Clinical Features and Immunophenotypes of Double-Hit Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry Analysis
2.3. Double Hit Analysis
2.4. Statistical Analysis
3. Results
3.1. Comparison of Clinical and Biological Features between the DH and Non-DH Groups
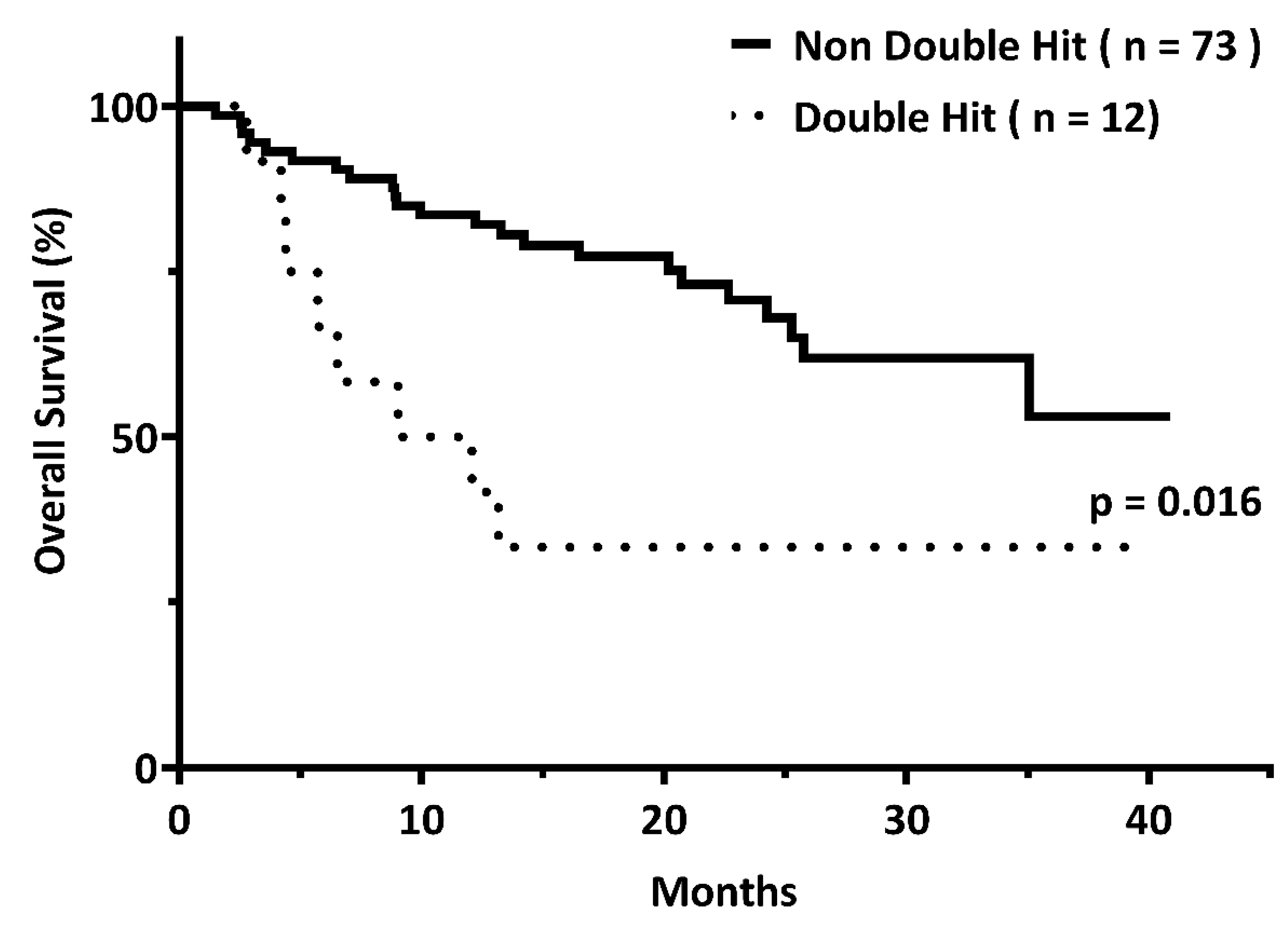
3.2. Outcome Comparison between the DH and Non-DH Groups
3.3. DH Genetics as an Independent Factor Associated with Inferior OS
3.4. Clinical Data of DH (+) DLBCL Patients
3.5. Bulky Disease with DE Immunopositivity for Identifying DH DLBCL
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, N.L.; Wilson, W.H.; Jung, S.H.; Hsi, E.D.; Maurer, M.J.; Pederson, L.D.; Polley, M.C.; Pitcher, B.N.; Cheson, B.D.; Kahl, B.S.; et al. Dose-Adjusted EPOCH-R Compared with R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J. Clin. Oncol. 2019, 37, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Leppa, S.; Jorgensen, J.; Tierens, A.; Meriranta, L.; Ostlie, I.; de Nully Brown, P.; Fagerli, U.M.; Larsen, T.S.; Mannisto, S.; Munksgaard, L.; et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv. 2020, 4, 1906–1915. [Google Scholar] [CrossRef]
- Oki, Y.; Noorani, M.; Lin, P.; Davis, R.E.; Neelapu, S.S.; Ma, L.; Ahmed, M.; Rodriguez, M.A.; Hagemeister, F.B.; Fowler, N.; et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br. J. Haematol. 2014, 166, 891–901. [Google Scholar] [CrossRef]
- Petrich, A.M.; Gandhi, M.; Jovanovic, B.; Castillo, J.J.; Rajguru, S.; Yang, D.T.; Shah, K.A.; Whyman, J.D.; Lansigan, F.; Hernandez-Ilizaliturri, F.J.; et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood 2014, 124, 2354–2361. [Google Scholar] [CrossRef] [Green Version]
- Sehn, L.H.; Berry, B.; Chhanabhai, M.; Fitzgerald, C.; Gill, K.; Hoskins, P.; Klasa, R.; Savage, K.J.; Shenkier, T.; Sutherland, J.; et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 2007, 109, 1857–1861. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, A.L.; Lymphoma, G.; Eastern Cooperative Oncology, G.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Pasqualucci, L.; Dalla-Favera, R. Genetics of diffuse large B-cell lymphoma. Blood 2018, 131, 2307–2319. [Google Scholar] [CrossRef]
- Hilton, L.K.; Tang, J.; Ben-Neriah, S.; Alcaide, M.; Jiang, A.; Grande, B.M.; Rushton, C.K.; Boyle, M.; Meissner, B.; Scott, D.W.; et al. The double-hit signature identifies double-hit diffuse large B-cell lymphoma with genetic events cryptic to FISH. Blood 2019, 134, 1528–1532. [Google Scholar] [CrossRef] [Green Version]
- Savage, K.J.; Johnson, N.A.; Ben-Neriah, S.; Connors, J.M.; Sehn, L.H.; Farinha, P.; Horsman, D.E.; Gascoyne, R.D. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009, 114, 3533–3537. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.W.; King, R.L.; Staiger, A.M.; Ben-Neriah, S.; Jiang, A.; Horn, H.; Mottok, A.; Farinha, P.; Slack, G.W.; Ennishi, D.; et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 2018, 131, 2060–2064. [Google Scholar] [CrossRef]
- Hu, S.; Xu-Monette, Z.Y.; Tzankov, A.; Green, T.; Wu, L.; Balasubramanyam, A.; Liu, W.M.; Visco, C.; Li, Y.; Miranda, R.N.; et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: A report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013, 121, 4021–4031. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Kim, S.; Koh, J.; Yim, J.; Lee, C.; Heo, D.S.; Kim, T.M.; Paik, J.H.; Jeon, Y.K. Immunophenotypic Landscape and Prognosis of Diffuse Large B-Cell Lymphoma with MYC/BCL2 Double Expression: An Analysis of A Prospectively Immunoprofiled Cohort. Cancers 2020, 12, 3305. [Google Scholar] [CrossRef]
- Riedell, P.A.; Smith, S.M. Double hit and double expressors in lymphoma: Definition and treatment. Cancer 2018, 124, 4622–4632. [Google Scholar] [CrossRef] [Green Version]
- Landsburg, D.J.; Falkiewicz, M.K.; Maly, J.; Blum, K.A.; Howlett, C.; Feldman, T.; Mato, A.R.; Hill, B.T.; Li, S.; Medeiros, L.J.; et al. Outcomes of Patients with Double-Hit Lymphoma Who Achieve First Complete Remission. J. Clin. Oncol. 2017, 35, 2260–2267. [Google Scholar] [CrossRef] [Green Version]
- Pfreundschuh, M.; Ho, A.D.; Cavallin-Stahl, E.; Wolf, M.; Pettengell, R.; Vasova, I.; Belch, A.; Walewski, J.; Zinzani, P.L.; Mingrone, W.; et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: An exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008, 9, 435–444. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Muller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, M.R.; Stevens, W.B.C.; van den Brand, M.; van Krieken, J.H.; Scheijen, B. Molecular Genetics of Relapsed Diffuse Large B-Cell Lymphoma: Insight into Mechanisms of Therapy Resistance. Cancers 2020, 12, 3553. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; Rosenwald, A.; Campo, E. Understanding MYC-driven aggressive B-cell lymphomas: Pathogenesis and classification. Blood 2013, 122, 3884–3891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapproth, K.; Wirth, T. Advances in the understanding of MYC-induced lymphomagenesis. Br. J. Haematol. 2010, 149, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Bric, A.; Teruya-Feldstein, J.; Herbst, A.; Nilsson, J.A.; Cordon-Cardo, C.; Cleveland, J.L.; Tansey, W.P.; Lowe, S.W. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 2005, 436, 807–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calado, D.P.; Sasaki, Y.; Godinho, S.A.; Pellerin, A.; Kochert, K.; Sleckman, B.P.; de Alboran, I.M.; Janz, M.; Rodig, S.; Rajewsky, K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 2012, 13, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Green, T.M.; Young, K.H.; Visco, C.; Xu-Monette, Z.Y.; Orazi, A.; Go, R.S.; Nielsen, O.; Gadeberg, O.V.; Mourits-Andersen, T.; Frederiksen, M.; et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012, 30, 3460–3467. [Google Scholar] [CrossRef]
- Sewastianik, T.; Prochorec-Sobieszek, M.; Chapuy, B.; Juszczynski, P. MYC deregulation in lymphoid tumors: Molecular mechanisms, clinical consequences and therapeutic implications. Biochim. Biophys. Acta 2014, 1846, 457–467. [Google Scholar] [CrossRef]
- Laude, M.C.; Lebras, L.; Sesques, P.; Ghesquieres, H.; Favre, S.; Bouabdallah, K.; Croizier, C.; Guieze, R.; Drieu La Rochelle, L.; Gyan, E.; et al. First-line treatment of double-hit and triple-hit lymphomas: Survival and tolerance data from a retrospective multicenter French study. Am. J. Hematol. 2021, 96, 302–311. [Google Scholar] [CrossRef]
- Basci, S.; Yigenoglu, T.N.; Bakirtas, M.; Uncu Ulu, B.; Yaman, S.; Dal, M.S.; Kizil Cakar, M.; Altuntas, F. The effect of bulky mass on prognosis in diffuse large-B-cell lymphoma: Still poor? Leuk. Res. 2021, 102, 106521. [Google Scholar] [CrossRef]
| All Patients (n = 92) | Double Hit (n = 14) | Non-Double Hit (n = 78) | p-Value | |
|---|---|---|---|---|
| Gender, n (%) | 0.909 a | |||
| Male | 48 (52.2%) | 8 (57.1%) | 40 (51.3%) | |
| Female | 44 (47.8%) | 6 (42.9%) | 38 (48.7%) | |
| Age when diagnosed, n (%) | 0.518 a | |||
| <65 | 50 (54.3%) | 6 (42.9%) | 44 (56.4%) | |
| ≥65 | 42 (45.7%) | 8 (57.1%) | 34 (43.6%) | |
| Performance status, n (%) | 0.060 b | |||
| ≤2 | 81 (88.0%) | 10 (71.4%) | 71 (91.0%) | |
| >2 | 11 (12.0%) | 4 (28.6%) | 7 (9.0%) | |
| Stage, n (%) | 0.134 b | |||
| Limited (Stage 1–2) | 30 (32.6%) | 2 (14.3%) | 28 (35.9%) | |
| Advanced (Stage 3–4) | 62 (67.4%) | 12 (85.7%) | 50 (64.1%) | |
| Bulky disease, n (%) | 0.013 b | |||
| No | 61 (66.3%) | 5 (35.7%) | 56 (71.8%) | |
| Yes | 31 (33.7%) | 9 (64.3%) | 22 (28.2%) | |
| R-IPI score, n (%) | 0.640 a | |||
| Lower risk (0–2) | 48 (52.2%) | 6 (42.9%) | 42 (53.8%) | |
| Higher risk (3–5) | 44 (47.8%) | 8 (57.1%) | 36 (46.2%) | |
| LDH (U/L), median (range) | 315 (111–4461) | 360 (169–1760) | 303 (111–4461) | 0.277 c |
| Uric acid (mg/dL), median (range) | 5.5 (1.5–19.0) | 5.2 (3.5–16.4) | 5.6 (1.5–19.0) | 0.721 c |
| Maximal SUV (/1 h), median (range) | 12.6 (2.5–27.4) | 13.1 (4.1–25.2) | 12.4 (2.5–27.4) | 0.436 c |
| CCI score, median (range) | 4 (2–11) | 5 (2–11) | 4 (2–11) | 0.659 c |
| Double expresser, n (%) | 0.044 b | |||
| No | 68 (73.9%) | 7 (50.0%) | 61 (78.2%) | |
| Yes | 24 (26.1%) | 7 (50.0%) | 17 (21.8%) | |
| Cell of origin, n (%) | 0.122 b | |||
| GCB | 30 (34.1%) | 7 (53.8%) | 23 (30.7%) | |
| Non-GCB | 58 (65.9%) | 6 (46.2%) | 52 (69.3%) | |
| Treatment, n (%) | 0.501 a | |||
| R + intensified chemotherapy | 13 (14.1%) | 2 (14.3%) | 11 (14.1%) | |
| R-CHOP | 65 (70.7%) | 8 (57.1%) | 57 (73.1%) | |
| R-COP | 7 (7.6%) | 2 (14.3%) | 5 (6.4%) | |
| Palliative care | 7 (7.6%) | 2 (14.3%) | 5 (6.4%) | |
| Treatment response, n (%) | 0.687 a | |||
| Non-CR | 31 (36.5%) | 5 (41.7%) | 26 (35.6%) | |
| CR | 54 (63.5%) | 7 (58.3%) | 47 (64.4%) | |
| Mortality, n (%) | 0.028 a | |||
| Alive | 54 (58.7%) | 4 (28.6%) | 50 (64.1%) | |
| Death | 38 (41.3%) | 10 (71.4%) | 28 (35.9%) | |
| Causes of death, n (%) | 0.663 a | |||
| Disease related | 22 (57.9%) | 7 (70.0%) | 15 (53.6%) | |
| Complication | 11 (28.9%) | 2 (20.0%) | 9 (32.1%) | |
| Others | 5 (13.2%) | 1 (10.0%) | 4 (14.3%) |
| Clinical Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age, years | ||||||
| <65 | 1.00 | 1.00 | ||||
| ≥65 | 2.74 | 1.32–5.67 | 0.007 | 1.46 | 0.57–3.71 | 0.432 |
| Gender | ||||||
| Male | 1.00 | |||||
| Female | 0.78 | 0.38–1.59 | 0.489 | |||
| Performance status | ||||||
| ≤2 | 1.00 | |||||
| >2 | 2.17 | 0.76–6.22 | 0.150 | |||
| Stage | ||||||
| Limited (Stage: 1–2) | 1.00 | 1.00 | ||||
| Advanced (Stage: 3–4) | 6.58 | 2.00–21.66 | 0.002 | 5.00 | 1.36–18.35 | 0.015 |
| Bulky, n (%) | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.17 | 1.07–4.42 | 0.032 | 1.80 | 0.78–4.16 | 0.169 |
| R-IPI score | ||||||
| Non-poor risk (0–2) | 1.00 | 1.00 | ||||
| Poor risk (3–5) | 3.21 | 1.51–6.83 | 0.002 | 1.21 | 0.52–2.85 | 0.655 |
| CCI score | 1.35 | 1.13–1.60 | 0.001 | 1.30 | 1.03–1.63 | 0.025 |
| LDH (U/L) | 1.00 | 1.00–1.00 | 0.197 | |||
| Uric acid (mg/dL) | 1.13 | 1.02–1.25 | 0.019 | |||
| Maximal SUV (/1 h) | 1.09 | 1.02–1.17 | 0.016 | |||
| Double hit | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.63 | 1.16–5.93 | 0.020 | 1.62 | 0.65–4.06 | 0.300 |
| Double expresser | ||||||
| No | 1.00 | |||||
| Yes | 1.47 | 0.69–3.12 | 0.320 | |||
| Cell of origin | ||||||
| GCB | 1.00 | |||||
| Non-GCB | 1.11 | 0.51–2.42 | 0.800 | |||
| No. | Age | Stage | R-IPI | Bulky | DE | Frontline Treatment | CR | Alive | Cause of Death | PFS (Days) | OS (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | 4 | 5 | Yes | Yes | Palliative care | No | No | Disease related | 14 | 14 |
| 2 | 64 | 1 | 1 | No | No | R-CHOP × 6 | Yes | Yes | 1203 | 1203 | |
| 3 | 75 | 2 | 2 | Yes | No | R-COP × 8 | No | No | Disease related | 257 | 368 |
| 4 | 67 | 3 | 2 | Yes | No | R-CHOP × 4 | Yes | No | Hepatitis B flare-up | 174 | 174 |
| 5 | 54 | 4 | 2 | No | No | R-CHOP × 8 | Yes | Yes | 1176 | 1176 | |
| 6 | 77 | 3 | 3 | Yes | No | R-COP × 2 | No | No | Disease related | 85 | 85 |
| 7 | 54 | 4 | 2 | No | Yes | (R-CHOP + IT) × 5 | Yes | No | Septic shock | 128 | 128 |
| 8 | 46 | 4 | 2 | No | No | (R-CHOP + IT, R-MA) × 2 | No | No | Disease related | 223 | 275 |
| 9 | 80 | 4 | 4 | Yes | Yes | Palliative care | No | No | Disease related | 43 | 43 |
| 10 | 71 | 3 | 4 | Yes | No | R-CEOP × 5 | No | No | Disease related | 158 | 401 |
| 11 | 48 | 4 | 3 | Yes | Yes | R-CHOP × 1 | No | No | Disease related | 58 | 134 |
| 12 | 70 | 4 | 3 | Yes | Yes | R-CHOP × 6 | Yes | Yes | 866 | 866 | |
| 13 | 66 | 4 | 3 | No | Yes | R-CHOP × 6 | Yes | No | Aortic stenosis with heart failure | 199 | 199 |
| 14 | 41 | 3 | 3 | Yes | Yes | R-EPOCH × 6 | Yes | Yes | 1157 | 1157 |
| Double Hit | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Yes | No | |||||||
| Double expresser | Yes | 24 | 7 | 17 | 50.0 | 78.2 | 29.2 | 89.7 | 73.9 |
| No | 68 | 7 | 61 | ||||||
| Bulky disease | Yes | 31 | 9 | 22 | 64.3 | 71.8 | 29.0 | 91.8 | 70.7 |
| No | 61 | 5 | 56 | ||||||
| Double expresser + bulky disease | Yes | 13 | 5 | 8 | 35.7 | 89.7 | 38.5 | 88.6 | 81.5 |
| No | 79 | 9 | 70 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-H.; Gau, J.-P.; Teng, C.-L.J.; Shih, Y.-H.; Su, Y.-C.; Wang, R.-C.; Chen, T.-C. Clinical Features and Immunophenotypes of Double-Hit Diffuse Large B-Cell Lymphoma. Diagnostics 2022, 12, 1106. https://doi.org/10.3390/diagnostics12051106
Wu C-H, Gau J-P, Teng C-LJ, Shih Y-H, Su Y-C, Wang R-C, Chen T-C. Clinical Features and Immunophenotypes of Double-Hit Diffuse Large B-Cell Lymphoma. Diagnostics. 2022; 12(5):1106. https://doi.org/10.3390/diagnostics12051106
Chicago/Turabian StyleWu, Cheng-Han, Jyh-Pyng Gau, Chieh-Lin Jerry Teng, Yu-Hsuan Shih, Yu-Chen Su, Ren-Ching Wang, and Tsung-Chih Chen. 2022. "Clinical Features and Immunophenotypes of Double-Hit Diffuse Large B-Cell Lymphoma" Diagnostics 12, no. 5: 1106. https://doi.org/10.3390/diagnostics12051106
APA StyleWu, C.-H., Gau, J.-P., Teng, C.-L. J., Shih, Y.-H., Su, Y.-C., Wang, R.-C., & Chen, T.-C. (2022). Clinical Features and Immunophenotypes of Double-Hit Diffuse Large B-Cell Lymphoma. Diagnostics, 12(5), 1106. https://doi.org/10.3390/diagnostics12051106






