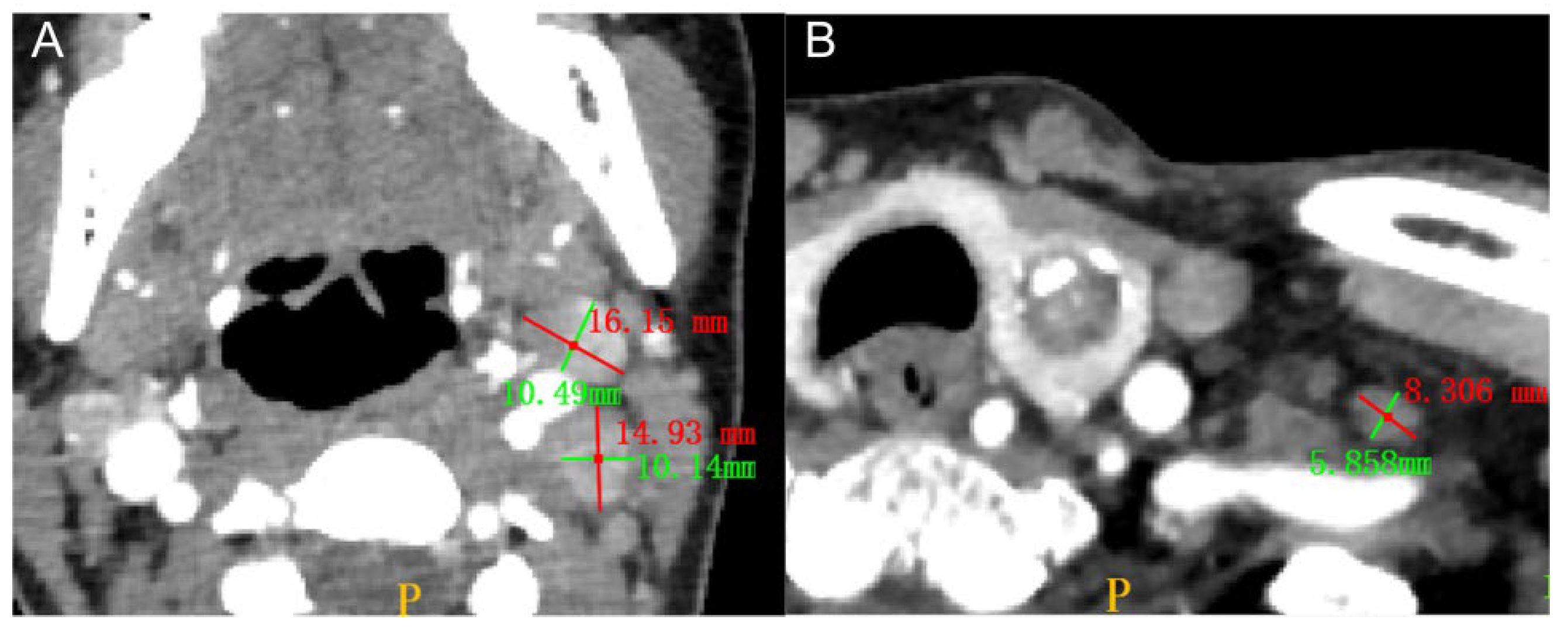
2.3. Radiologist Assessment of the Primary Tumors and LNMs
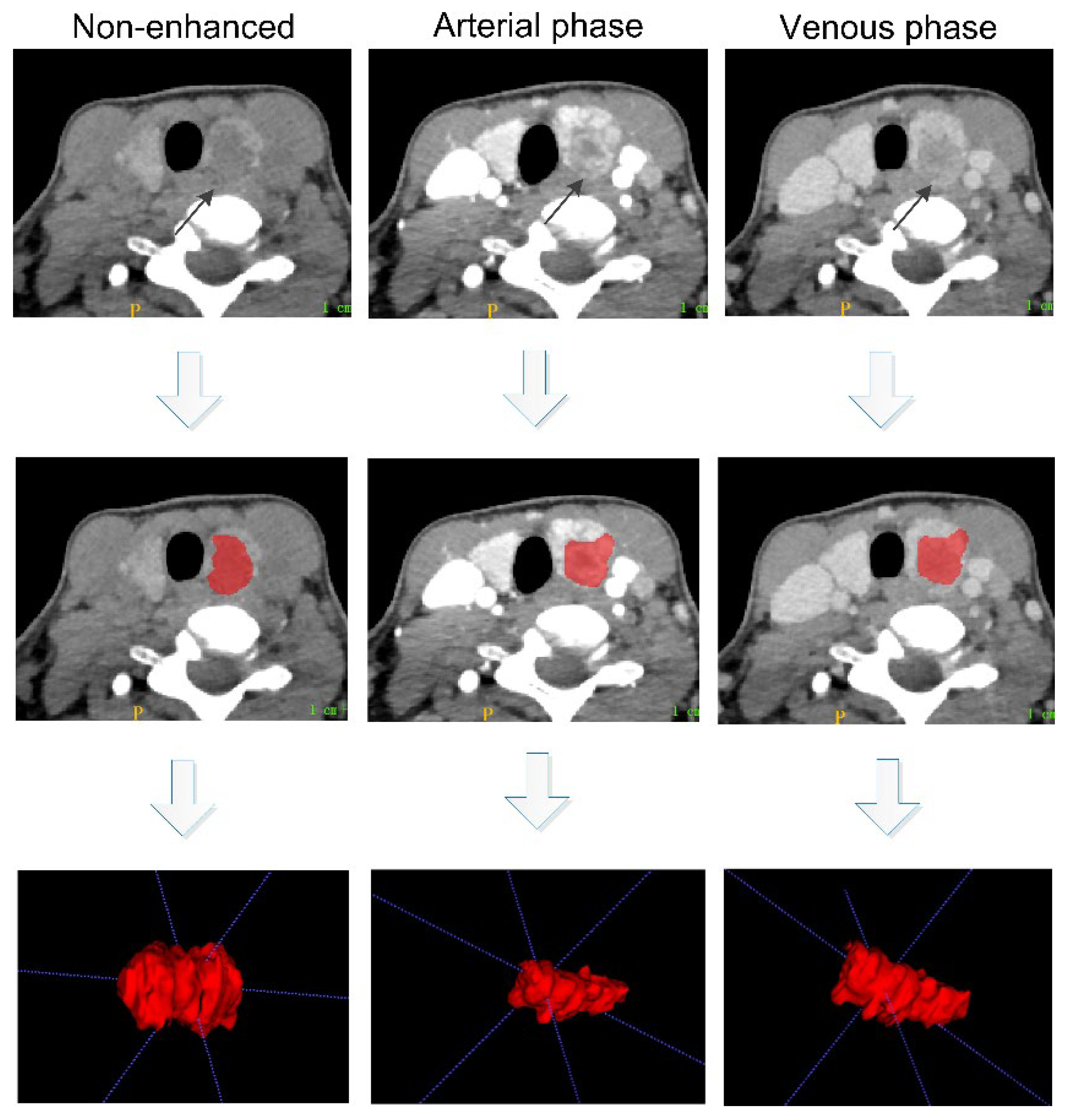
2.4. Tumor Segmentation and Feature Extraction
2.5. Feature Selection and Model Construction
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and the Clinical–Radiological Models
3.2. Feature Selection and Radiomic Models
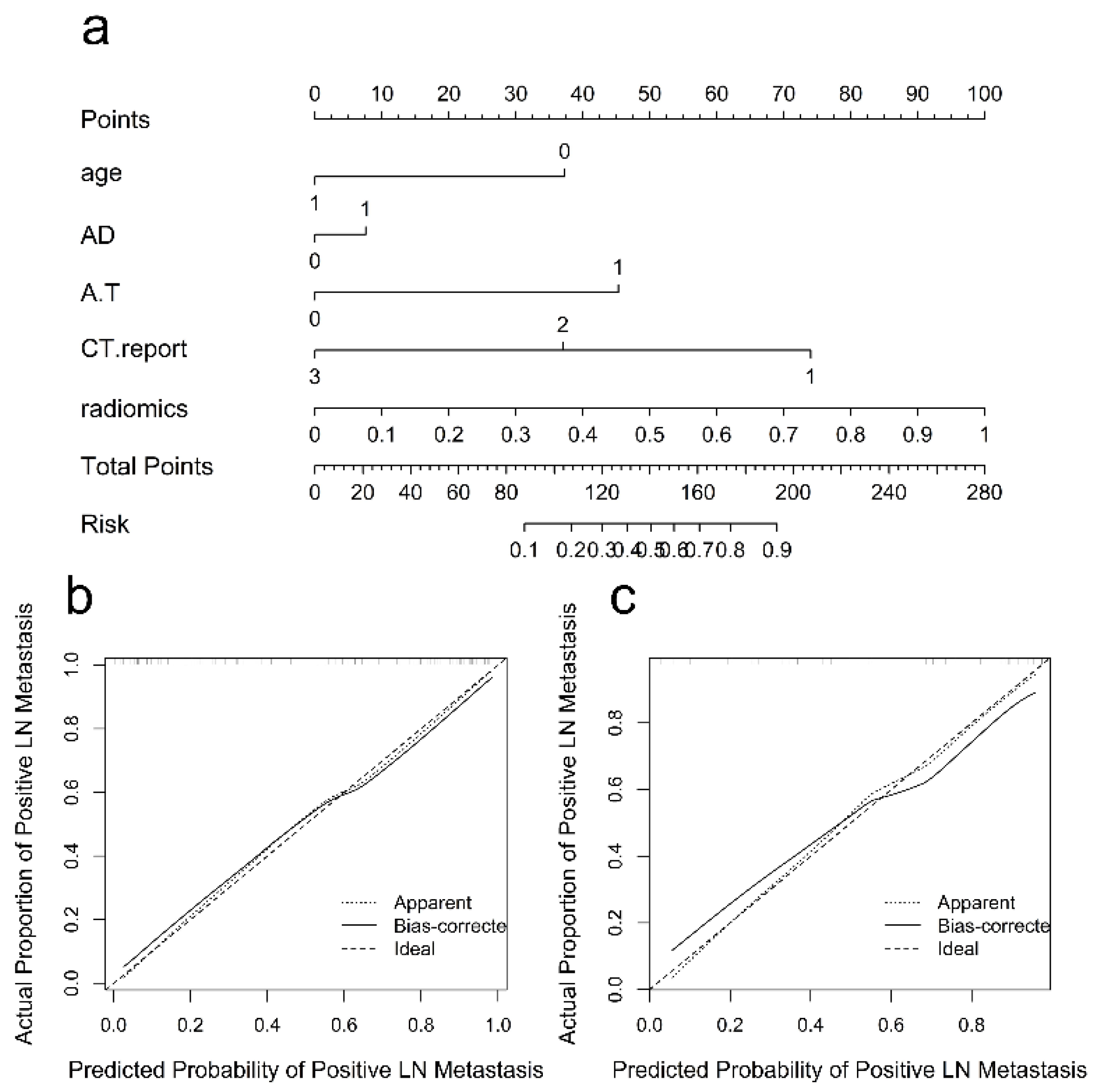
3.3. Radiomics Nomogram and Clinical Utility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgren, C.I.; Hall, P.; Dickman, P.W.; Zedenius, J. Clinically significant prognostic factors for differentiated thyroid carcinoma. Cancer 2006, 106, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.K.; Jung, K.Y.; Kang, S.M.; Kwon, S.Y.; Woo, J.S.; Cho, S.H.; Chung, E.J. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2009, 20, 147–152. [Google Scholar] [CrossRef]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Abbasian Ardakani, A.; Reiazi, R.; Mohammadi, A. A clinical decision support system using ultrasound textures and radiologic features to distinguish metastasis from tumor-free cervical lymph nodes in patients with papillary thyroid carcinoma. J. Ultrasound Med. 2018, 37, 2527–2535. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, J.; Qiu, Q.; Wang, Y.; Bai, T.; Yin, Y. Prediction of immunohistochemistry of suspected thyroid nodules by use of machine learning-based radiomics. AJR Am. J. Roentgenol. 2019, 213, 1348–1357. [Google Scholar] [CrossRef]
- Roh, J.L.; Park, J.Y.; Kim, J.M.; Song, C.J. Use of preoperative ultrasonography as guidance for neck dissection in patients with papillary thyroid carcinoma. J. Surg. Oncol. 2009, 99, 28–31. [Google Scholar] [CrossRef]
- Cho, S.J.; Suh, C.H.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Diagnostic performance of CT in detection of metastatic cervical lymph nodes in patients with thyroid cancer: A systematic review and meta-analysis. Eur. Radiol. 2019, 29, 4635–4647. [Google Scholar] [CrossRef] [PubMed]
- Renkonen, S.; Lindén, R.; Bäck, L.; Silén, R.; Mäenpää, H.; Tapiovaara, L.; Aro, K. Accuracy of preoperative MRI to assess lateral neck metastases in papillary thyroid carcinoma. Eur. Arch. Otorhinolaryngol. 2017, 274, 3977–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, N.D.; Weissman, J.L.; Talbot, J.M.; Andersen, P.E.; Wax, M.K.; Cohen, J.I. MRI detection of cervical metastasis from differentiated thyroid carcinoma. Laryngoscope 2001, 111, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Raghavan, P.; Mukherjee, S.; Jameson, M.J.; Patrie, J.; Xin, W.; Xian, J.; Wang, Z.; Levine, P.A.; Wintermark, M. Accuracy of MRI for the diagnosis of metastatic cervical lymphadenopathy in patients with thyroid cancer. Radiol. Med. 2015, 120, 959–966. [Google Scholar] [CrossRef]
- Lee, D.W.; Song, C.M.; Ji, Y.B.; Kim, J.Y.; Choi, Y.Y.; Lee, J.Y.; Tae, K. Efficacy of 18F-fluorodeoxyglucose PET/CT for detecting lymph node metastasis in papillary thyroid carcinoma. OTO Open 2018, 2, 2473974X18788545. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Kim, C.Y.; Son, S.H.; Kim, D.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Preoperative prediction of cervical lymph node metastasis using primary tumor SUVmax on 18F-FDG PET/CT in patients with papillary thyroid carcinoma. PLoS ONE 2015, 10, e0144152. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.S.; Baek, C.H.; Son, Y.I.; Choi, J.Y.; Kim, H.J.; Ko, Y.H.; Chung, J.H.; Baek, H.J. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: Comparison with ultrasound and contrast-enhanced CT. Clin. Endocrinol. 2006, 65, 402–407. [Google Scholar] [CrossRef]
- Suh, C.H.; Baek, J.H.; Choi, Y.J.; Lee, J.H. Performance of CT in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid cancer: A systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2017, 38, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ouyang, D.; Li, H.; Zhang, R.; Lv, Y.; Yang, A.; Xie, C. Papillary thyroid cancer: Dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology 2014, 275, 167–176. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.K.; Yu, H.W.; Choi, J.Y.; Park, S.Y.; Moon, J.H. Computed tomography for detecting cervical lymph node metastasis in patients who have papillary thyroid microcarcinoma with tumor characteristics appropriate for active surveillance. Thyroid 2019, 29, 1653–1659. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mărginean, L.; Ștefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the differentiation of gliomas from brain metastases: The radiomics analysis of the peritumoral zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef]
- Ștefan, P.A.; Lupean, R.A.; Mihu, C.M.; Lebovici, A.; Oancea, M.D.; Hîțu, L.; Duma, D.; Csutak, C. Ultrasonography in the diagnosis of adnexal lesions: The role of texture analysis. Diagnostics 2021, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, S.; Dong, D.; Wei, J.; Fang, C.; Zhou, X.; Sun, K.; Li, L.; Li, B.; Wang, M.; et al. The applications of radiomics in precision diagnosis and treatment of oncology: Opportunities and challenges. Theranostics 2019, 9, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Q.; Liang, C.H.; He, L.; Tian, J.; Liang, C.S.; Chen, X.; Ma, Z.L.; Liu, Z.Y. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Q.; Yang, W.; Lu, Z.; Deng, C.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. Eur. Radiol. 2018, 28, 582–591. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Yan, L.; Zheng, J.; Liang, C.; Liu, Z. Radiomics-based predictive risk score: A scoring system for preoperatively predicting risk of lymph node metastasis in patients with resectable non-small cell lung cancer. Chin. J. Cancer Res. 2019, 31, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Chen, C.; Zhang, X.; Zha, X.; Lv, W.; Xie, J.; Huang, W.; Sun, Z.; Hu, Y.; et al. Radiomics signature on computed tomography imaging: Association with lymph node metastasis in patients with gastric cancer. Front. Oncol. 2019, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.W.; Zhang, Y.D.; Zhang, H.; Zhu, F.P.; Wang, K.; Xia, Y.X.; Zhang, Y.D.; Jiang, W.J.; Li, X.C.; Wang, X.H. Biliary tract cancer at CT: A radiomics-based model to predict lymph node metastasis and survival outcomes. Radiology 2019, 290, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Li, J.; Huang, Y.; Zhou, J.; Liu, T.; Guo, Y.; Yu, J.; Zhou, S.; Wang, Y.; Chang, C. Ultrasound-based radiomic nomogram for predicting lateral cervical lymph node metastasis in papillary thyroid carcinoma. Acad. Radiol. 2021, 28, 1675–1684. [Google Scholar] [CrossRef]
- Zhou, S.C.; Liu, T.T.; Zhou, J.; Huang, Y.X.; Guo, Y.; Yu, J.H.; Wang, Y.Y.; Chang, C. An ultrasound radiomics nomogram for preoperative prediction of central neck lymph node metastasis in papillary thyroid carcinoma. Front. Oncol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, S.; Yu, J.; Guo, Y.; Wang, Y.; Zhou, J.; Chang, C. Prediction of lymph node metastasis in patients with papillary thyroid carcinoma: A radiomics method based on preoperative ultrasound images. Technol. Cancer Res. Treat. 2019, 18, 1533033819831713. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Zhong, L.; Dong, D.; Fang, M.; Dai, Q.; Leng, S.; Zhang, L.; Sun, W.; Tian, J.; Zheng, J.; et al. Radiomic analysis for preoperative prediction of cervical lymph node metastasis in patients with papillary thyroid carcinoma. Eur. J. Radiol. 2019, 118, 231–238. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, G.Y.; Hu, H.; Ge, Y.Q.; Si, Y.; Shen, M.P.; Xu, X.Q.; Wu, F.Y. Radiomics analysis of dual-energy CT-derived iodine maps for diagnosing metastatic cervical lymph nodes in patients with papillary thyroid cancer. Eur. Radiol. 2020, 30, 6251–6262. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Gao, E.L.; Zhang, W.; Yang, H.; Guo, G.L.; Zhang, X.H.; Wang, O.C. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: A retrospective study. World J. Surg. Oncol. 2012, 10, 67. [Google Scholar]
- Kim, E.; Park, J.S.; Son, K.R.; Kim, J.H.; Jeon, S.J.; Na, D.G. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: Comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid 2008, 18, 411–418. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.; Kwak, J.Y.; Kim, M.J.; Chang, H.S.; Kim, E.K. Preoperative staging of papillary thyroid carcinoma: Comparison of ultrasound imaging and CT. AJR Am. J. Roentgenol. 2009, 193, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Zhang, Y.D.; Hou, Y.; Yan, X.; Wang, Y.; Zhou, M.; Yao, Y.F.; Yang, G. FeAture explorer (FAE): A tool for developing and comparing radiomics models. PLoS ONE 2020, 15, e0237587. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Tufano, R.P.; Clayman, G.; Heller, K.S.; Inabnet, W.B.; Kebebew, E.; Shaha, A.; Steward, D.L.; Tuttle, R.M. American thyroid association surgical affairs committee writing task force. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: A critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid 2015, 25, 15–27. [Google Scholar] [PubMed]
- Sohn, S.Y.; Choi, J.H.; Kim, N.K.; Joung, J.Y.; Cho, Y.Y.; Park, S.M.; Kim, T.H.; Jin, S.M.; Bae, J.C.; Lee, S.Y.; et al. The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid 2014, 24, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pradhan, P.K.; Gambhir, S.; Sabaretnam, M.; Gupta, A.; Babu, S. Preoperative contrast-enhanced computerized tomography should not delay radioiodine ablation in differentiated thyroid carcinoma patients. J. Surg. Res. 2015, 193, 731–737. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean society of thyroid radiology consensus statement and recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef]
- Alabousi, M.; Alabousi, A.; Adham, S.; Pozdnyakov, A.; Ramadan, S.; Chaudhari, H.; Young, J.E.M.; Gupta, M.; Harish, S. Diagnostic test accuracy of ultrasonography vs computed tomography for papillary thyroid cancer cervical lymph node metastasis: A systematic review and meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 107–118. [Google Scholar] [CrossRef]
- Hîțu, L.; Șîțu, P.A.; Piciu, D. Total tumor diameter and unilateral multifocality as independent predictor factors for metastatic papillary thyroid microcarcinoma. J. Clin. Med. 2021, 10, 3707. [Google Scholar] [CrossRef]
- Fournier, L.; Costaridou, L.; Bidaut, L.; Michoux, N.; Lecouvet, F.E.; de Geus-Oei, L.F.; Boellaard, R.; Oprea-Lager, D.E.; Obuchowski, N.A.; Caroli, A.; et al. Incorporating radiomics into clinical trials: Expert consensus endorsed by the European Society of Radiology on considerations for data-driven compared to biologically driven quantitative biomarkers. Eur. Radiol. 2021, 31, 6001–6012. [Google Scholar] [CrossRef]
- Larue, R.T.H.M.; van Timmeren, J.E.; de Jong, E.E.C.; Feliciani, G.; Leijenaar, R.T.H.; Schreurs, W.M.J.; Sosef, M.N.; Raat, F.H.P.J.; van der Zande, F.H.R.; Das, M.; et al. Influence of gray level discretization on radiomic feature stability for different CT scanners, tube currents and slice thicknesses: A comprehensive phantom study. Acta Oncol. 2017, 56, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Shafiq-Ul-Hassan, M.; Latifi, K.; Zhang, G.; Ullah, G.; Gillies, R.; Moros, E. Voxel size and gray level normalization of CT radiomic features in lung cancer. Sci. Rep. 2018, 8, 10545. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | LNM (+) (N = 100) | LNM (−) (N = 78) | p-Value |
|---|---|---|---|
| Age, mean ± SD, years | 42.55 ± 14.28 | 49.45 ± 9.95 | |
| <45 NO. (%) | 56 (56.00) | 24 (30.77) | 0.001 |
| ≥45 NO. (%) | 44 (44.00) | 54 (69.23) | |
| Sex NO. (%) | |||
| Male | 28 (28.00) | 15 (19.23) | 0.175 |
| Female | 72 (72.00) | 63 (80.77) | |
| BMI NO. (%) | |||
| Normal | 48 (48.00) | 35 (44.87) | 0.678 |
| Abnormal | 52 (52.00) | 43 (55.13) | |
| TG NO. (%) | |||
| Normal | 66 (66.00) | 56 (71.79) | 0.409 |
| Abnormal | 34 (34.00) | 22 (28.21) | |
| TGAb NO. (%) | |||
| Normal | 30 (30.00) | 29 (37.18) | 0.313 |
| Abnormal | 70 (70.00) | 49 (62.82) | |
| TPOAb NO. (%) | |||
| Normal | 51 (51.00) | 37 (47.44) | 0.637 |
| Abnormal | 49 (49.00) | 41 (52.56) | |
| FT3 NO. (%) | |||
| Normal | 71 (71.00) | 64 (82.05) | 0.087 |
| Abnormal | 29 (29.00) | 14 (17.95) | |
| FT4 NO. (%) | |||
| Normal | 45 (45.00) | 33 (42.31) | 0.719 |
| Abnormal | 55 (55.00) | 45 (57.69) | |
| TSH NO. (%) | |||
| Normal | 58 (58.00) | 50 (64.10) | 0.408 |
| Abnormal | 42 (42.00) | 28 (35.90) | |
| AD NO. (%) | |||
| <6 mm | 4 (4.00) | 22 (28.21) | <0.001 |
| ≥6 mm | 96 (96.00) | 56 (71.79) | |
| TD NO. (%) | |||
| <6 mm | 10 (10.00) | 27 (34.62) | <0.001 |
| ≥6 mm | 90 (90.00) | 51 (65.38) | |
| A/T NO. (%) | |||
| <1 | 15 (15.00) | 28 (35.90) | 0.001 |
| ≥1 | 85 (85.00) | 50 (64.10) | |
| HT NO. (%) | |||
| Not involved | 94 (94.00) | 75 (96.15) | 0.515 |
| Involved | 6 (6.00) | 3 (3.85) | |
| NG NO. (%) | |||
| Not involved | 70 (70.00) | 62 (79.49) | 0.151 |
| Involved | 30 (30.00) | 16 (20.51) | |
| Capsule NO. (%) | |||
| Not involved | 36 (36.00) | 41 (52.56) | 0.027 |
| Involved | 64 (64.00) | 37 (47.44) | |
| Calcification NO. (%) | |||
| Negative | 48 (48.00) | 45 (57.69) | 0.199 |
| Positive | 52 (52.00) | 33 (42.31) | |
| Location NO. (%) | |||
| Left lobe | 52 (52.00) | 32 (41.03) | |
| Isthmus | 0 (0.00) | 6 (7.69) | 0.012 |
| Right lobe | 48 (48.00) | 40 (51.28) | |
| CT reported-lymph node status 1 NO. (%) | |||
| LNM-positive | 61 (61.00) | 15 (19.23) | |
| LNM-suspicious | 23 (23.00) | 17 (21.79) | <0.001 |
| LNM-negative | 16 (16.00) | 46 (58.97) | |
| CT reported-lymph node status 2 NO. (%) | |||
| LNM-positive | 59 (59.00) | 35 (44.87) | |
| LNM-suspicious | 18 (18.00) | 12 (15.38) | 0.053 |
| LNM-negative | 23 (23.00) | 31 (39.74) | |
| CT reported-lymph node status 3 NO. (%) | |||
| LNM-positive | 58 (58.00) | 27 (34.62) | |
| LNM-suspicious | 13 (13.00) | 4 (5.13) | <0.001 |
| LNM-negative | 29 (29.00) | 47 (60.26) |
| Characteristics | Training Cohort (N = 125) | p-Value | Test Cohort (N = 53) | p-Value | ||
|---|---|---|---|---|---|---|
| LNM (+) (N = 70) | LNM (−) (N = 55) | LNM (+) (N = 30) | LNM (−) (N = 23) | |||
| Age, mean ± SD, years | 42.64 ± 13.927 | 49.15 ± 9.519 | 42.33 ± 15.320 | 50.17 ± 11.092 | ||
| <45 NO. (%) | 42 (60.00) | 17 (30.91) | 0.001 | 14 (46.67) | 7 (30.43) | 0.231 |
| ≥45 NO. (%) | 28 (40.00) | 38 (69.09) | 16 (53.33) | 16 (69.57) | ||
| Sex NO. (%) | ||||||
| Male | 21 (30.00) | 10 (18.18) | 0.129 | 7 (23.33) | 5 (21.74) | 0.891 |
| Female | 49 (70.00) | 45 (81.82) | 23 (76.67) | 18 (78.26) | ||
| BMI NO. (%) | ||||||
| Normal | 34 (48.57) | 26 (47.27) | 0.885 | 14 (46.67) | 9 (39.13) | 0.583 |
| Abnormal | 36 (51.43) | 29 (52.73) | 16 (53.33) | 14 (60.87) | ||
| TG NO. (%) | ||||||
| Normal | 46 (65.71) | 41 (74.55) | 0.287 | 20 (45.83) | 15 (65.22) | 0.912 |
| Abnormal | 24 (34.29) | 14 (25.45) | 10 (54.17) | 8 (34.78) | ||
| TGAb NO. (%) | ||||||
| Normal | 22 (31.43) | 20 (36.36) | 0.562 | 8 (26.67) | 9 (39.13) | 0.335 |
| Abnormal | 48 (68.57) | 35 (63.64) | 22 (73.33) | 14 (60.87) | ||
| TPOAb NO. (%) | ||||||
| Normal | 36 (40.00) | 26 (41.67) | 0.645 | 15 (50.00) | 11 (47.83) | 0.875 |
| Abnormal | 34 (60.00) | 29 (58.33) | 15 (50.00) | 12 (52.17) | ||
| FT3 NO. (%) | ||||||
| Normal | 48 (68.57) | 45 (81.82) | 0.092 | 23 (76.67) | 19 (82.61) | 0.597 |
| Abnormal | 22 (31.43) | 10 (18.18) | 7 (23.33) | 4 (17.39) | ||
| FT4 NO. (%) | ||||||
| Normal | 30 (42.86) | 23 (41.82) | 0.907 | 15 (50.00) | 10 (43.48) | 0.637 |
| Abnormal | 40 (57.14) | 32 (58.18) | 15 (50.00) | 13 (56.52) | ||
| TSH NO. (%) | ||||||
| Normal | 42 (60.00) | 36 (65.45) | 0.532 | 16 (53.33) | 14 (60.87) | 0.583 |
| Abnormal | 28 (40.00) | 19 (34.55) | 14 (46.67) | 9 (39.13) | ||
| AD NO. (%) | ||||||
| <6 mm | 3 (4.29) | 12 (21.82) | 0.003 | 1 (3.33) | 10 (43.48) | <0.001 |
| ≥6 mm | 67 (95.71) | 43 (78.18) | 29 (96.67) | 13 (56.52) | ||
| TD NO. (%) | ||||||
| <6 mm | 9 (12.86) | 18 (32.73) | 0.007 | 1 (3.33) | 9 (39.13) | 0.001 |
| ≥6 mm | 61 (87.14) | 37 (67.27) | 29 (76.67) | 14 (60.87) | ||
| A/T NO. (%) | ||||||
| <1 | 8 (11.43) | 18 (32.73) | 0.004 | 7 (23.33) | 10 (43.48) | 0.119 |
| ≥1 | 62 (88.57) | 37 (67.27) | 23 (76.67) | 13 (56.52) | ||
| HT NO. (%) | ||||||
| Not involved | 65 (92.86) | 53 (96.36) | 0.397 | 29 (96.67) | 22 (95.65) | 0.848 |
| Involved | 5 (7.14) | 2 (3.64) | 1 (3.33) | 1 (4.35) | ||
| NG NO. (%) | ||||||
| Not involved | 49 (70.00) | 43 (78.18) | 0.303 | 21 (70.00) | 19 (82.61) | 0.290 |
| Involved | 21 (30.00) | 12 (21.82) | 9 (30.00) | 4 (17.39) | ||
| Capsule NO. (%) | ||||||
| Not involved | 25 (35.71) | 26 (47.27) | 0.192 | 11 (36.67) | 15 (65.22) | 0.039 |
| Involved | 45 (64.29) | 29 (52.73) | 19 (63.33) | 8 (34.78) | ||
| Calcification NO. (%) | ||||||
| Negative | 35 (50.00) | 34 (61.82) | 0.187 | 13 (43.33) | 11 (47.83) | 0.745 |
| Positive | 35 (50.00) | 21 (38.18) | 17 (56.67) | 12 (52.17) | ||
| Location NO. (%) | ||||||
| Left lobe | 34 (48.57) | 22 (40.00) | 18 (60.00) | 10 (43.48) | ||
| Isthmus | 0 (0.00) | 5 (9.09) | 0.032 | 0 (0.00) | 1 (4.35) | 0.301 |
| Right lobe | 36 (51.43) | 28 (50.91) | 12 (40.00) | 12 (52.17) | ||
| CT reported-lymph node status 1 NO. (%) | ||||||
| LNM-positive | 43 (61.43) | 9 (16.36) | 18 (60.00) | 6 (26.09) | ||
| LNM-suspicious | 17 (24.29) | 12 (21.82) | <0.001 | 6 (20.00) | 5 (21.74) | 0.026 |
| LNM-negative | 10 (14.29) | 34 (61.82) | 6 (20.00) | 12 (52.17) | ||
| CT reported-lymph node status 2 NO. (%) | ||||||
| LNM-positive | 44 (62.86) | 24 (43.64) | 15 (50.00) | 11 (47.83) | ||
| LNM-suspicious | 10 (14.29) | 8 (14.55) | 0.06 | 8 (26.67) | 4 (17.39) | 0.574 |
| LNM-negative | 16 (22.86) | 23 (41.82) | 7 (23.33) | 8 (34.78) | ||
| CT reported-lymph node status 3 NO. (%) | ||||||
| LNM-positive | 39 (55.71) | 17 (30.91) | 19 (63.33) | 10 (43.48) | ||
| LNM-suspicious | 9 (12.86) | 3 (5.45) | 0.002 | 4 (13.33) | 1 (4.35) | 0.079 |
| LNM-negative | 22 (40.00) | 35 (63.64) | 7 (23.33) | 12 (52.17) | ||
| Characteristics | Training Cohort (N = 125) | Test Cohort (N = 53) | p-Value |
|---|---|---|---|
| Age, mean ± SD, years | 45.50 ± 12.566 | 45.74 ± 14.084 | |
| <45 NO. (%) | 59 (47.20) | 21 (39.62) | 0.353 |
| ≥45 NO. (%) | 66 (52.80) | 32 (60.38) | |
| Sex NO. (%) | |||
| Male | 31 (24.80) | 12 (22.64) | 0.758 |
| Female | 94 (75.20) | 41 (77.36) | |
| BMI NO. (%) | |||
| Normal | 60 (48.00) | 23 (43.40) | 0.573 |
| Abnormal | 65 (52.00) | 30 (56.60) | |
| TG NO. (%) | |||
| Normal | 87 (69.60) | 35 (66.04) | 0.640 |
| Abnormal | 38 (30.40) | 18 (33.94) | |
| TGAb NO. (%) | |||
| Normal | 42 (33.60) | 17 (32.08) | 0.843 |
| Abnormal | 83 (66.40) | 36 (67.92) | |
| TPOAb NO. (%) | |||
| Normal | 62 (49.60) | 26 (49.06) | 0.947 |
| Abnormal | 63 (50.40) | 27 (50.94) | |
| FT3 NO. (%) | |||
| Normal | 93 (74.40) | 42 (79.25) | 0.490 |
| Abnormal | 32 (25.60) | 11 (20.75) | |
| FT4 NO. (%) | |||
| Normal | 53 (42.40) | 25 (47.17) | 0.558 |
| Abnormal | 72 (57.60) | 28 (52.83) | |
| TSH NO. (%) | |||
| Normal | 78 (62.40) | 30 (56.60) | 0.469 |
| Abnormal | 47 (37.60) | 23 (43.40) | |
| AD NO. (%) | |||
| <6 mm | 15 (12.00) | 11 (20.75) | 0.130 |
| ≥6 mm | 110 (88.00) | 42 (79.25) | |
| TD NO. (%) | |||
| <6 mm | 27 (21.60) | 10 (18.87) | 0.681 |
| ≥6 mm | 98 (78.40) | 43 (81.13) | |
| A/T NO. (%) | |||
| <1 | 26 (20.80) | 17 (32.08) | 0.108 |
| ≥1 | 99 (79.20) | 36 (67.92) | |
| HT NO. (%) | |||
| Not involved | 118 (94.40) | 51 (96.23) | 0.611 |
| Involved | 7 (5.60) | 2 (3.77) | |
| NG NO. (%) | |||
| Not involved | 92 (73.60) | 40 (75.47) | 0.794 |
| Involved | 33 (26.40) | 13 (24.53) | |
| Capsule NO. (%) | |||
| Not involved | 51 (40.80) | 26 (49.06) | 0.309 |
| Involved | 74 (59.20) | 27 (50.94) | |
| Calcification NO. (%) | |||
| Negative | 69 (55.20) | 24 (45.28) | 0.226 |
| Positive | 56 (44.80) | 29 (54.72) | |
| Location NO. (%) | |||
| Left lobe | 56 (44.80) | 28 (52.83) | |
| Isthmus | 5 (4.00) | 1 (1.89) | 0.531 |
| Right lobe | 64 (51.20) | 24 (45.28) | |
| CT reported-lymph node status1 NO. (%) | |||
| LNM-positive | 52 (41.60) | 24 (45.28) | |
| LNM-suspicious | 29 (23.20) | 11 (20.75) | 0.890 |
| LNM-negative | 44 (35.20) | 18 (33.96) | |
| CT reported-lymph node status 2 NO. (%) | |||
| LNM-positive | 68 (54.40) | 26 (49.06) | |
| LNM-suspicious | 18 (14.40) | 12 (22.64) | 0.406 |
| LNM-negative | 39 (31.20) | 15 (28.30) | |
| CT reported-lymph node status 3 NO. (%) | |||
| LNM-positive | 56 (44.80) | 29 (54.72) | |
| LNM-suspicious | 12 (9.60) | 5 (9.43) | 0.450 |
| LNM-negative | 57 (45.60) | 19 (35.85) | |
| LNM status NO. (%) | |||
| Negative | 70 (56.00) | 30 (56.60) | 0.941 |
| Positive | 55 (44.00) | 23 (43.40) |
| Model Categories | Training | Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | AUC | p-Value | Sensitivity | Specificity | Accuracy | AUC | p-Value | |
| Clinical–radiological model 1 | 74.29 | 76.36 | 75.20 | 0.781 | 0.003 | 80.00 | 65.22 | 73.58 | 0.758 | 0.017 |
| Clinical–radiological model 2 | 85.71 | 63.64 | 76.00 | 0.796 | 0.036 | 73.33 | 69.57 | 71.70 | 0.729 | 0.024 |
| Clinical–radiological model 3 | 74.29 | 78.18 | 76.00 | 0.800 | 0.045 | 73.33 | 65.22 | 69.81 | 0.743 | 0.052 |
| Noncontrast model | 84.29 | 58.18 | 72.80 | 0.786 | 0.068 | 80.00 | 65.22 | 73.58 | 0.781 | 0.141 |
| Arterial contrast model | 71.43 | 78.18 | 74.40 | 0.808 | 0.212 | 66.67 | 86.96 | 75.47 | 0.791 | 0.296 |
| Venous contrast model | 87.14 | 63.64 | 76.80 | 0.827 | 0.343 | 86.67 | 60.87 | 75.47 | 0.790 | 0.224 |
| Three-phase radiomics model | 78.57 | 65.45 | 72.80 | 0.790 | 0.011 | 80.00 | 78.26 | 79.25 | 0.813 | 0.116 |
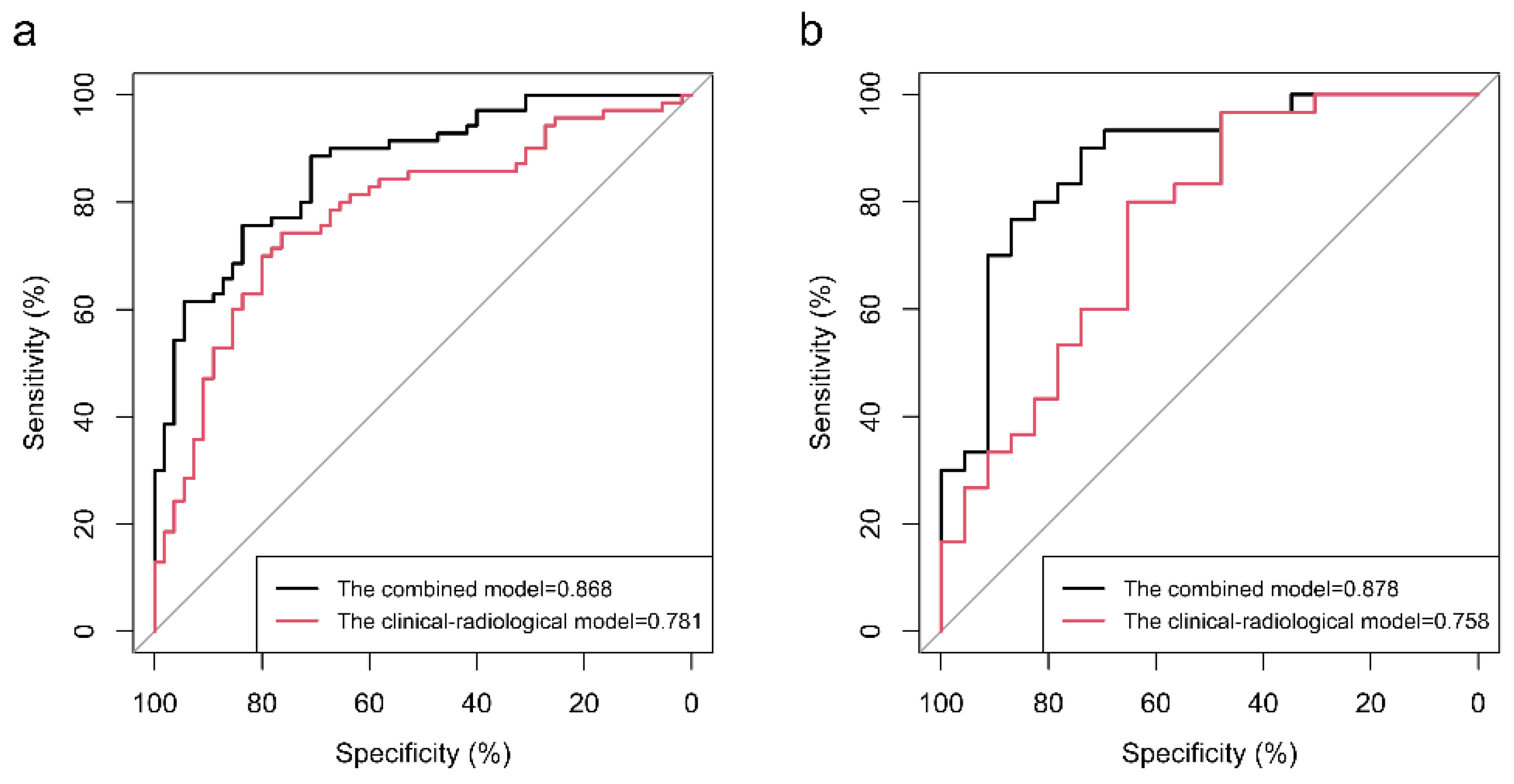
| Combined model | 88.57 | 70.91 | 86.83 | 0.868 | -- | 90.00 | 73.91 | 83.02 | 0.878 | -- |
| LNM Location Categories | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC |
|---|---|---|---|---|---|---|
| Central LNM prediction | 78.79 | 72.73 | 75.58 | 74.29 | 77.42 | 0.833 |
| Lateral LNM prediction | 66.67 | 81.48 | 74.07 | 78.26 | 70.97 | 0.823 |
| Central and lateral LNM prediction | 87.50 | 85.50 | 86.25 | 85.37 | 87.18 | 0.960 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Yang, F.; Zhang, F.; Wang, X.; Tan, Y.; Qiao, Y.; Zhang, H. Radiomics Profiling Identifies the Value of CT Features for the Preoperative Evaluation of Lymph Node Metastasis in Papillary Thyroid Carcinoma. Diagnostics 2022, 12, 1119. https://doi.org/10.3390/diagnostics12051119
Yang G, Yang F, Zhang F, Wang X, Tan Y, Qiao Y, Zhang H. Radiomics Profiling Identifies the Value of CT Features for the Preoperative Evaluation of Lymph Node Metastasis in Papillary Thyroid Carcinoma. Diagnostics. 2022; 12(5):1119. https://doi.org/10.3390/diagnostics12051119
Chicago/Turabian StyleYang, Guoqiang, Fan Yang, Fengyan Zhang, Xiaochun Wang, Yan Tan, Ying Qiao, and Hui Zhang. 2022. "Radiomics Profiling Identifies the Value of CT Features for the Preoperative Evaluation of Lymph Node Metastasis in Papillary Thyroid Carcinoma" Diagnostics 12, no. 5: 1119. https://doi.org/10.3390/diagnostics12051119
APA StyleYang, G., Yang, F., Zhang, F., Wang, X., Tan, Y., Qiao, Y., & Zhang, H. (2022). Radiomics Profiling Identifies the Value of CT Features for the Preoperative Evaluation of Lymph Node Metastasis in Papillary Thyroid Carcinoma. Diagnostics, 12(5), 1119. https://doi.org/10.3390/diagnostics12051119






