Comparison of Nonclassic and Classic Phenotype of Hypertrophic Cardiomyopathy Focused on Prognostic Cardiac Magnetic Resonance Parameters: A Single-Center Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition and Analysis
2.3. Statistical Analysis
3. Results
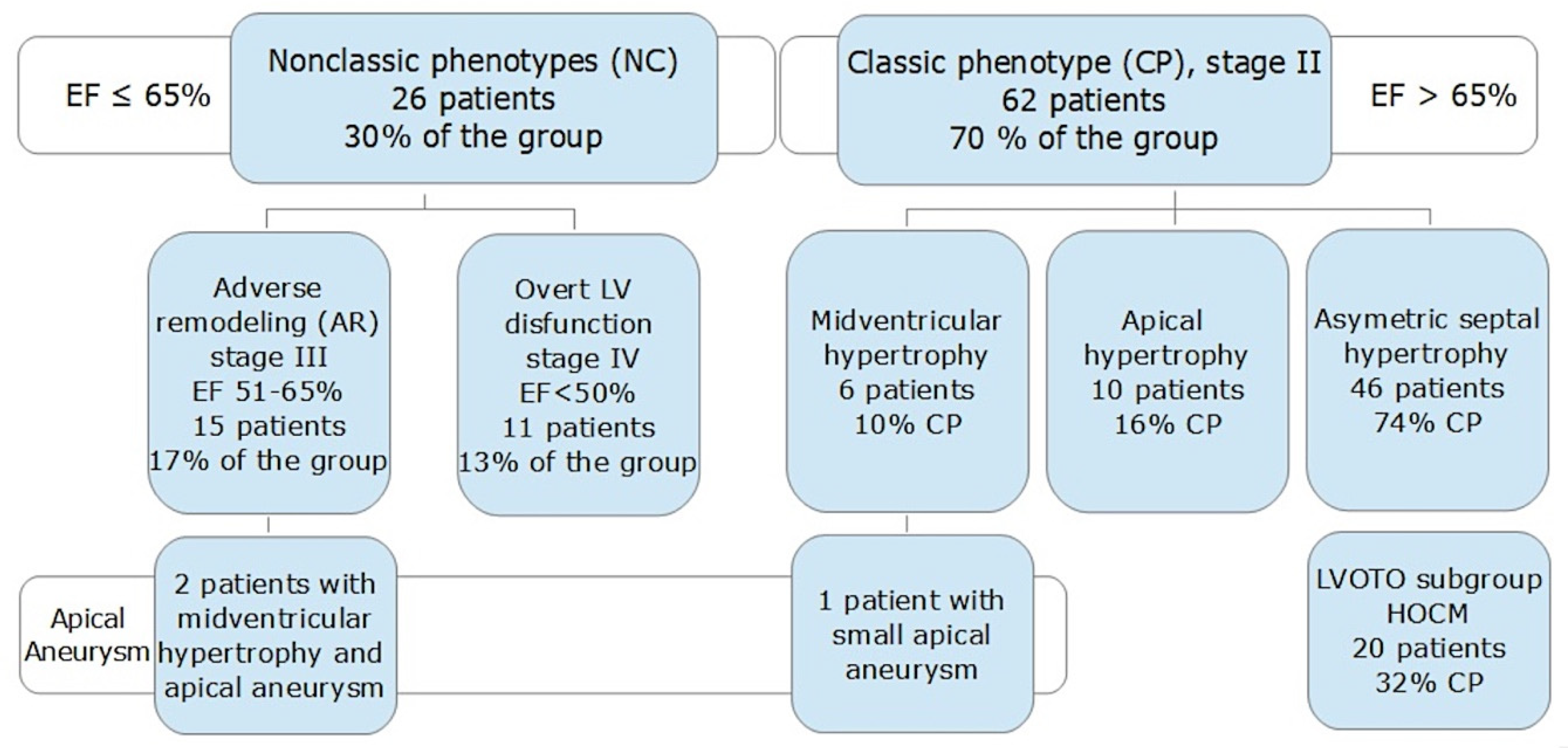
3.1. The Study Population
3.2. Left Ventricular Aneurysm
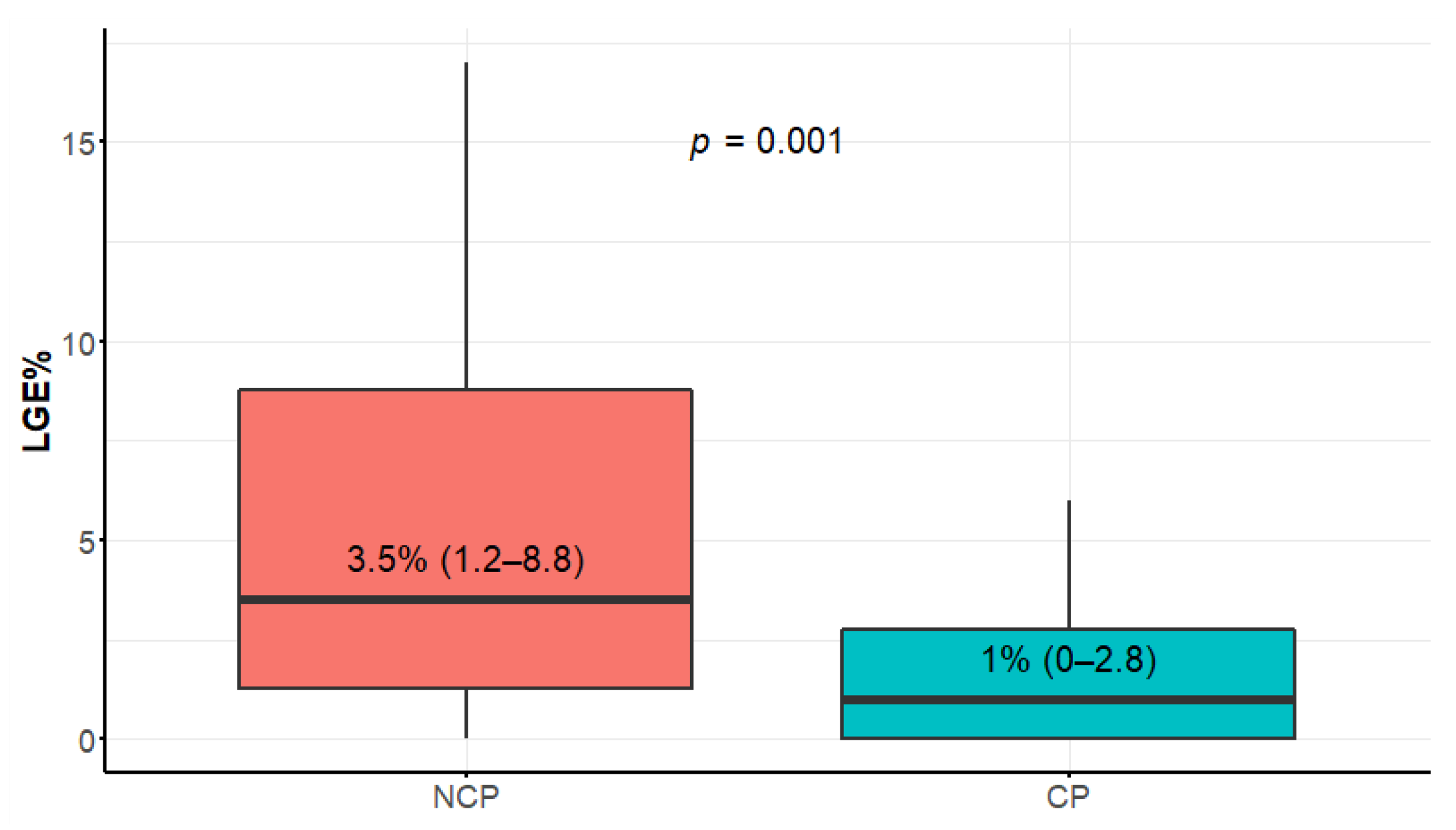
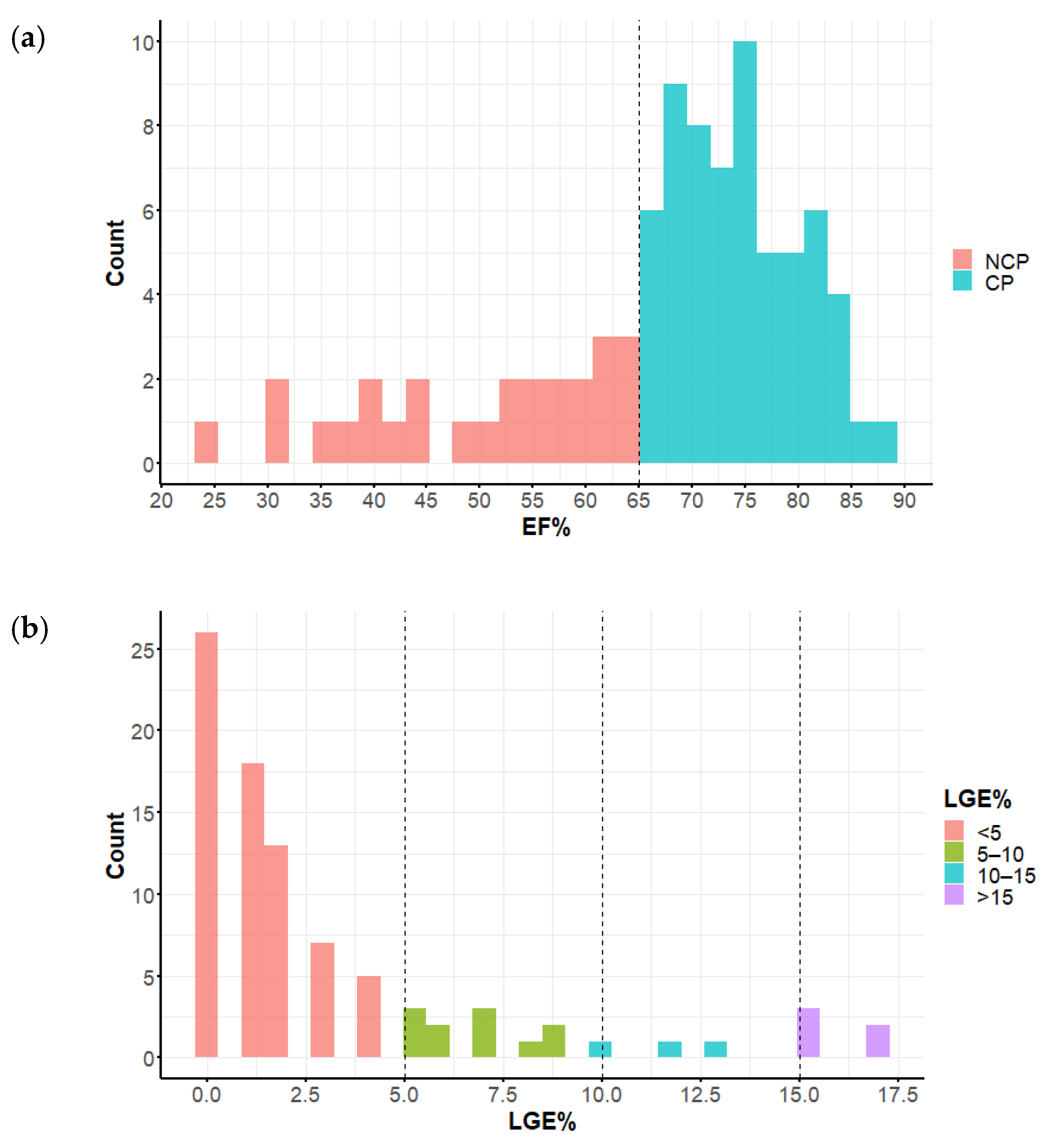
3.3. Late Gadolinium Enhancement Extent
3.4. LV Mass Index
3.5. Myocardial Contraction Fraction (MCF) and Left Ventricular Global Function Index (LVGFI)
3.6. Left Atrial Parameters
3.7. Volume–Time Curve Parameters
3.8. Right Ventricular Involvement
3.9. Left Ventricular Outflow Tract and Mitral Apparatus
4. Discussion
4.1. HCM Risk Score, LVMT, and Indexed Maximal Wall Thickness (IMWT)
4.2. Left Ventricular Apical Aneurysm
4.3. LGE Extent
4.4. LV Mass Index
4.5. Myocardial Contraction Fraction (MCF) and Left Ventricular Global Function Index (LVGFI)
4.6. Left Atrial CMR Parameters
4.7. Volume–Time Curve Parameters
4.8. Right Ventricular Involvement
4.9. Left Ventricular Outflow Tract and Mitral Apparatus
4.10. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UK COM. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Writing Committee Members; Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Musumeci, B.; Tini, G.; Russo, D.; Sclafani, M.; Cava, F.; Tropea, A.; Adduci, C.; Palano, F.; Francia, P.; Autore, C. Left Ventricular Remodeling in Hypertrophic Cardiomyopathy: An Overview of Current Knowledge. J. Clin. Med. 2021, 10, 1547. [Google Scholar] [CrossRef] [PubMed]
- Autore, C.; Musumeci, M.B. The natural history of hypertrophic cardiomyopathy. Eur. Heart J. Suppl. 2020, 22, L11–L14. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Garberich, R.F.; Maron, M.S. What Do Patients With Hypertrophic Cardiomyopathy Die from? Am. J. Cardiol. 2016, 117, 434–435. [Google Scholar] [CrossRef] [Green Version]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of Disease Progression in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2012, 5, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Olivotto, I.; Cecchi, F.; Casey, S.A.; Dolara, A.; Traverse, J.H.; Maron, B.J. Impact of Atrial Fibrillation on the Clinical Course of Hypertrophic Cardiomyopathy. Circulation 2001, 104, 2517–2524. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease With Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Greulich, S.; Seitz, A.; Herter, D.; Günther, F.; Probst, S.; Bekeredjian, R.; Gawaz, M.; Sechtem, U.; Mahrholdt, H. Long-term risk of sudden cardiac death in hypertrophic cardiomyopathy: A cardiac magnetic resonance outcome study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 732–741. [Google Scholar] [CrossRef]
- Ramchand, J.; Fava, A.M.; Chetrit, M.; Desai, M.Y. Advanced imaging for risk stratification of sudden death in hypertrophic cardiomyopathy. Heart 2020, 106, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Stachera, M.; Przybyło, P.; Sznajder, K.; Gierlotka, M. Cardiac Magnetic Resonance in assessment of Hypertrophic Cardiomyopathy Phenotypes and Stages—Pictorial Review. Pol. J. Radiol. 2021, 86, e672. [Google Scholar] [CrossRef]
- Huang, S.; Xu, H.; Diao, K.; Shi, K.; He, Y.; He, S.; Zhang, Y.; Gao, Y.; Shen, M.; Guo, Y.; et al. Left ventricular global function index by magnetic resonance imaging—A novel marker for differentiating cardiac amyloidosis from hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 4707. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, H.-Y.; Diao, K.-Y.; Shi, K.; He, Y.; He, S.; Zhang, Y.; Gao, Y.; Shen, M.-T.; Guo, Y.-K.; et al. Myocardial contraction fraction: A volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J. Am. Coll. Cardiol. 2002, 40, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Mewton, N.; Opdahl, A.; Choi, E.-Y.; Almeida, A.L.; Kawel, N.; Wu, C.O.; Burke, G.L.; Liu, S.; Liu, K.; Bluemke, D.A.; et al. Left Ventricular Global Function Index by Magnetic Resonance Imaging—A Novel Marker for Assessment of Cardiac Performance for the Prediction of Cardiovascular Events. Hypertension 2013, 61, 770–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, B.J.; Haas, T.S.; Maron, M.S.; Lesser, J.R.; Browning, J.A.; Chan, R.H.; Olivotto, I.; Garberich, R.F.; Schwartz, R.S. Left Atrial Remodeling in Hypertrophic Cardiomyopathy and Susceptibility Markers for Atrial Fibrillation Identified by Cardiovascular Magnetic Resonance. Am. J. Cardiol. 2014, 113, 1394–1400. [Google Scholar] [CrossRef]
- Farhad, H.; Seidelmann, S.B.; Vigneault, D.; Abbasi, S.A.; Yang, E.; Day, S.M.; Colan, S.D.; Russell, M.W.; Towbin, J.; Sherrid, M.V.; et al. Left Atrial structure and function in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. J. Cardiovasc. Magn. Reson. 2017, 19, 107. [Google Scholar] [CrossRef] [Green Version]
- Spirito, P.; Iascone, M.; Ferrazzi, P. Mitral valve abnormalities in hypertrophic cardiomyopathy: A primary expression of the disease? Getting closer to the answer. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1107–1108. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral Valve Abnormalities Identified by Cardiovascular Magnetic Resonance Represent a Primary Phenotypic Expression of Hypertrophic Cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel-Claussen, J.; Tomas, M.S.; Newatia, A.; Boyce, D.; Pinheiro, A.; Abraham, R.; Abraham, T.; Bluemke, D.A. Cardiac MRI evaluation of hypertrophic cardiomyopathy: Left ventricular outflow tract/aortic valve diameter ratio predicts severity of LVOT obstruction. J. Magn. Reson. Imaging JMRI 2012, 36, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic Value of Quantitative Contrast-Enhanced Cardiovascular Magnetic Resonance for the Evaluation of Sudden Death Risk in Patients With Hypertrophic Cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Arenja, N.; Fritz, T.; Andre, F.; Riffel, J.H.; aus dem Siepen, F.; Ochs, M.; Paffhausen, J.; Hegenbart, U.; Schönland, S.; Müller-Hennessen, M.; et al. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images—reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Méndez, C.; Rodríguez, E.; Barriales, R.; Ochoa, J.P.; Monserrat, L. Phenotypes of hypertrophic cardiomyopathy. An illustrative review of MRI findings. Insights Imaging 2018, 9, 1007–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauffal, V.; Marstrand, P.; Han, L.; Parikh, V.N.; Helms, A.S.; Ingles, J.; Jacoby, D.; Lakdawala, N.K.; Kapur, S.; Michels, M.; et al. Worldwide differences in primary prevention implantable cardioverter defibrillator utilization and outcomes in hypertrophic cardiomyopathy. Eur. Heart J. 2021, 42, 3932–3944. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Carrick, R.T.; Patel, P.P.; Koethe, B.; Wells, S.; Maron, M.S. Outcomes in Patients With Hypertrophic Cardiomyopathy and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy With Left Ventricular Systolic Dysfunction. Circulation 2020, 141, 1371–1383. [Google Scholar] [CrossRef]
- Brinkley, D.M.; Wells, Q.S.; Stevenson, L.W. Avoiding Burnout From Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2020, 75, 3044–3047. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Li, Y.; Xu, Y.; Wan, K.; Chen, Y. Variable and Limited Predictive Value of the European Society of Cardiology Hypertrophic Cardiomyopathy Sudden-Death Risk Model: A Meta-analysis. Can. J. Cardiol. 2019, 35, 1791–1799. [Google Scholar] [CrossRef]
- Wells, S.B.; Maron, M.; Patel, P.; Maron, B.; Rowin, E. End-Stage Hypertrophic Cardiomyopathy Revisited: Impact of Contemporary Therapies. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 762. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, M.S.; Autore, C.; Lesser, J.R.; Rega, L.; Casolo, G.; De Santis, M.; Quarta, G.; Nistri, S.; Cecchi, F.; et al. Assessment and Significance of Left Ventricular Mass by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Hindieh, W.; Weissler-Snir, A.; Hammer, H.; Adler, A.; Rakowski, H.; Chan, R.H. Discrepant Measurements of Maximal Left Ventricular Wall Thickness Between Cardiac Magnetic Resonance Imaging and Echocardiography in Patients With Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e006309. [Google Scholar] [CrossRef] [Green Version]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients With Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Rowin, E.J.; Maron, B.J. How to Image Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohy, Z.; Szabo, L.; Toth, A.; Czimbalmos, C.; Horvath, R.; Horvath, V.; Suhai, F.I.; Geller, L.; Merkely, B.; Vago, H. Prognostic significance of cardiac magnetic resonance-based markers in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 2027–2036. [Google Scholar] [CrossRef]
- Chuang, M.L.; Gona, P.; Salton, C.J.; Yeon, S.B.; Kissinger, K.V.; Blease, S.J.; Levy, D.; O’Donnell, C.J.; Manning, W.J. Usefulness of the left ventricular myocardial contraction fraction in healthy men and women to predict cardiovascular morbidity and mortality. Am. J. Cardiol. 2012, 109, 1454–1458. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.; Akwo, E.A.; Bluemke, D.A.; Lima, J.A.C.; Shimbo, D.; Maurer, M.S.; Bertoni, A.G. Association between reduced myocardial contraction fraction and cardiovascular disease outcomes: The Multi-Ethnic Study of Atherosclerosis. Int. J. Cardiol. 2019, 293, 10–16. [Google Scholar] [CrossRef]
- Romeo, F.J.; Seropian, I.M.; Arora, S.; Vavalle, J.P.; Falconi, M.; Oberti, P.; Kotowicz, V.; Agatiello, C.R.; Berrocal, D.H. Prognostic impact of myocardial contraction fraction in patients undergoing transcatheter aortic valve replacement for aortic stenosis. Cardiovasc. Diagn. Ther. 2020, 10, 12–23. [Google Scholar] [CrossRef]
- Tendler, A.; Helmke, S.; Teruya, S.; Alvarez, J.; Maurer, M.S. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2015, 22, 61–66. [Google Scholar] [CrossRef]
- Rubin, J.; Steidley, D.E.; Carlsson, M.; Ong, M.-L.; Maurer, M.S. Myocardial Contraction Fraction by M-Mode Echocardiography Is Superior to Ejection Fraction in Predicting Mortality in Transthyretin Amyloidosis. J. Card. Fail. 2018, 24, 504–511. [Google Scholar] [CrossRef]
- Arenja, N.; Andre, F.; Riffel, J.H.; Siepen, F.A.D.; Hegenbart, U.; Schönland, S.; Kristen, A.V.; Katus, H.A.; Buss, S.J. Prognostic value of novel imaging parameters derived from standard cardiovascular magnetic resonance in high risk patients with systemic light chain amyloidosis. J. Cardiovasc. Magn. Reson. 2019, 21, 53. [Google Scholar] [CrossRef] [Green Version]
- Arenja, N.; Riffel, J.H.; Fritz, T.; André, F.; aus dem Siepen, F.; Mueller-Hennessen, M.; Giannitsis, E.; Katus, H.A.; Friedrich, M.G.; Buss, S.J. Diagnostic and Prognostic Value of Long-Axis Strain and Myocardial Contraction Fraction Using Standard Cardiovascular MR Imaging in Patients with Nonischemic Dilated Cardiomyopathies. Radiology 2017, 283, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Arenja, N.; Buss, S.J. Myocardial Contraction Fraction Performance in Left Ventricular Hypertrophy. Curr. Trends Clin. Med. Imaging 2019, 2, 555622. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z.; Zhao, L.; Chen, X.; He, S. Myocardial contraction fraction predicts mortality for patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 17026. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.J.; Hoeger, C.W.; Latif, F.; Takayama, H.; Ginns, J.; Maurer, M.S. Myocardial Contraction Fraction Predicts Cardiovascular Events in Patients With Hypertrophic Cardiomyopathy and Normal Ejection Fraction. J. Card. Fail. 2019, 25, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Kowallick, J.T.; Vieira, M.S.; Kutty, S.; Lotz, J.; Hasenfuß, G.; Chiribiri, A.; Schuster, A. Left Atrial Performance in the Course of Hypertrophic Cardiomyopathy: Relation to Left Ventricular Hypertrophy and Fibrosis. Investig. Radiol. 2017, 52, 177–185. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, G.; Jiang, Y.; Song, L.; Zhao, S.; Lu, M. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: A feasibility and reproducibility study. J. Cardiovasc. Magn. Reson. 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Samiei, S.; Venkatesh, B.A.; Opdahl, A.; Helle-Valle, T.M.; Zareian, M.; Almeida, A.L.C.; Choi, E.-Y.; Wu, C.; Alonso, A.; et al. CMR-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Penela, D.; Sorgente, A.; Cappato, R. State-of-the-Art Treatments for Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. J. Clin. Med. 2021, 10, 3025. [Google Scholar] [CrossRef]
- Ziółkowska, L.; Mazurkiewicz, Ł.; Petryka, J.; Kowalczyk-Domagała, M.; Boruc, A.; Bieganowska, K.; Ciara, E.; Piekutowska-Abramczuk, D.; Śpiewak, M.; Miśko, J.; et al. The Indices of Cardiovascular Magnetic Resonance Derived Atrial Dynamics May Improve the Contemporary Risk Stratification Algorithms in Children with Hypertrophic Cardiomyopathy. J. Clin. Med. 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Cardiac MRI—Circle Cardiovascular Imaging. Available online: https://www.circlecvi.com/cvi42/cardiac-mri/ (accessed on 22 November 2021).
- Fatema, K.; Barnes, M.E.; Bailey, K.R.; Abhayaratna, W.P.; Cha, S.; Seward, J.B.; Tsang, T.S.M. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: A prospective study. Eur. J. Echocardiogr. 2008, 10, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.; Fovargue, L.; Tøndel, K.; Porter, B.; Sieniewicz, B.; Gould, J.; Rinaldi, C.A.; Ismail, T.; Chiribiri, A.; Carr-White, G. The emerging role of cardiac magnetic resonance imaging in the evaluation of patients with HFpEF. Curr. Heart Fail. Rep. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śpiewak, M.; Kłopotowski, M.; Mazurkiewicz, Ł.; Kowalik, E.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Klisiewicz, A.; Witkowski, A.; Marczak, M. Predictors of right ventricular function and size in patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 21054. [Google Scholar] [CrossRef]
- Rosu, R.O.; Lupsor, A.; Necula, A.; Cismaru, G.; Cainap, S.S.; Iacob, D.; Lazea, C.; Cismaru, A.; Negru, A.G.; Pop, D.; et al. Anatomical-MRI Correlations in Adults and Children with Hypertrophic Cardiomyopathy. Diagnostics 2022, 12, 489. [Google Scholar] [CrossRef]
- Śpiewak, M.; Kłopotowski, M.; Kowalik, E.; Mazurkiewicz, Ł.; Kożuch, K.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Witkowski, A.; Klisiewicz, A.; Marczak, M. Comparison of mitral regurgitation severity assessments based on magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Sci. Rep. 2021, 11, 19902. [Google Scholar] [CrossRef]






| Parameter | NCP | CP | All Patients | p Value |
|---|---|---|---|---|
| Number of patients, n | 26 | 62 | 88 | |
| age (years) | 60 (56–68) | 61 (46–70) | 60 (52–70) | 0.9054 |
| BSA (m2) | 2.0 (1.9–2.2) | 1.9 (1.8–2.1) | 1.9 (1.8–2.1) | 0.0350 |
| BMI (kg/m2) | 29 (25–33) | 29 (26–31) | 29 (26–31) | 0.8370 |
| HCM risk score (%) | 3.3 (1.8–4.6) | 2.6 (1.4–4.0) | 2.9 (1.5–4.2) | 0.3918 |
| Male sex, n (%) | 21 (80%) | 33 (53%) | 54 (61%) | 0.02912 |
| LV CMR parameters | ||||
| LVEDV (mL) | 172 (153–257) | 140 (126–166) | 150 (128–179) | 0.0005 |
| LVESV (mL) | 86 (61–144) | 38 (27–47) | 44 (30–64) | <0.0001 |
| SV (mL) | 93 (72–104) | 105 (92–121) | 102 (88–116) | 0.0042 |
| CO (L/min) | 7 (5–7) | 7 (5–7) | 7 (5–7) | 0.7316 |
| LV mass ((g) | 241 (197–309) | 174 (149–210) | 194 (157–243) | 0.0009 |
| LVMT (mm) | 20 (17–23) | 20 (17–22) | 20 (17–22) | 0.54 |
| Right Ventricle | ||||
| RVMT (mm) | 4.5 (3–6) | 3 (2–4) | 3 (2–5) | 0.0013 |
| Volume–time curve | ||||
| PER (mL/s) | 500 (370–563) | 479 (428–605) | 491 (428–586) | 0.3800 |
| PET (ms) | 128 (114–149) | 110 (91–125) | 115 (100–131) | 0.0012 |
| PFR (mL/s) | 360 (290–438) | 362 (305–437) | 362 (299–438) | 0.6706 |
| PFT (s) | 521 (475–690) | 565 (490–875) | 559 477–801) | 0.0924 |
| Late Gadolinium Enhancement | ||||
| LGE% (%) | 3.5 (1.2–8.8) | 1 (0–2.8) | 2 (0–4) | 0.0010 |
| LGE volume (mL) | 9 (3–20) | 2 (0–5) | 3 (1–9) | 0.0004 |
| Indexed (to BMI) and complex parameters | ||||
| LVEDVI | 87 (75–120) | 75 (65–83) | 79 (68–90) | 0.0041 |
| LVESVI | 39 (29–67) | 18 (14–25) | 24 (17–31) | <0.0001 |
| LVMI | 111 (101–150) | 93 (80–113) | 98 (83–120) | <0.0001 |
| PFR/LVEDV | 2 (1.5–2.5) | 2.5 (2,2–3) | 2.4 (2–2,9) | 0.0004 |
| PFR/SV | 3.9 (3.4–4.7) | 3.3 (3–3.9) | 3.6 (3–4.2) | 0.0024 |
| Wall to volume ratio | 0.23 (0.17–0.28) | 0.27 (0.21–0.31) | 0.26 (0.2–0.31) | 0.0401 |
| LVEDV/EDM | 0.75 (0.65–0.89) | 0.79 (0.65–0.93) | 0.78 (0.65–0.91) | 0.7454 |
| IMWT | 10 (9–12) | 10 (9–11) | 10 (9–11) | 0.8227 |
| MCF | 40% (32–53) | 63% (52–72) | 56% (42–68) | <0.0001 |
| LVGFI | 26 (19–31) | 41 (36–45) | 38 (30–44) | <0.0001 |
| Left Atrium | ||||
| LA 3Ch dimension (mm) | 40 (36–49) | 39 (37–44) | 39 (367–46) | 0.3726 |
| LAA max 4Ch (cm2) | 32 (24–35) | 28 (24–33) | 28 (24–34) | 0.2119 |
| LAL max 4Ch (mm) | 71 (57–79) | 62 (57–69) | 64 (57–72) | 0.0242 |
| LAA min 4Ch (cm2) | 22 (18–27) | 17 (14–22) | 18 (15–25) | 0.0090 |
| LAL min 4Ch (mm) | 61 (51–74) | 53 (44–59) | 54 (47–63) | 0.0030 |
| LAA max 2Ch (cm2) | 26 (24–34) | 25 (23–28) | 25 (23–31) | 0.1244 |
| LAL max 2Ch (mm) | 63 (58–72) | 61 (56–65) | 61 (57–68) | 0.1397 |
| LAA min 2Ch (cm2) | 19 (16–27) | 16 (13–20) | 17 (13–22) | 0.0075 |
| LAL min 2Ch (mm) | 53 (48–64) | 50 (43–54) | 51 (45–58) | 0.0263 |
| LAV max (mL) | 118 (79–135) | 94 (78–120) | 97 (78–125) | 0.2665 |
| LAV min (mL) | 67 (44–92) | 44 (34–62) | 47 (36–74) | 0.0089 |
| LAEF (%) | 38 (26–43) | 53 (42–60) | 48 (37–57) | 0.0001 |
| LAVI (mL/m2) | 58 (36–69) | 49 (41–62) | 51 (41–65) | 0.4027 |
| LVOT and mitral apparatus | ||||
| LVOT (mm) | 21 (17–24) | 15 (10–17) | 16 (13–20) | <0.0001 |
| Ao (mm) | 26 (23–28) | 23 (21–25) | 23 (21–26) | 0.0025 |
| LVOT/Ao | 0.86 (0.77–0.89) | 0.65 (0.45–0.75) | 0.72 (0.58–0.83) | <0.0001 |
| AML (mm) | 26 (23–30) | 24 (22–26) | 25 (22–27) | 0.1925 |
| PML (mm) | 14 (13–16) | 14 (13–16) | 14 (13–16) | 0.8585 |
| AML/LVOT | 1.27 (1.08–1.45) | 1.56 (1.33–2.5) | 1.44 (1.26–2.07) | <0.0001 |
| LVOTO visible in 3Ch | 0% | 20 (32%) | 20 (23%) | 0.0010 |
| LV Segment | Mean Thickness | LGE Presence | ||||
|---|---|---|---|---|---|---|
| NCP | CP | p Value | NCP | CP | p Value | |
| 1 | 14 | 12 | 0.0160 | 10 (39%) | 18 (29%) | 0.3862 |
| 2 | 14 | 14 | 0.5491 | 18 (69%) | 30 (48%) | 0.0732 |
| 3 | 14 | 14 | 0.7420 | 19 (73%) | 21 (34%) | 0.0008 |
| 4 | 13 | 12 | 0.2454 | 8 (31%) | 11 (18%) | 0.1754 |
| 5 | 12 | 12 | 0.4928 | 10 (39%) | 13 (21%) | 0.0884 |
| 6 | 11 | 11 | 0.3750 | 6 (23%) | 5 (8%) | 0.0520 |
| 7 | 12 | 10 | 0.0176 | 10 (39%) | 11 (18%) | 0.0375 |
| 8 | 13 | 10 | 0.0027 | 15 (58%) | 21 (34%) | 0.0381 |
| 9 | 15 | 11 | 0.0004 | 15 (58%) | 27 (44%) | 0.2255 |
| 10 | 14 | 11 | 0.0031 | 13 (50%) | 15 (24%) | 0.0177 |
| 11 | 13 | 10 | 0.0346 | 7 (27%) | 9 (15%) | 0.1686 |
| 12 | 11 | 10 | 0.1372 | 3 (12%) | 5 (8%) | 0.6050 |
| 13 | 13 | 10 | 0.1222 | 5 (19%) | 8 (13%) | 0.4453 |
| 14 | 11 | 10 | 0.0621 | 2 (8%) | 9 (15%) | 0.3772 |
| 15 | 11 | 10 | 0.0396 | 4 (15%) | 7 (11%) | 0.5962 |
| 16 | 12 | 10 | 0.1244 | 3 (12%) | 5 (8%) | 0.6050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachera, M.; Przybyło, P.; Sznajder, K.; Gierlotka, M. Comparison of Nonclassic and Classic Phenotype of Hypertrophic Cardiomyopathy Focused on Prognostic Cardiac Magnetic Resonance Parameters: A Single-Center Observational Study. Diagnostics 2022, 12, 1104. https://doi.org/10.3390/diagnostics12051104
Stachera M, Przybyło P, Sznajder K, Gierlotka M. Comparison of Nonclassic and Classic Phenotype of Hypertrophic Cardiomyopathy Focused on Prognostic Cardiac Magnetic Resonance Parameters: A Single-Center Observational Study. Diagnostics. 2022; 12(5):1104. https://doi.org/10.3390/diagnostics12051104
Chicago/Turabian StyleStachera, Magdalena, Paweł Przybyło, Katarzyna Sznajder, and Marek Gierlotka. 2022. "Comparison of Nonclassic and Classic Phenotype of Hypertrophic Cardiomyopathy Focused on Prognostic Cardiac Magnetic Resonance Parameters: A Single-Center Observational Study" Diagnostics 12, no. 5: 1104. https://doi.org/10.3390/diagnostics12051104
APA StyleStachera, M., Przybyło, P., Sznajder, K., & Gierlotka, M. (2022). Comparison of Nonclassic and Classic Phenotype of Hypertrophic Cardiomyopathy Focused on Prognostic Cardiac Magnetic Resonance Parameters: A Single-Center Observational Study. Diagnostics, 12(5), 1104. https://doi.org/10.3390/diagnostics12051104






