Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Abstraction
2.3. Outcomes
2.4. Data Synthesis and Statistical Analysis
- The year of publication (continuous moderator).
- The number of study participants (continuous moderator).
- The age of the participants (categorical moderator). We formulated the following ranges in the latter case: <60, 60–80 and >80 years old. Finally, we inspected funnel plots and used Egger’s regression test [11] and the Duval and Tweedie’s trim and fill method where applicable [12] to quantify whether publication bias could have influenced the results.
3. Results
3.1. Search Results
3.2. Study and Studied Subjects Characteristics
3.3. Lesion Detection Rates (DR) by Capsule Type
3.4. Completion Rates (CR) by Capsule Type
3.5. Retention Rates (RR) by Capsule Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Amadi, C.; Gerson, L.B. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 1157–1168.e2. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.F.; Pennazio, M.; Rondonotti, E.; Wolf, D.; Buras, M.R.; Albert, J.G.; Cohen, S.A.; Cotter, J.; D’Haens, G.; Eliakim, R.; et al. Capsule Retention in Crohn’s Disease: A Meta-analysis. Inflamm. Bowel Dis. 2020, 26, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mitselos, I.V.; Katsanos, K.; Tsianos, E.V.; Eliakim, R.; Christodoulou, D. Clinical Use of Patency Capsule: A Comprehensive Review of the Literature. Inflamm. Bowel Dis. 2018, 24, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Cortegoso Valdivia, P.; Elosua, A.; Houdeville, C.; Pennazio, M.; Fernández-Urién, I.; Dray, X.; Toth, E.; Eliakim, R.; Koulaouzidis, A. Clinical feasibility of panintestinal (or panenteric) capsule endoscopy: A systematic review. Eur. J. Gastroenterol. Hepatol. 2021, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Geropoulos, G.; Aquilina, J.; Kakos, C.; Anestiadou, E.; Giannis, D. Magnetically controlled capsule endoscopy versus conventional gastroscopy: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2021, 55, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-F.; Wu, Z.-X.; He, S.; Zhou, Y.-Y.; Zhao, Y.-B.; He, J.-L.; Peng, X.; Yang, Z.-X.; Lv, Q.-J.; Yang, H.; et al. Fully automated magnetically controlled capsule endoscopy for examination of the stomach and small bowel: A prospective, feasibility, two-centre study. Lancet Gastroenterol. Hepatol. 2021, 6, 914–921. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; McNamara, D.; Despott, E.J.; Adler, S.; Cash, B.D.; Fernández-Urién, I.; Ivekovic, H.; Keuchel, M.; McAlindon, M.; Saurin, J.-C.; et al. Performance measures for small-bowel endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy 2019, 51, 574–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Liao, Z.; Gao, R.; Xu, C.; Li, Z.-S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest. Endosc. 2010, 71, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Pan, J.; Liu, Y.-W.; Sun, F.-Y.; Qian, Y.-Y.; Jiang, X.; Zou, W.-B.; Xia, J.; Jiang, B.; Ru, N.; et al. Adverse events of video capsule endoscopy over the past two decades: A systematic review and proportion meta-analysis. BMC Gastroenterol. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, A.; Dabos, K.; Philipper, M.; Toth, E.; Keuchel, M. How should we do capsule endoscopy reading: A practical guide. Ther. Adv. Gastrointest. Endosc. 2021, 14, 26317745211001983. [Google Scholar] [CrossRef] [PubMed]
- Dray, X.; Iakovidis, D.; Houdeville, C.; Jover, R.; Diamantis, D.; Histace, A.; Koulaouzidis, A. Artificial intelligence in small bowel capsule endoscopy—Current status, challenges and future promise. J. Gastroenterol. Hepatol. 2021, 36, 12–19. [Google Scholar] [CrossRef] [PubMed]

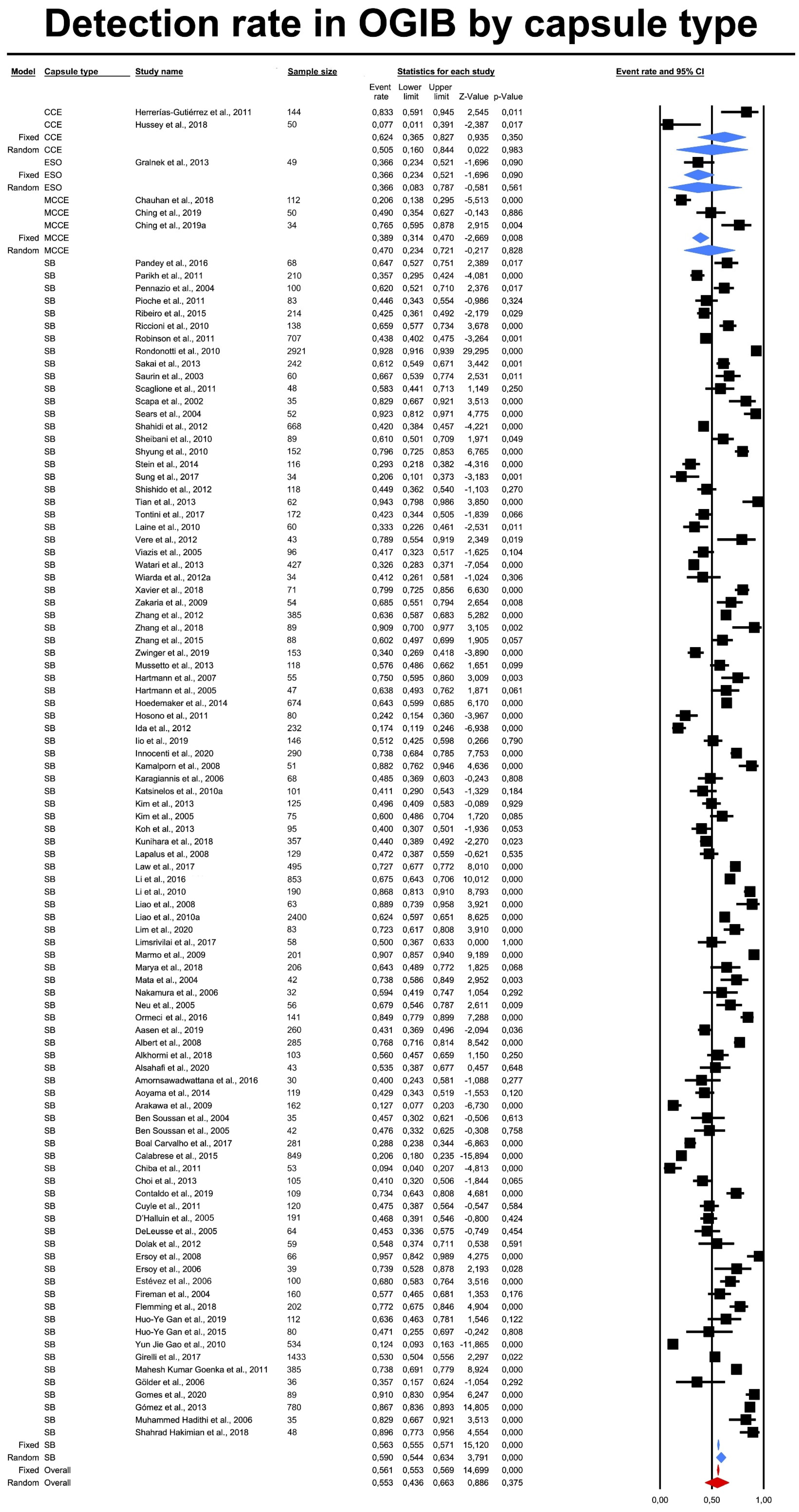
| Capsule Type | Effect Size and 95%CI | Test Z | Heterogenity (from Fixed Effect Analysis) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Point Estimate | Lower Limit | Upper Limit | z Value | p Value | Q Value | df(Q) | p Value | I2 | |
| OGIB | ||||||||||
| CCE | 2 | 0.50 | 0.16 | 0.84 | 0.02 | 0.98 | 11.30 | 1.00 | 0.00 | 91.15 |
| ESO | 1 | 0.37 | 0.08 | 0.79 | −0.58 | 0.56 | 0.00 | 0.00 | 1.00 | 0.00 |
| MCCE | 3 | 0.47 | 0.23 | 0.72 | −0.22 | 0.83 | 31.78 | 2.00 | 0.00 | 93.71 |
| SBCE | 95 | 0.59 | 0.54 | 0.63 | 3.79 | 0.00 | 2814.10 | 94.00 | 0.00 | 96.66 |
| Total between | 1.79 | 3.00 | 0.62 | |||||||
| Overall | 101 | 0.55 | 0.44 | 0.66 | 0.89 | 0.38 | 2880.59 | 100.00 | 0.00 | 96.53 |
| CD | ||||||||||
| CCE | 2 | 0.82 | 0.60 | 0.93 | 2.66 | 0.01 | 2.37 | 1.00 | 0.12 | 57.86 |
| Combi | 1 | 0.52 | 0.19 | 0.83 | 0.09 | 0.93 | 0.00 | 0.00 | 1.00 | 0.00 |
| PCC | 4 | 0.68 | 0.48 | 0.83 | 1.74 | 0.08 | 12.94 | 3.00 | 0.00 | 76.81 |
| SBCE | 36 | 0.62 | 0.55 | 0.68 | 3.42 | 0.00 | 301.31 | 35.00 | 0.00 | 88.38 |
| Total between | 3.71 | 3.00 | 0.29 | |||||||
| Overall | 43 | 0.66 | 0.53 | 0.77 | 2.38 | 0.02 | 345.07 | 42.00 | 0.00 | 87.83 |
| NL | ||||||||||
| CCE | 12 | 0.67 | 0.55 | 0.78 | 2.73 | 0.01 | 366.85 | 11.00 | 0.00 | 97.00 |
| SBCE | 7 | 0.56 | 0.38 | 0.72 | 0.62 | 0.53 | 104.46 | 6.00 | 0.00 | 94.26 |
| Total between | 1.19 | 1.00 | 0.27 | |||||||
| Overall | 19 | 0.63 | 0.52 | 0.73 | 2.27 | 0.02 | 478.65 | 18.00 | 0.00 | 96.24 |
| CeD | ||||||||||
| SBCE | 9 | 0.52 | 0.40 | 0.64 | 0.37 | 0.71 | 39.63 | 8.00 | 0.00 | 79.81 |
| Total between | 0.00 | 0.00 | 1.00 | |||||||
| Overall | 9 | 0.52 | 0.40 | 0.64 | 0.37 | 0.71 | 39.63 | 8.00 | 0.00 | 79.81 |
| CS | ||||||||||
| CCE | 11 | 0.60 | 0.42 | 0.75 | 1.09 | 0.28 | 155.06 | 10.00 | 0.00 | 93.55 |
| ESO | 7 | 0.68 | 0.48 | 0.84 | 1.74 | 0.08 | 113.49 | 6.00 | 0.00 | 94.71 |
| MCCE | 4 | 0.68 | 0.40 | 0.87 | 1.29 | 0.20 | 107.81 | 3.00 | 0.00 | 97.22 |
| PCC | 2 | 0.84 | 0.43 | 0.97 | 1.66 | 0.10 | 2.32 | 1.00 | 0.13 | 56.93 |
| SBCE | 41 | 0.55 | 0.46 | 0.64 | 1.02 | 0.31 | 907.32 | 40.00 | 0.00 | 95.59 |
| Total between | 3.82 | 4.00 | 0.43 | |||||||
| Overall | 65 | 0.62 | 0.51 | 0.73 | 2.07 | 0.04 | 1371.25 | 64.00 | 0.00 | 95.33 |
| All indications | ||||||||||
| CCE | 38 | 0.64 | 0.58 | 0.70 | 4.71 | 0.00 | 738.68 | 37.00 | 0.00 | 94.99 |
| Combi | 2 | 0.58 | 0.34 | 0.79 | 0.65 | 0.51 | 3.39 | 1.00 | 0.07 | 70.46 |
| ESO | 9 | 0.59 | 0.46 | 0.70 | 1.39 | 0.16 | 160.41 | 8.00 | 0.00 | 95.01 |
| MCCE | 17 | 0.47 | 0.38 | 0.56 | −0.61 | 0.54 | 616.48 | 16.00 | 0.00 | 97.40 |
| PCC | 5 | 0.69 | 0.53 | 0.82 | 2.24 | 0.03 | 17.71 | 4.00 | 0.00 | 77.42 |
| SBCE | 202 | 0.57 | 0.55 | 0.60 | 5.49 | 0.00 | 4316.24 | 201.00 | 0.00 | 95.34 |
| Total between | 11.92 | 5.00 | 0.04 | |||||||
| Overall | 273 | 0.59 | 0.52 | 0.65 | 2.65 | 0.01 | 7360.86 | 272.00 | 0.00 | 96.30 |
| Capsule Type | Effect Size and 95%CI | Test Z | Heterogenity (from Fixed Effect Analysis) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Point Estimate | Lower Limit | Upper Limit | z Value | p Value | Q Value | df(Q) | p Value | I2 | |
| OGIB | ||||||||||
| CCE | 1 | 0.742 | 0.398 | 0.926 | 1.407 | 0.159 | 0.000 | 0.000 | 1.000 | 0.000 |
| ESO | 1 | 0.978 | 0.803 | 0.998 | 3.107 | 0.002 | 0.000 | 0.000 | 1.000 | 0.000 |
| MCCE | 1 | 0.978 | 0.667 | 0.999 | 2.396 | 0.017 | 0.000 | 0.000 | 1.000 | 0.000 |
| SBCE | 56 | 0.891 | 0.868 | 0.910 | 1.894 | 0.000 | 508.477 | 55.000 | 0.000 | 89.183 |
| Total between | 5.031 | 3.000 | 0.170 | |||||||
| Overall | 59 | 0.906 | 0.753 | 0.968 | 3848 | 0.000 | 519.524 | 58.000 | 0.000 | 88.836 |
| CD | ||||||||||
| CCE | 2 | 0.702 | 0.632 | 0.764 | 5.300 | 1.16 × 10−7 | 4.517604 | 1.000 | 0.034 | 77.864 |
| Combi | 1 | 0.991 | 0.875 | 0.999 | 3.328 | 8.74 × 10−4 | 6.67 × 10−14 | 0.000 | 1.000 | 0.000 |
| PC | 2 | 0.893 | 0.827 | 0.935 | 7.480 | 7.46 × 10−14 | 0.195398 | 1.000 | 0.658 | 0.000 |
| PCC | 4 | 0.879 | 0.828 | 0.916 | 9.521 | 0 | 3.270906 | 3.000 | 0.352 | 8.282 |
| SBCE | 24 | 0.717 | 0.696 | 0.737 | 18.231 | 0 | 0.0187 | 23.000 | 0.000 | 87.704 |
| Total between | 6.957 | 4.000 | 0.138 | |||||||
| Overall | 33 | 0.865 | 0.766 | 0.927 | 5.410 | 6.30 × 10−8 | 242.297 | 32.000 | 0.000 | 86.793 |
| NL | ||||||||||
| CCE | 11 | 0.921 | 0.860 | 0.957 | 7.467 | 0.000 | 155.2934 | 10.000 | 0.000 | 93.561 |
| PC | 1 | 0.496 | 0.127 | 0.870 | −0.015 | 0.988 | 2.07 × 10−17 | 0.000 | 1.000 | 0.000 |
| SBCE | 6 | 0.707 | 0.512 | 0.848 | 2.073 | 0.038 | 56.14724 | 5.000 | 0.000 | 91.095 |
| Total between | 12.024 | 2.000 | 0.002 | |||||||
| Overall | 18 | 0.782 | 0.455 | 0.939 | 1.718 | 0.086 | 409.476 | 17.000 | 0.000 | 95.84835 |
| CeD | ||||||||||
| SBCE | 5 | 0.940 | 0.836 | 0.980 | 4.817 | 1.46 × 10−6 | 19.41264 | 4.000 | 0.001 | 79.395 |
| Overall | 5 | 0.940 | 0.836 | 0.980 | 4.817 | 1.46 × 10−6 | 19.41264 | 4.000 | 0.001 | 79.395 |
| CS | ||||||||||
| CCE | 8 | 0.888 | 0.790 | 0.944 | 5.442 | 0.000 | 57.4281 | 7.000 | 0.000 | 87.811 |
| ESO | 4 | 0.817 | 0.642 | 0.918 | 3.211 | 0.001 | 50.21082 | 3.000 | 0.000 | 94.025 |
| MCCE | 3 | 0.997 | 0.982 | 1.000 | 6.225 | 0.000 | 9.61 × 10−3 | 2.000 | 0.995 | 0.000 |
| PC | 1 | 0.870 | 0.562 | 0.972 | 2.257 | 0.024 | 0 | 0.000 | 1.000 | 0.000 |
| PCC | 2 | 0.960 | 0.831 | 0.992 | 3.911 | 0.000 | 7.12 × 10−4 | 1.000 | 0..979 | 0.000 |
| SBCE | 23 | 0.902 | 0.859 | 0.932 | 10.607 | 0.000 | 90.13725 | 22.000 | 0.000 | 75.593 |
| Total between | 18.916 | 5.000 | 0.002 | |||||||
| Overall | 41 | 0.928 | 0.845 | 0.968 | 5.847 | 5.01 × 10−9 | 277.4474 | 40.000 | 0.000 | 85.583 |
| All indications | ||||||||||
| CCE | 42 | 0.857 | 0.818 | 0.889 | 12.255 | 0.000 | 485.6277 | 41.000 | 0.000 | 91.557 |
| Combi | 3 | 0.953 | 0.872 | 0.984 | 5.390 | 0.000 | 467.6693 | 2.000 | 0.000 | 99.572 |
| ESO | 5 | 0.859 | 0.712 | 0.938 | 3.936 | 0.000 | 58.78379 | 4.000 | 0.000 | 93.195 |
| MCCE | 13 | 0.959 | 0.924 | 0.978 | 9.615 | 0.000 | 389.2855 | 12.000 | 0.000 | 96.917 |
| PC | 12 | 0.846 | 0.764 | 0.903 | 6.333 | 0.000 | 397.1725 | 11.000 | 0.000 | 97.230 |
| PCC | 5 | 0.920 | 0.825 | 0.965 | 5.399 | 0.000 | 8.72 | 4.000 | 0.069 | 54.123 |
| SBCE | 177 | 0.876 | 0.860 | 0.890 | 27.449 | 0.000 | 2356.912 | 176.000 | 0.000 | 92.533 |
| Total between | 20.125 | 6.000 | 0.003 | |||||||
| Overall | 257 | 0.896 | 0.857 | 0.925 | 11.640 | 0.000 | 4681.25 | 256.000 | 0.000 | 94.531 |
| Capsule Type | Effect Size and 95%CI | Test Z | Heterogenity (from Fixed Effect Analysis) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Point Estimate | Lower Limit | Upper Limit | z Value | p Value | Q Value | df(Q) | p Value | I2 | |
| OGIB | ||||||||||
| CCE | 3 | 0.04 | 0.01 | 0.21 | −3.39 | 0.00 | 1.48 | 2.00 | 0.48 | 0.00 |
| Combi | 2 | 0.01 | 0.00 | 0.03 | −9.10 | 0.00 | 45.28 | 1.00 | 0.00 | 97.79 |
| MCCE | 2 | 0.06 | 0.01 | 0.37 | −2.42 | 0.02 | 1.09 | 1.00 | 0.30 | 7.94 |
| PC | 1 | 0.02 | 0.00 | 0.12 | −3.98 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| SBCE | 60 | 0.01 | 0.01 | 0.02 | −30.98 | 0.00 | 102.89 | 59.00 | 0.00 | 42.66 |
| Total between | 3.22 | 4.00 | 0.52 | |||||||
| Overall | 68 | 0.02 | 0.01 | 0.03 | −13.02 | 0.00 | 157.76 | 67.00 | 0.00 | 57.53 |
| CD | ||||||||||
| CCE | 1 | 0.01 | 0.00 | 0.16 | −2.95 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| Combi | 2 | 0.04 | 0.01 | 0.22 | −3.27 | 0.00 | 5.08 | 1.00 | 0.02 | 80.33 |
| MCCE | 1 | 0.11 | 0.01 | 0.71 | −1.37 | 0.17 | 0.00 | 0.00 | 1.00 | 0/00 |
| PC | 4 | 0.08 | 0.02 | 0.26 | −3.42 | 0.00 | 34.61 | 3.00 | 0.00 | 91.33 |
| PCC | 3 | 0.03 | 0.01 | 0.17 | −3.64 | 0.00 | 3.94 | 2.00 | 0.14 | 49.29 |
| SBCE | 39 | 0.04 | 0.03 | 0.07 | −11.86 | 0.00 | 390.55 | 38.00 | 0.00 | 90.27 |
| Total between | 2.46 | 5.00 | 0.78 | |||||||
| Overall | 50 | 0.04 | 0.02 | 0.09 | −8.67 | 0.00 | 520.57 | 49.00 | 0.00 | 90.59 |
| NL | ||||||||||
| CCE | 11 | 0.00 | 0.00 | 0.01 | −12.08 | 0.00 | 9.58 | 10.00 | 0.48 | 0.00 |
| Combi | 1 | 0.01 | 0.00 | 0.06 | −4.40 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| MCCE | 1 | 0.01 | 0.00 | 0.19 | −2.91 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| PC | 1 | 0.00 | 0.00 | 0.04 | −4.11 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| SBCE | 9 | 0.04 | 0.02 | 0.07 | −9.64 | 0.00 | 16.55 | 8.00 | 0.04 | 51.65 |
| Total between | 21.23 | 4.00 | 0.00 | |||||||
| Overall | 23 | 0.01 | 0.00 | 0.04 | −5.82 | 0.00 | 69.32 | 22.00 | 0.00 | 68.26 |
| CeD | ||||||||||
| SBCE | 6 | 0.06 | 0.00 | 0.48 | −2.01 | 0.04 | 50.01 | 5.00 | 0.00 | 90.00 |
| Overall | 6 | 0.06 | 0.00 | 0.48 | −2.01 | 0.04 | 50.01 | 5.00 | 0.00 | 90.00 |
| CS | ||||||||||
| CCE | 7 | 0.02 | 0.00 | 0.06 | −5.79 | 0.00 | 1.77 | 6.00 | 0.94 | 0.00 |
| Combi | 2 | 0.04 | 0.01 | 0.22 | −3.30 | 0.00 | 27.51 | 1.00 | 0.00 | 96.36 |
| ESO | 3 | 0.25 | 0.05 | 0.69 | −1.13 | 0.26 | 51.67 | 2.00 | 0.00 | 96.13 |
| MCCE | 5 | 0.01 | 0.00 | 0.03 | −6.07 | 0.00 | 1.81 | 4.00 | 0.77 | 0.00 |
| PC | 1 | 0.00 | 0.00 | 0.07 | −3.31 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| PCC | 1 | 0.01 | 0.00 | 0.21 | −2.78 | 0.01 | 0.00 | 0.00 | 1.00 | 0.00 |
| SBCE | 33 | 0.03 | 0.01 | 0.04 | −12.51 | 0.00 | 157.35 | 32.00 | 0.00 | 79.66 |
| Total between | 12.96 | 6.00 | 0.04 | |||||||
| Overall | 52 | 0.02 | 0.01 | 0.06 | −6.80 | 0.00 | 426.64 | 51.00 | 0.00 | 88.05 |
| All indications | ||||||||||
| CCE | 42 | 0.01 | 0.00 | 0.01 | −17.99 | 0.00 | 156.98 | 41.00 | 0.00 | 73.88 |
| Combi | 3 | 0.02 | 0.00 | 0.08 | −5.39 | 0.00 | 5.92 | 2.00 | 0.05 | 66.22 |
| ESO | 9 | 0.04 | 0.01 | 0.11 | −5.62 | 0.00 | 146.12 | 8.00 | 0.00 | 94.52 |
| MCCE | 17 | 0.01 | 0.00 | 0.02 | −11.69 | 0.00 | 49.67 | 16.00 | 0.00 | 67.79 |
| PC | 12 | 0.05 | 0.03 | 0.10 | −7.76 | 0.00 | 145.24 | 11.00 | 0.00 | 92.43 |
| PCC | 5 | 0.02 | 0.01 | 0.08 | −5.62 | 0.00 | 7.29 | 4.00 | 0.12 | 45.16 |
| SBCE | 184 | 0.02 | 0.01 | 0.02 | −37.76 | 0.00 | 937.46 | 183.00 | 0.00 | 80.48 |
| Total between | 21.50 | 6.00 | 0.00 | |||||||
| Overall | 272 | 0.02 | 0.01 | 0.03 | −14.18 | 0.00 | 1832.05 | 271.00 | 0.00 | 85.21 |
| Indications | Detection Rate | Completion Rate | Retention Rate |
|---|---|---|---|
| OGIB | 55% | 90.6% | 2% |
| CD | 66% | 86.5% | 4% |
| NL | 63% | 78.2% | 1% |
| CeD | 52% | 94.0% | 6% |
| CS | 62% | 92.8% | 2% |
| All Indications | 59% | 89.6% | 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortegoso Valdivia, P.; Skonieczna-Żydecka, K.; Elosua, A.; Sciberras, M.; Piccirelli, S.; Rullan, M.; Tabone, T.; Gawel, K.; Stachowski, A.; Lemiński, A.; et al. Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1105. https://doi.org/10.3390/diagnostics12051105
Cortegoso Valdivia P, Skonieczna-Żydecka K, Elosua A, Sciberras M, Piccirelli S, Rullan M, Tabone T, Gawel K, Stachowski A, Lemiński A, et al. Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(5):1105. https://doi.org/10.3390/diagnostics12051105
Chicago/Turabian StyleCortegoso Valdivia, Pablo, Karolina Skonieczna-Żydecka, Alfonso Elosua, Martina Sciberras, Stefania Piccirelli, Maria Rullan, Trevor Tabone, Katarzyna Gawel, Adam Stachowski, Artur Lemiński, and et al. 2022. "Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 5: 1105. https://doi.org/10.3390/diagnostics12051105
APA StyleCortegoso Valdivia, P., Skonieczna-Żydecka, K., Elosua, A., Sciberras, M., Piccirelli, S., Rullan, M., Tabone, T., Gawel, K., Stachowski, A., Lemiński, A., Marlicz, W., Fernández-Urién, I., Ellul, P., Spada, C., Pennazio, M., Toth, E., & Koulaouzidis, A. (2022). Indications, Detection, Completion and Retention Rates of Capsule Endoscopy in Two Decades of Use: A Systematic Review and Meta-Analysis. Diagnostics, 12(5), 1105. https://doi.org/10.3390/diagnostics12051105











