MRI-Based Radiomic Features Help Identify Lesions and Predict Histopathological Grade of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition Protocol
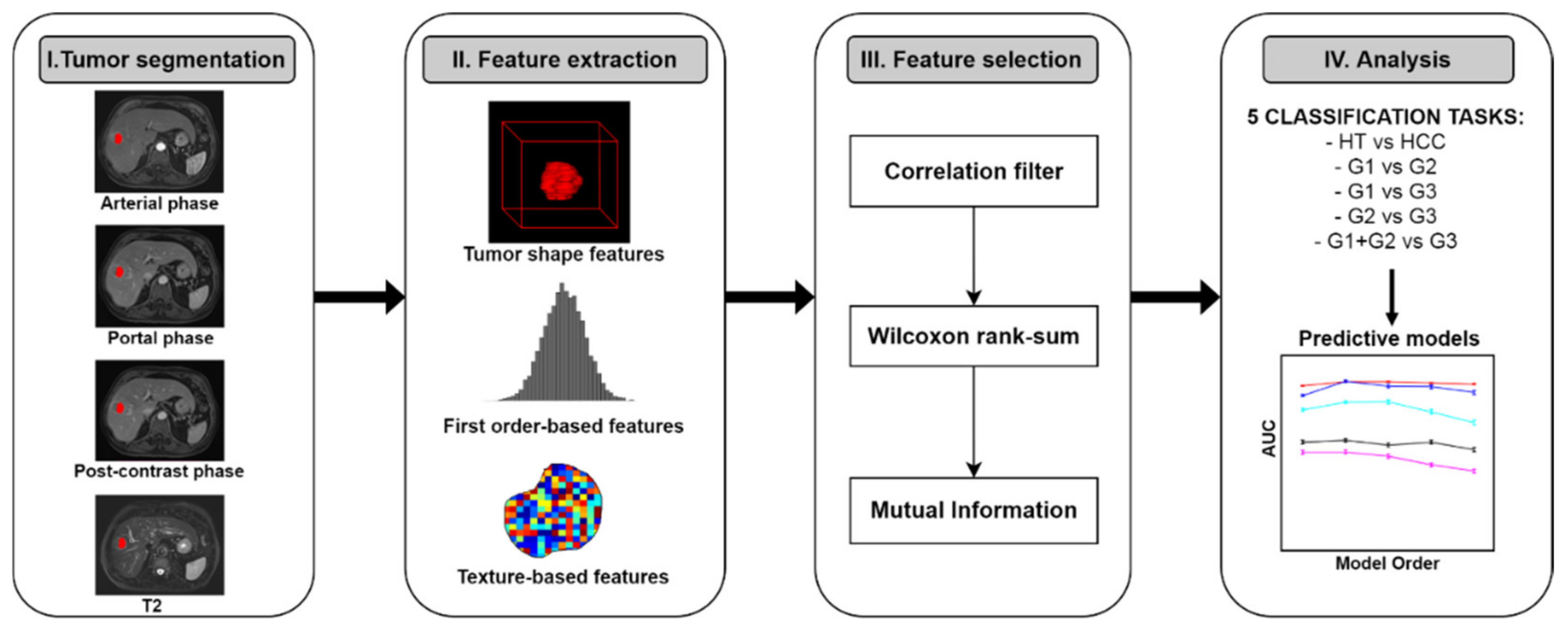
2.3. Image Preprocessing and 3D ROIs Segmentations
2.4. Radiomic Analysis
2.4.1. Radiomic Features Extraction
2.4.2. Radiomic Feature Selection
2.4.3. Multivariable Prediction Models Building and Analysis
3. Results
3.1. Radiomic Features Selection
3.2. Multivariable Prediction Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ozer Etik, D.; Suna, N.; Boyacioglu, A.S. Management of Hepatocellular Carcinoma: Prevention, Surveillance, Diagnosis, and Staging. Exp. Clin. Transplant. 2017, 15, 31–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crissien, A.M.; Frenette, C. Current Management of Hepatocellular Carcinoma. Gastroenterol. Hepatol. N. Y. 2014, 10, 153–161. [Google Scholar] [PubMed]
- Martins-Filho, S.N.; Paiva, C.; Azevedo, R.S.; Alves, V.A.F. Histological Grading of Hepatocellular Carcinoma—A Systematic Review of Literature. Front. Med. 2017, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Atupelage, C.; Nagahashi, H.; Kimura, F.; Yamaguchi, M.; Tokiya, A.; Hashiguchi, A.; Sakamoto, M. Computational Hepatocellular Carcinoma Tumor Grading Based on Cell Nuclei Classification. J. Med. Imaging 2014, 1, 034501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasaki, K.; Matsuda, M.; Ohkura, Y.; Kawamura, Y.; Inoue, M.; Hashimoto, M.; Ikeda, K.; Kumada, H.; Watanabe, G. In Hepatocellular Carcinomas, Any Proportion of Poorly Differentiated Components Is Associated with Poor Prognosis after Hepatectomy. World J. Surg. 2014, 38, 1147–1153. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Cho, S.G.; Lee, K.Y.; Kim, J.M.; Lee, J.W. Association between Relative Liver Enhancement on Gadoxetic Acid Enhanced Magnetic Resonance Images and Histologic Grade of Hepatocellular Carcinoma. Medicine 2017, 96, e7580. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Gleisner, A.L.; Anders, R.A.; Assumpcao, L.; Maley, W.; Choti, M.A. Preoperative Assessment of Hepatocellular Carcinoma Tumor Grade Using Needle Biopsy: Implications for Transplant Eligibility. Ann. Surg. 2007, 245, 435–442. [Google Scholar] [CrossRef]
- Silva, M.A.; Hegab, B.; Hyde, C.; Guo, B.; Buckels, J.A.C.; Mirza, D.F. Needle Track Seeding Following Biopsy of Liver Lesions in the Diagnosis of Hepatocellular Cancer: A Systematic Review and Meta-Analysis. Gut 2008, 57, 1592–1596. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Zhang, X.; Li, J.; Wei, X.; Cheng, J.; Ling, Q.; Xie, H.; Zhou, L.; Xu, X.; et al. Diagnostic Value of Preoperative Needle Biopsy for Tumor Grading Assessment in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0144216. [Google Scholar] [CrossRef]
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology 2014, 273, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Lee, J.-M.; Sirlin, C.B. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part I. Development, Growth, and Spread: Key Pathologic and Imaging Aspects. Radiology 2014, 272, 635–654. [Google Scholar] [CrossRef]
- Coskun, M. Hepatocellular Carcinoma in the Cirrhotic Liver: Evaluation Using Computed Tomography and Magnetic Resonance Imaging. Exp. Clin. Transpl. 2017, 15, 36–44. [Google Scholar] [CrossRef]
- Niendorf, E.; Spilseth, B.; Wang, X.; Taylor, A. Contrast Enhanced MRI in the Diagnosis of HCC. Diagnostics 2015, 5, 383–398. [Google Scholar] [CrossRef]
- Kobayashi, S.; Matsui, O.; Gabata, T.; Koda, W.; Minami, T.; Ryu, Y.; Kozaka, K.; Kitao, A. Relationship between Signal Intensity on Hepatobiliary Phase of Gadolinium Ethoxybenzyl Diethylenetriaminepentaacetic Acid (Gd-EOB-DTPA)-Enhanced MR Imaging and Prognosis of Borderline Lesions of Hepatocellular Carcinoma. Eur. J. Radiol. 2012, 81, 3002–3009. [Google Scholar] [CrossRef]
- Chan, M.V.; Huo, Y.R.; Trieu, N.; Mitchelle, A.; George, J.; He, E.; Lee, A.U.; Chang, J.; Yang, J. Non-Contrast MRI for Hepatocellular Carcinoma Detection: A Systematic Review and Meta-Analysis—A Potential Surveillance Tool? Clin. Gastroenterol. Hepatol. 2021, 20, 44–56. [Google Scholar] [CrossRef]
- Lan, H.; Lin, G.; Zhong, W. A Meta-analysis of the Added Value of Diffusion Weighted Imaging in Combination with Contrast-enhanced Magnetic Resonance Imaging for the Diagnosis of Small Hepatocellular Carcinoma Lesser or Equal to 2 Cm. Oncol. Lett. 2020, 20, 2739–2748. [Google Scholar] [CrossRef]
- Tang, A.; Singal, A.G.; Mitchell, D.G.; Hecht, E.M.; Fowler, K.J.; Kulik, L.; Parikh, N.D.; Kono, Y.; Sirlin, C.B. Introduction to the Liver Imaging Reporting and Data System for Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 1228–1238. [Google Scholar] [CrossRef]
- Marks, R.M.; Masch, W.R.; Chernyak, V. LI-RADS: Past, Present, and Future, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am. J. Roentgenol. 2021, 216, 295–304. [Google Scholar] [CrossRef]
- Chang, W.-C.; Chen, R.-C.; Chou, C.-T.; Lin, C.-Y.; Yu, C.-Y.; Liu, C.-H.; Chou, J.-M.; Hsu, H.-H.; Huang, G.-S. Histological Grade of Hepatocellular Carcinoma Correlates with Arterial Enhancement on Gadoxetic Acid-Enhanced and Diffusion-Weighted MR Images. Abdom. Imaging 2014, 39, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Park, M.-S.; Jeon, H.-M.; Kim, Y.-E.; Chung, W.-S.; Chung, Y.E.; Kim, M.-J.; Kim, K.W. Prediction of the Histopathological Grade of Hepatocellular Carcinoma Using Qualitative Diffusion-Weighted, Dynamic, and Hepatobiliary Phase MRI. Eur. Radiol. 2012, 22, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Brancato, V.; Aiello, M.; Basso, L.; Monti, S.; Palumbo, L.; Di Costanzo, G.; Salvatore, M.; Ragozzino, A.; Cavaliere, C. Evaluation of a Multiparametric MRI Radiomic-Based Approach for Stratification of Equivocal PI-RADS 3 and Upgraded PI-RADS 4 Prostatic Lesions. Sci. Rep. 2021, 11, 643. [Google Scholar] [CrossRef]
- Wang, X.-H.; Long, L.-H.; Cui, Y.; Jia, A.Y.; Zhu, X.-G.; Wang, H.-Z.; Wang, Z.; Zhan, C.-M.; Wang, Z.-H.; Wang, W.-H. MRI-Based Radiomics Model for Preoperative Prediction of 5-Year Survival in Patients with Hepatocellular Carcinoma. Br. J. Cancer 2020, 122, 978–985. [Google Scholar] [CrossRef]
- Ji, G.-W.; Zhu, F.-P.; Xu, Q.; Wang, K.; Wu, M.-Y.; Tang, W.-W.; Li, X.-C.; Wang, X.-H. Machine-Learning Analysis of Contrast-Enhanced CT Radiomics Predicts Recurrence of Hepatocellular Carcinoma after Resection: A Multi-Institutional Study. EBioMedicine 2019, 50, 156–165. [Google Scholar] [CrossRef]
- Ning, P.; Gao, F.; Hai, J.; Wu, M.; Chen, J.; Zhu, S.; Wang, M.; Shi, D. Application of CT Radiomics in Prediction of Early Recurrence in Hepatocellular Carcinoma. Abdom. Radiol. 2020, 45, 64–72. [Google Scholar] [CrossRef]
- Kloth, C.; Thaiss, W.M.; Kärgel, R.; Grimmer, R.; Fritz, J.; Ioanoviciu, S.D.; Ketelsen, D.; Nikolaou, K.; Horger, M. Evaluation of Texture Analysis Parameter for Response Prediction in Patients with Hepatocellular Carcinoma Undergoing Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE) Using Biphasic Contrast-Enhanced CT Image Data. Acad. Radiol. 2017, 24, 1352–1363. [Google Scholar] [CrossRef]
- Lewis, S.; Hectors, S.; Taouli, B. Radiomics of Hepatocellular Carcinoma. Abdom. Radiol. 2021, 46, 111–123. [Google Scholar] [CrossRef]
- Borhani, A.A.; Catania, R.; Velichko, Y.S.; Hectors, S.; Taouli, B.; Lewis, S. Radiomics of Hepatocellular Carcinoma: Promising Roles in Patient Selection, Prediction, and Assessment of Treatment Response. Abdom. Radiol. 2021, 46, 3674–3685. [Google Scholar] [CrossRef]
- Castaldo, A.; De Lucia, D.R.; Pontillo, G.; Gatti, M.; Cocozza, S.; Ugga, L.; Cuocolo, R. State of the Art in Artificial Intelligence and Radiomics in Hepatocellular Carcinoma. Diagnostics 2021, 11, 1194. [Google Scholar] [CrossRef]
- Miranda Magalhaes Santos, J.M.; Clemente Oliveira, B.; Araujo-Filho, J.d.A.B.; Assuncao, A.N., Jr.; de M. Machado, F.A.; Carlos Tavares Rocha, C.; Horvat, J.V.; Menezes, M.R.; Horvat, N. State-of-the-Art in Radiomics of Hepatocellular Carcinoma: A Review of Basic Principles, Applications, and Limitations. Abdom. Radiol. 2020, 45, 342–353. [Google Scholar] [CrossRef]
- Harding-Theobald, E.; Louissaint, J.; Maraj, B.; Cuaresma, E.; Townsend, W.; Mendiratta-Lala, M.; Singal, A.G.; Su, G.L.; Lok, A.S.; Parikh, N.D. Systematic Review: Radiomics for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Aliment. Pharm. 2021, 54, 890–901. [Google Scholar] [CrossRef]
- Masokano, I.B.; Liu, W.; Xie, S.; Marcellin, D.F.H.; Pei, Y.; Li, W. The Application of Texture Quantification in Hepatocellular Carcinoma Using CT and MRI: A Review of Perspectives and Challenges. Cancer Imaging 2020, 20, 67. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Ouhmich, F.; Gonzalez-Cabrera, C.; Felli, E.; Saviano, A.; Agnus, V.; Savadjiev, P.; Baumert, T.F.; Pessaux, P.; Marescaux, J.; et al. Radiomics in Hepatocellular Carcinoma: A Quantitative Review. Hepatol. Int. 2019, 13, 546–559. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, M.; Liu, Y.; Jiang, N.; Meng, Q.; Wang, J.; Yao, Z.; Gan, W.; Dai, H. Texture Analysis of MR Images to Identify the Differentiated Degree in Hepatocellular Carcinoma: A Retrospective Study. BMC Cancer 2020, 20, 611. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Wang, K.; Chen, S.; Wang, G.; Liu, Z.; Liang, C. Malignancy Characterization of Hepatocellular Carcinomas Based on Texture Analysis of Contrast-Enhanced MR Images. J. Magn. Reson. Imaging 2017, 45, 1476–1484. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Wang, S.; Li, H.; Zhang, C.; Yin, S.; Xie, C.; Dai, Y. Radiomics Analysis of Susceptibility Weighted Imaging for Hepatocellular Carcinoma: Exploring the Correlation between Histopathology and Radiomics Features. MRMS 2021, 20, 253–263. [Google Scholar] [CrossRef]
- Shi, G.; Han, X.; Wang, Q.; Ding, Y.; Liu, H.; Zhang, Y.; Dai, Y. Evaluation of Multiple Prognostic Factors of Hepatocellular Carcinoma with Intra-Voxel Incoherent Motions Imaging by Extracting the Histogram Metrics. CMAR 2020, 12, 6019–6031. [Google Scholar] [CrossRef]
- Erickson, B.J.; Kirk, S.; Lee, Y.; Bathe, O.; Kearns, M.; Gerdes, C.; Rieger-Christ, K.; Lemmerman, J. Radiology Data from The Cancer Genome Atlas Liver Hepatocellular Carcinoma [TCGA-LIHC] Collection. Cancer Imaging Arch. 2016. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- TCGA-LIHC. Available online: https://wiki.cancerimagingarchive.net/display/Public/TCGA-LIHC (accessed on 30 September 2021).
- Weinreb, J.; Kumari, S.; Phillips, G.; Pochaczevsky, R. Portal Vein Measurements by Real-Time Sonography. AJR Am. J. Roentgenol. 1982, 139, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Silva, R.A.; de Queiroz, L.C.; Azeredo, L.M.; Silva, L.C.d.S.; Lambertucci, J.R. Ultrasound in Schistosomiasis Mansoni. Mem. Inst. Oswaldo. Cruz. 2010, 105, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Geleto, G.; Getnet, W.; Tewelde, T. Mean Normal Portal Vein Diameter Using Sonography among Clients Coming to Radiology Department of Jimma University Hospital, Southwest Ethiopia. Ethiop. J. Health Sci. 2016, 26, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.D. AJCC Cancer Staging Manual; American Joint Committee on Cancer: Chicago, IL, USA, 1997. [Google Scholar]
- Green, F.; Page, D.; Fleming, I.D.; Fritz, A.; Balch, C.; Haller, D.; Morrow, M. Others AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2002; Volume 6, pp. 32–34. [Google Scholar]
- Scalco, E.; Belfatto, A.; Mastropietro, A.; Rancati, T.; Avuzzi, B.; Messina, A.; Valdagni, R.; Rizzo, G. T2w-MRI Signal Normalization Affects Radiomics Features Reproducibility. Med. Phys. 2020, 47, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Duron, L.; Balvay, D.; Vande Perre, S.; Bouchouicha, A.; Savatovsky, J.; Sadik, J.-C.; Thomassin-Naggara, I.; Fournier, L.; Lecler, A. Gray-Level Discretization Impacts Reproducible MRI Radiomics Texture Features. PLoS ONE 2019, 14, e0213459. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Vergara, J.R.; Estévez, P.A. A Review of Feature Selection Methods Based on Mutual Information. Neural. Comput. Applic. 2014, 24, 175–186. [Google Scholar] [CrossRef]
- Zaffalon, M.; Hutter, M. Robust Feature Selection by Mutual Information Distributions. arXiv 2014, arXiv:0206006. [Google Scholar]
- Roffo, G. Feature Selection Library (MATLAB Toolbox). arXiv 2018, arXiv:1607.01327. [Google Scholar]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A Radiomics Model from Joint FDG-PET and MRI Texture Features for the Prediction of Lung Metastases in Soft-Tissue Sarcomas of the Extremities. Phys. Med. Biol. 2015, 60, 5471–5496. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Statist. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. Improvements on Cross-Validation: The .632+ Bootstrap Method. J. Am. Stat. Assoc. 1997, 92, 548. [Google Scholar] [CrossRef]
- Sahiner, B.; Chan, H.-P.; Hadjiiski, L. Classifier Performance Prediction for Computer-Aided Diagnosis Using a Limited Dataset: Classifier Performance Prediction Using a Limited Data Set. Med. Phys. 2008, 35, 1559–1570. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, H.; Lu, B.; You, J.; Piao, J.; Yang, P.; Li, J. Differentiation of Small Hepatocellular Carcinoma From Dysplastic Nodules in Cirrhotic Liver: Texture Analysis Based on MRI Improved Performance in Comparison Over Gadoxetic Acid-Enhanced MR and Diffusion-Weighted Imaging. Front. Oncol. 2020, 9, 1382. [Google Scholar] [CrossRef]
- Stocker, D.; Marquez, H.P.; Wagner, M.W.; Raptis, D.A.; Clavien, P.-A.; Boss, A.; Fischer, M.A.; Wurnig, M.C. MRI Texture Analysis for Differentiation of Malignant and Benign Hepatocellular Tumors in the Non-Cirrhotic Liver. Heliyon 2018, 4, e00987. [Google Scholar] [CrossRef]
- Starmans, M.; Klein, S.; van der Voort, S.R.; Thomeer, M.G.; Miclea, R.L.; Niessen, W.J. Classification of Malignant and Benign Liver Tumors Using a Radiomics Approach. In Image Processing, Proceedings of the Medical Imaging 2018, Houston, TX, USA, 2 March 2018; Angelini, E.D., Landman, B.A., Eds.; SPIE: Urberin, Washington, DC, USA, 2018; p. 48. [Google Scholar]
- Hectors, S.J.; Wagner, M.; Bane, O.; Besa, C.; Lewis, S.; Remark, R.; Chen, N.; Fiel, M.I.; Zhu, H.; Gnjatic, S.; et al. Quantification of Hepatocellular Carcinoma Heterogeneity with Multiparametric Magnetic Resonance Imaging. Sci. Rep. 2017, 7, 2452. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Schroeder, J.L.; Huang, P.; Chen, Y.; Coquia, S.F.; Kawamoto, S.; Fishman, E.K. Preliminary Data Using Computed Tomography Texture Analysis for the Classification of Hypervascular Liver Lesions: Generation of a Predictive Model on the Basis of Quantitative Spatial Frequency Measurements—A Work in Progress. J. Comput. Assist. Tomogr. 2015, 39, 383–395. [Google Scholar] [CrossRef]
- Choi, J.M.; Yu, J.-S.; Cho, E.-S.; Kim, J.H.; Chung, J.-J. Texture Analysis of Hepatocellular Carcinoma on Magnetic Resonance Imaging: Assessment for Performance in Predicting Histopathologic Grade. J. Comput. Assist. Tomogr. 2020, 44, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Hectors, S.J.; Lewis, S.; Besa, C.; King, M.J.; Said, D.; Putra, J.; Ward, S.; Higashi, T.; Thung, S.; Yao, S.; et al. MRI Radiomics Features Predict Immuno-Oncological Characteristics of Hepatocellular Carcinoma. Eur. Radiol. 2020, 30, 3759–3769. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, C.; Zhang, Y.; Wang, Z. Magnetic Resonance Radiomics Signatures for Predicting Poorly Differentiated Hepatocellular Carcinoma: A SQUIRE-Compliant Study. Medicine 2021, 100, e25838. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, A.; Cui, J.; Chen, A.; Song, Q.; Xie, L. Radiomics-Based Classification of Hepatocellular Carcinoma and Hepatic Haemangioma on Precontrast Magnetic Resonance Images. BMC Med. Imaging 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Zhang, H.; He, X.; Liu, Y.; Zhou, J.; Guo, D. Texture Analysis Based on Preoperative Magnetic Resonance Imaging (MRI) and Conventional MRI Features for Predicting the Early Recurrence of Single Hepatocellular Carcinoma after Hepatectomy. Acad. Radiol. 2019, 26, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Y.; Huang, W.; Li, H.; Zhu, J.; Li, W.; Li, B. Texture-Based Classification of Different Single Liver Lesion Based on SPAIR T2W MRI Images. BMC Med. Imaging 2017, 17, 42. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, B.; Wang, S.; Wang, L.; Wu, J.; Ma, X.; Zhao, X. Model-based Three-dimensional Texture Analysis of Contrast-enhanced Magnetic Resonance Imaging as a Potential Tool for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Oncol. Lett. 2019, 18, 720–732. [Google Scholar] [CrossRef]
- Forghani, R.; Savadjiev, P.; Chatterjee, A.; Muthukrishnan, N.; Reinhold, C.; Forghani, B. Radiomics and Artificial Intelligence for Biomarker and Prediction Model Development in Oncology. Comput. Struct. Biotechnol. J. 2019, 17, 995–1008. [Google Scholar] [CrossRef]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine Learning Algorithm Validation with a Limited Sample Size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Norvell, D. Study Types and Bias—Don’t Judge a Study by the Abstract’s Conclusion Alone. Evid. Based Spine Care J. 2010, 1, 7–10. [Google Scholar] [CrossRef]
- Tripepi, G.; Jager, K.J.; Dekker, F.W.; Zoccali, C. Selection Bias and Information Bias in Clinical Research. Nephron. Clin. Pr. 2010, 115, c94–c99. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Initiative, for the I.B.S. Image Biomarker Standardisation Initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Parmar, C.; Rios Velazquez, E.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B.; et al. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Lei, L.; Yuan, G.; He, S. The Diagnostic Performance of Gadoxetic Acid Disodium-Enhanced Magnetic Resonance Imaging and Contrast-Enhanced Multi-Detector Computed Tomography in Detecting Hepatocellular Carcinoma: A Meta-Analysis of Eight Prospective Studies. Eur. Radiol. 2019, 29, 6519–6528. [Google Scholar] [CrossRef]
- Saltz, J.; Almeida, J.; Gao, Y.; Sharma, A.; Bremer, E.; DiPrima, T.; Saltz, M.; Kalpathy-Cramer, J.; Kurc, T. Towards Generation, Management, and Exploration of Combined Radiomics and Pathomics Datasets for Cancer Research. AMIA Jt. Summits Transl. Sci. Proc. 2017, 2017, 85–94. [Google Scholar]
- Gupta, R.; Kurc, T.; Sharma, A.; Almeida, J.S.; Saltz, J. The Emergence of Pathomics. Curr. Pathobiol. Rep. 2019, 7, 73–84. [Google Scholar] [CrossRef]
- Wu, M.; Tan, H.; Gao, F.; Hai, J.; Ning, P.; Chen, J.; Zhu, S.; Wang, M.; Dou, S.; Shi, D. Predicting the Grade of Hepatocellular Carcinoma Based on Non-Contrast-Enhanced MRI Radiomics Signature. Eur. Radiol. 2019, 29, 2802–2811. [Google Scholar] [CrossRef]
- Tselikas, L.; Sun, R.; Ammari, S.; Dercle, L.; Yevich, S.; Hollebecque, A.; Ngo-Camus, M.; Nicotra, C.; Deutsch, E.; Deschamps, F.; et al. Role of Image-Guided Biopsy and Radiomics in the Age of Precision Medicine. Chin. Clin. Oncol. 2019, 8, 57. [Google Scholar] [CrossRef]




| Clinical Characteristic | Value |
|---|---|
| Age (mean ± SD) | 57.8 ± 15.3 |
| Sex (n (%)) | |
| Male | 26 (68.4) |
| Female | 12 (31.6) |
| Risk factors (n (%)) | |
| HBV | 3 (7.9) |
| HBV|tobacco | 2 (5.3) |
| HCV | 5 (13.2) |
| HCV|tobacco | 2 (5.3) |
| HCV|alcohol | 1 (2.63) |
| Alcohol | 9 (23.7) |
| Tobacco | 1 (2.6) |
| Tobacco|BCP | 1 (2.6) |
| NAFLD | 2 (5.3) |
| Hemochromatosis | 1 (2.6) |
| No history of RF | 10 (26.3) |
| NA | 1 (2.6) |
| PH 1 (n (%)) | |
| Y | 9 (23.7) |
| N | 29 (76.3) |
| Histologic grade (n (%)) | |
| G1 | 7 (18.4) |
| G2 | 15 (39.5) |
| G3 | 16 (42.1) |
| AJCC stage 2 (n (%)) | |
| I | 15 (39.5) |
| II | 12 (31.6) |
| III | 10 (26.3) |
| IV | 1 (2.6) |
| Classification Task | Top 5 Selected Features |
|---|---|
| HCC/HT | T2 gldm Dependence Non Uniformity Normalized |
| T2 glszm Small Area Low Gray Level Emphasis | |
| T2 glrlm Long Run High Gray Level Emphasis | |
| ART firstorder Minimum | |
| ART gldm Large Dependence Low Gray Level Emphasis | |
| G1 + G2/G3 | PORT gldm Large Dependence Low Gray Level Emphasis |
| ART glszm Size Zone Non Uniformity Normalized | |
| PORT glcm Maximum Probability | |
| PORT glszm Small Area Low Gray Level Emphasis | |
| T2 glszm Low Gray Level Zone Emphasis | |
| G1/G2 | PORT ngtdm Strength |
| T2 gldm Low Gray Level Emphasis | |
| ART firstorder 10Percentile | |
| ART firstorder Skewness | |
| TARD firstorder Maximum | |
| G1/G3 | SHAPE Surface Volume Ratio |
| T2 gldm Large Dependence High Gray Level Emphasis | |
| PORT glcm Maximum Probability | |
| ART glcm Cluster Shade | |
| ART firstorder Skewness | |
| G2/G3 | PORT gldm Large Dependence Low Gray Level Emphasis |
| PORT glszm Zone Percentage | |
| PORT ngtdm Complexity | |
| PORT glszm Large Area Low Gray Level Emphasis | |
| TARD glrlm Long Run Low Gray Level Emphasis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancato, V.; Garbino, N.; Salvatore, M.; Cavaliere, C. MRI-Based Radiomic Features Help Identify Lesions and Predict Histopathological Grade of Hepatocellular Carcinoma. Diagnostics 2022, 12, 1085. https://doi.org/10.3390/diagnostics12051085
Brancato V, Garbino N, Salvatore M, Cavaliere C. MRI-Based Radiomic Features Help Identify Lesions and Predict Histopathological Grade of Hepatocellular Carcinoma. Diagnostics. 2022; 12(5):1085. https://doi.org/10.3390/diagnostics12051085
Chicago/Turabian StyleBrancato, Valentina, Nunzia Garbino, Marco Salvatore, and Carlo Cavaliere. 2022. "MRI-Based Radiomic Features Help Identify Lesions and Predict Histopathological Grade of Hepatocellular Carcinoma" Diagnostics 12, no. 5: 1085. https://doi.org/10.3390/diagnostics12051085
APA StyleBrancato, V., Garbino, N., Salvatore, M., & Cavaliere, C. (2022). MRI-Based Radiomic Features Help Identify Lesions and Predict Histopathological Grade of Hepatocellular Carcinoma. Diagnostics, 12(5), 1085. https://doi.org/10.3390/diagnostics12051085








