Predictors of Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Statistical Analysis
3. Results
3.1. Patients Characteristics
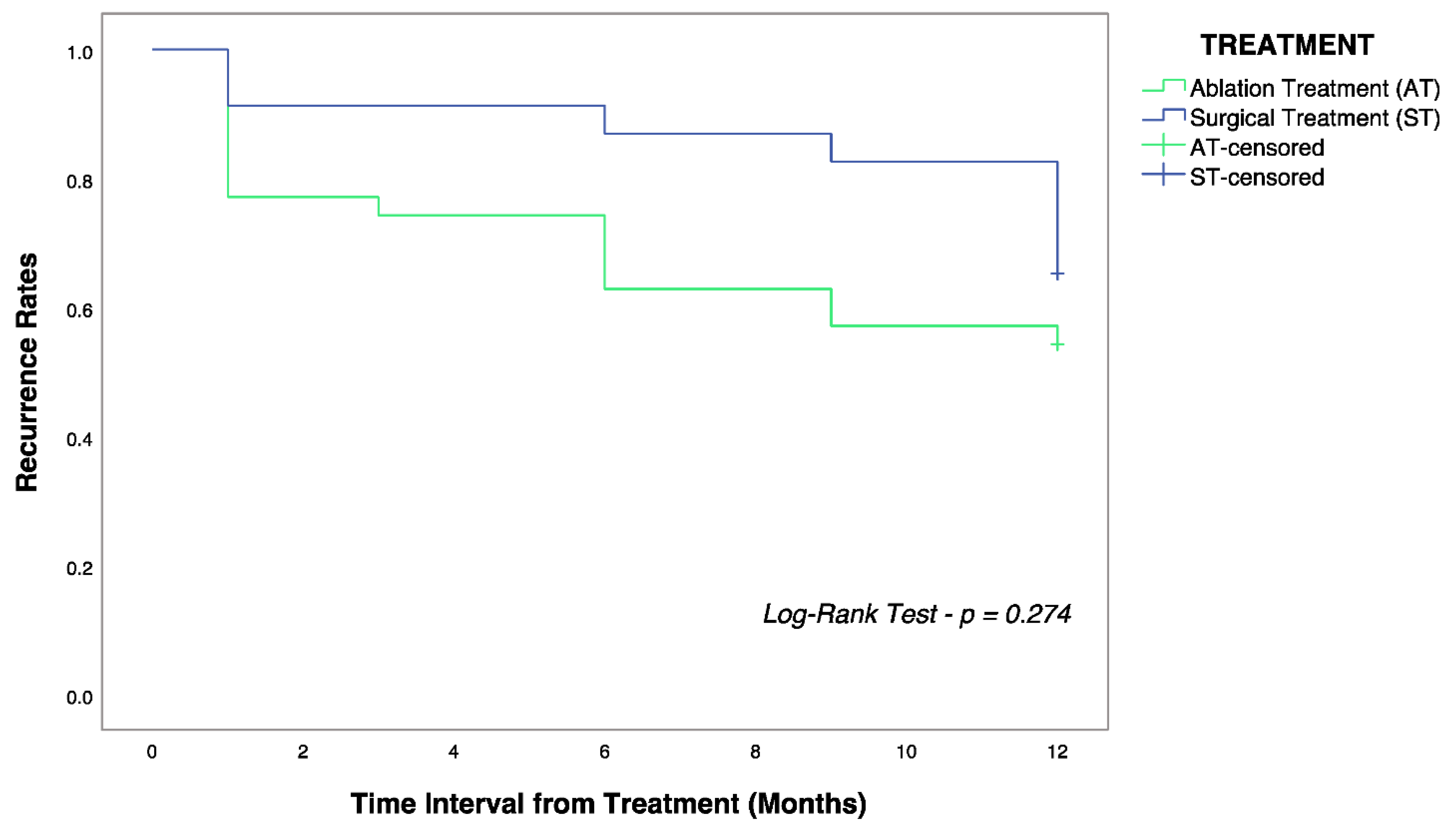
3.2. Analysis on HCC Recurrence
4. Discussion
4.1. Surgical Resection vs. Local Ablation
4.2. Male Gender
4.3. Tumor Dimension
4.4. α-Fetoprotein
4.5. Platelet Count
4.6. Lymphocyte-Monocyte Ratio
4.7. Neutrophil-Lymphocyte Ratio
4.8. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philips, C.A.; Rajesh, S.; Nair, D.C.; Ahamed, R.; Abduljaleel, J.K.; Augustine, P. Hepatocellular Carcinoma in 2021: An Exhaustive Update. Cureus 2021, 13, e19274. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Lange, N.; Dufour, J.F. Changing Epidemiology of HCC: How to Screen and Identify Patients at Risk? Dig. Dis. Sci. 2019, 64, 903–909. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef]
- Karagozian, R.; Derdák, Z.; Baffy, G. Obesity-associated mechanisms of hepatocarcinogenesis. Metab. Clin. Exp. 2014, 63, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Liver Italian Program (CLIP) Investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology 1998, 28, 751–755. [Google Scholar] [CrossRef]
- McGlynn, K.A.; London, W.T. The Global Epidemiology of Hepatocellular Carcinoma: Present and Future. Clin. Liver Dis. 2011, 15, 223–243. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Campigotto, M.; Giuffrè, M.; Colombo, A.; Visintin, A.; Aversano, A.; Budel, M.; Masutti, F.; Abazia, C.; Crocé, L.S. Comparison between hepatocellular carcinoma prognostic scores: A 10-year single-center experience and brief review of the current literature. World J. Hepatol. 2020, 12, 1239–1257. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.E.; de Lope, C.R.; Bruix, J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin. Liver Dis. 2010, 30, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Makuuchi, M.; Sano, K. The surgical approach to HCC: Our progress and results in Japan. Liver Transplant. 2004, 10, S46–S52. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, E.; Gan, J.; Song, X.; Bao, Z.; Zhang, H.; Chen, M. Long-term survival of hepatocellular carcinoma after percutaneous radiofrequency ablation guided by ultrasound. World J. Surg. Oncol. 2017, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yan, K.; Goldberg, S.N.; Ahmed, M.; Lee, J.C.; Wu, W.; Chen, M.H. Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J. Gastroenterol. 2016, 22, 2993–3005. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; KiyoshiHasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Meniconi, R.L.; Komatsu, S.; Perdigao, F.; Boëlle, P.Y.; Soubrane, O.; Scatton, O. Recurrent hepatocellular carcinoma: A Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery 2015, 157, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Ercolani, G.; Grazi, G.L.; Ravaioli, M.; Del Gaudio, M.; Gardini, A.; Cescon, M.; Cavallari, A. Liver Resection for Hepatocellular Carcinoma on Cirrhosis. Ann. Surg. 2003, 237, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.G.; Domenicali, M.; Giacomoni, P.; Dall’Aglioa, A.C.; Contia, F.; Borghia, A.; Bevilacquaa, V.; Napolia, L.; Miricia, F.; Cucchettib, A.; et al. Is there an association between commonly employed biomarkers of liver fibrosis and liver stiffness in the general population? Ann. Hepatol. 2020, 19, 380–387. [Google Scholar] [CrossRef]
- Tian, Y.; Lyu, H.; He, Y.; Xia, Y.; Li, J.; Shen, F. Comparison of Hepatectomy for Patients with Metabolic Syndrome-Related HCC and HBV-Related HCC. J. Gastrointest. Surg. 2018, 22, 615–623. [Google Scholar] [CrossRef]
- He, L.-L.; Liu, X.-L.; Zhang, S.; Li, M.G.; Wang, X.B.; Jiang, Y.Y.; Yang, Z.Y. Independent risk factors for disease recurrence after surgery in patients with hepatitis B virus-related hepatocellular carcinoma ≤3 cm in diameter. Gastroenterol. Rep. 2019, 7, 250–257. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Rong, W.; Li, Z.; Wu, F.; Liu, Y.; Wu, J. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: A non-randomized interventional clinical study. BMC Cancer 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Luo, P.; Wu, S.; Yu, Y.; Ming, X.; Li, S.; Zuo, X.; Tu, J. Current Status and Perspective Biomarkers in AFP Negative HCC: Towards Screening for and Diagnosing Hepatocellular Carcinoma at an Earlier Stage. Pathol. Oncol. Res. 2020, 26, 599–603. [Google Scholar] [CrossRef]
- Pascut, D.; Pratama, M.Y.; Gilardi, F.; Giuffrè, M.; Crocè, L.S.; Tiribelli, C. Weighted miRNA co-expression networks analysis identifies circulating miRNA predicting overall survival in hepatocellular carcinoma patients. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Bozzato, A.M.; Martingano, P.; Mucelli, R.A.P.; Cavallaro, M.F.M.; Cesarotto, M.; Marcello, C.; Tiribelli, C.; Pascut, D.; Pizzolato, R.; Mucelli, F.P.; et al. MicroRNAs Related to TACE Treatment Response: A Review of the Literature from a Radiological Point of View. Diagnostics 2022, 12, 374. [Google Scholar] [CrossRef]
- Gurian, E.; Di Silvestre, A.; Mitri, E.; Pascut, D.; Tiribelli, C.; Giuffrè, M.; Bonifacio, A. Repeated double cross-validation applied to the PCA-LDA classification of SERS spectra: A case study with serum samples from hepatocellular carcinoma patients. Anal. Bioanal. Chem. 2021, 413, 1303–1312. [Google Scholar] [CrossRef]
- Alhasan, A.; Cerny, M.; Olivié, D.; Billiard, J.S.; Bergeron, C.; Brown, K.; Tang, A. LI-RADS for CT diagnosis of hepatocellular carcinoma: Performance of major and ancillary features. Abdom. Radiol. 2019, 44, 517–528. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Moretti, R.; Giuffré, M.; Crocè, L.S.; Gazzin, S.; Tiribelli, C. Nonalcoholic Fatty Liver Disease and Altered Neuropsychological Functions in Patients with Subcortical Vascular Dementia. J. Pers. Med. 2022, 12, 1106. [Google Scholar] [CrossRef]
- Kishore, J.; Goel, M.; Khanna, P. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar] [CrossRef]
- Bauldry, S. Structural Equation Modeling. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 615–620. [Google Scholar] [CrossRef]
- Abd Elhafeez, S.; D’Arrigo, G.; Leonardis, D.; Fusaro, M.; Tripepi, G.; Roumeliotis, S. Methods to Analyze Time-to-Event Data: The Cox Regression Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 1302811. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, G.; Giuffrè, M.; Sambataro, D.; Palermo, A.; Vignigni, G.; Cesareo, R.; Di Bella, S. The Model for Early COvid-19 Recognition (MECOR) Score: A Proof-of-Concept for a Simple and Low-Cost Tool to Recognize a Possible Viral Etiology in Community-Acquired Pneumonia Patients during COVID-19 Outbreak. Diagnostics 2020, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, X.F.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; Lam, V.; Poultsides, G.A.; Popescu, I.; et al. Early Versus Late Recurrence of Hepatocellular Carcinoma After Surgical Resection Based on Post-recurrence Survival: An International Multi-institutional Analysis. J. Gastrointest. Surg. 2021, 25, 125–133. [Google Scholar] [CrossRef]
- Shah, S.A.; Greig, P.D.; Gallinger, S.; Cattral, M.S.; Dixon, E.; Kim, R.D.; Vollmer, C.M. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J. Am. Coll. Surg. 2006, 202, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Matsuyama, Y. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, X.; Lau, W.Y.; Shen, F.; Wang, R.Y.; Yuan, S.X.; Zhou, W.P. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br. J. Surg. 2014, 101, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Li, J.Q.; Zheng, Y.; Guo, R.P.; Liang, H.H.; Zhang, Y.Q.; Lau, W.Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 2006, 243, 321–328. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Chan, J.A.; Aloia, T.A.; Chun, Y.S.; Kaseb, A.O.; Passot, G.; Yamashita, S.; Vauthey, J.-N.; Conrad, C. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017, 123, 1817–1827. [Google Scholar] [CrossRef]
- Kudo, M.; Hasegawa, K.; Kawaguchi, Y.; Takayama, T.; Izumi, N.; Yamanaka, N.; Kokudo, N. A multicenter randomized controlled trial to evaluate the efficacy of surgery versus radiofrequency ablation for small hepatocellular carcinoma (SURF trial): Analysis of overall survival. J. Clin. Oncol. 2021, 39, 4093. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Spitsbergen, J.M.; Gong, Z. Males develop faster and more severe hepatocellular carcinoma than females in kras V12 transgenic zebrafish. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, K.; Wang, F.; Zhangyuan, G.; Yu, W.; Liu, Y.; Sun, B. Differences in the prognostic value of tumor size on hepatocellular cancer-specific survival stratified by gender in a SEER population-based study. United Eur. Gastroenterol. J. 2019, 7, 933–941. [Google Scholar] [CrossRef]
- Liang, T.; He, Y.; Mo, S.; Chen, Z.; Liao, X.; Zhou, X.; Yang, C.; Zhao, S.; Han, C.; Zhu, G.; et al. Gender disparity in hepatocellular carcinoma recurrence after curative hepatectomy. Ann. Hepatol. 2022, 27, 100695. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Zhang, X.; Xin, Y.; Wang, Y.; Li, X.; Ye, F. Predictors and patterns of recurrence after radiofrequency ablation for hepatocellular carcinoma within up-to-seven criteria: A multicenter retrospective study. Eur. J. Radiol. 2021, 138, 109623. [Google Scholar] [CrossRef]
- Bae, B.K.; Park, H.C.; Yoo, G.S.; Choi, M.K.; Oh, J.H.; Yu, J. The Significance of Systemic Inflammation Markers in Intrahepatic Recurrence of Early-Stage Hepatocellular Carcinoma after Curative Treatment. Cancers 2022, 14, 2081. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lu, S.N.; Hung, C.H.; Wang, J.H.; Chen, C.H.; Yen, Y.H.; Kee, K.M. Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS ONE 2020, 15, e0242113. [Google Scholar] [CrossRef]
- Liu, I.T.; Yen, C.S.; Wang, W.L.; Tsai, H.W.; Chu, C.Y.; Chang, M.Y.; Yen, C.J. Predict early recurrence of resectable hepatocellular carcinoma using multi-dimensional artificial intelligence analysis of liver fibrosis. Cancers 2021, 13, 5323. [Google Scholar] [CrossRef]
- Kuo, M.J.; Mo, L.R.; Chen, C.L. Factors predicting long-term outcomes of early-stage hepatocellular carcinoma after primary curative treatment: The role of surgical or nonsurgical methods. BMC Cancer 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Chan, A.W.H.; Zhong, J.; Berhane, S.; Toyoda, H.; Cucchetti, A.; Shi, K.; Johnson, P.J. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J. Hepatol. 2018, 69, 1284–1293. [Google Scholar] [CrossRef]
- Lee, I.-C.; Lei, H.-J.; Chau, G.-Y.; Yeh, Y.-C.; Wu, C.-J.; Su, C.W.; Huo, T.I.; Chao, Y.; Lin, H.C.; Hou, M.C.; et al. Predictors of long-term recurrence and survival after resection of HBV-related hepatocellular carcinoma: The role of HBsAg. Am. J. Cancer Res. 2021, 11, 3711–3725. [Google Scholar]
- Shirabe, K.; Kanematsu, T.; Matsumata, T.; Adachi, E.; Akazawa, K.; Sugimachi, K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: Univariate and multivariate analyses. Hepatology 1991, 14, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, L.; Chen, T.; Zheng, G.; Ye, C.; Jia, W. Factors influencing early recurrence of hepatocellular carcinoma after curative resection. J. Int. Med. Res. 2020, 48, 300060520945552. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Jiang, R.; Hou, J.; Mu, X.; Li, G.; Sun, B. Effect of Tumor Size on Cancer-Specific Survival in Small Hepatocellular Carcinoma. Mayo Clin. Proc. 2015, 90, 1187–1195. [Google Scholar] [CrossRef]
- Lee, J.; Jin, Y.J.; Shin, S.K.; Kwon, J.H.; Kim, S.G.; Suh, Y.J.; Kim, Y.S. Surgery versus radiofrequency ablation in patients with Child-Pugh class-A/single small (≤3 cm) hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 207–218. [Google Scholar] [CrossRef]
- Biselli, M.; Conti, F.; Gramenzi, A.; Frigerio, M.; Cucchetti, A.; Fatti, G.; Trevisani, F. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br. J. Cancer 2015, 112, 69–76. [Google Scholar] [CrossRef]
- Varona, M.A.; Soriano, A.; Aguirre-Jaime, A.; Garrido, S.; Oton, E.; Diaz, D.; Perera, A. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: Accuracy of the alpha-fetoprotein model in a single-center experience. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 84–89. [Google Scholar] [CrossRef]
- Lee, W.C. Value of alpha-fetoprotein in hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2021, 6, 52. [Google Scholar] [CrossRef]
- dos Santos Schraiber, L.; de Mattos, A.A.; Zanotelli, M.L.; Cantisani, G.P.C.; de Mello Brandão, A.B.; Marroni, C.A.; dos Santos Marcon, P. Alpha-fetoprotein Level Predicts Recurrence After Transplantation in Hepatocellular Carcinoma. Medicine 2016, 95, e2478. [Google Scholar] [CrossRef]
- Ma, W.J.; Wang, H.Y.; Teng, L.S. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J. Surg. Oncol. 2013, 11, 1–7. [Google Scholar] [CrossRef]
- Lai, Q.; Vitale, A.; Manzia, T.M.; Foschi, F.G.; Levi Sandri, G.B.; Gambato, M.; Melandro, F.; Russo, F.P.; Miele, L.; Viganò, L.; et al. Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group. Platelets and Hepatocellular Cancer: Bridging the Bench to the Clinics. Cancers 2019, 11, 1568. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F.; Davila, J.A.; Kramer, J.; Richardson, P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014, 146, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Bedogni, G.; Crocè, L.S. Spleen Stiffness Probability Index (SSPI): A simple and accurate method to detect esophageal varices in patients with compensated liver cirrhosis. Ann. Hepatol. 2020, 19, 53–61. [Google Scholar] [CrossRef]
- Wang, J.H.; Chang, K.C.; Kee, K.M.; Chen, P.-F.; Yen, Y.-H.; Tseng, P.-L.; Kuo, Y.-H.; Tsai, M.-C.; Hung, C.-H.; Chen, C.-H.; et al. Hepatocellular carcinoma surveillance at 4-vs. 12-month intervals for patients with chronic viral hepatitis: A randomized study in community. Am. J. Gastroenterol. 2013, 108, 416–424. [Google Scholar] [CrossRef]
- Pang, Q.; Qu, K.; Bi, J.B.; Liu, S.S.; Zhang, J.Y.; Song, S.D.; Liu, C. Thrombocytopenia for prediction of hepatocellular carcinoma recurrence: Systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 7895–7906. [Google Scholar] [CrossRef]
- Suner, A.; Carr, B.I.; Akkiz, H.; Uskudar, O.; Kuran, S.; Tokat, Y.; Tokmak, S.; Ballı, T.; Ulku, A.; AkCam, T.; et al. Inflammatory markers C-reactive protein and PLR in relation to HCC characteristics. J. Transl. Sci. 2019, 5, 10.15761/JTS.1000260, Epub 2018 June 22. [Google Scholar] [CrossRef]
- Lin, W.F.; Zhong, M.F.; Zhang, Y.R.; Wang, H.; Zhao, H.T.; Cheng, B.B.; Ling, C.Q. Prognostic Role of Platelet-to-Lymphocyte Ratio in Hepatocellular Carcinoma with Different BCLC Stages: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2018, 8, 5670949. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Wang, L.; Feng, S.; Qiu, Q.; Chen, D.; Xiao, Y. A new model based inflammatory index and tumor burden score (TBS) to predict the recurrence of hepatocellular carcinoma (HCC) after liver resection. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Jiang, R.; Liu, W.S.; Liu, Q.; Xu, M.; Feng, Q.-S.; Chen, L.-Z.; Bei, J.-X.; Chen, M.-Y.; Zeng, Y.-X. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS ONE 2013, 8, e83069. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishizuka, M.; Park, K.H.; Shiraki, T.; Sakuraoka, Y.; Mori, S.; Iso, Y.; Kato, M.; Aoki, T.; Kubota, K. Preoperative lymphocyte-to-monocyte ratio is useful for stratifying the prognosis of hepatocellular carcinoma patients with a low Cancer of the Liver Italian Program score undergoing curative resection. Ann. Gastroenterol. Surg. 2019, 3, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.; Agrawal, S.; Emond, J.C.; Halazun, K.J. Pretreatment neutrophil–lymphocyte ratio: Useful prognostic biomarker in hepatocellular carcinom. J. Hepatocell. Carcinoma 2018, 5, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Shi, X.J.; Chen, Y.G.; Wang, C.L.; Ma, Q.; Lv, G.Y. Elevated preoperative neutrophil-lymphocyte ratio is associated with poor prognosis in hepatocellular carcinoma patients treated with liver transplantation: A meta-analysis. Gastroenterol. Res. Pract. 2016, 2016, 4743808. [Google Scholar] [CrossRef] [PubMed]
| Variables | Altogether N = 58 | Surgery N = 23 | Ablation N = 35 | Significance |
|---|---|---|---|---|
| Gender, Male | 48 (82.8%) | 18 (78.3%) | 30 (85.7%) | NS |
| Age, years | 70 (65; 77) | 66 (61.5; 71) | 72 (69.5; 79) | p = 0.003 |
| Etiology, Viral | 27 (46.6%) | 13 (56.5%) | 14 (40%) | NS |
| Presence of Liver Cirrhosis | 51 (87.9%) | 19 (82.6%) | 31 (88.6%) | NS |
| Number of Nodules, number | 1 (55, 94.2%) 2 (3, 6.8%) | 1 (23, 100%) 2 (0, 0%) | 1 (32, 91.4%) 2 (3, 8.6%) | NA |
| Nodule Maximum Diameter, mm | 22 (15; 30) | 25 (20; 44) | 20 (14.5; 26) | p = 0.003 |
| BCLC | ||||
| Stage 0 Stage A | 28 (48.3%) 30 (51.7%) | 9 (39.1%) 14 (60.9%) | 19 (54.3%) 16 (45.7%) | NS |
| AST, IU/L | 25.5 (22; 37) | 24 (21.5; 39) | 26 (22; 36.5) | NS |
| ALT, IU/L | 22 (17; 35) | 21 (17; 38) | 23 (16; 29.5) | NS |
| ALP, IU/L | 92 (75; 112) | 90 (70; 102) | 92 (77; 119) | NS |
| GGT, IU/L | 56 (38; 112) | 53 (30; 116) | 58 (46; 112) | NS |
| Total Bilirubin, mg/dL | 0.89 (0.62; 1.20) | 0.7 (0.53; 0.82) | 1.1 (0.8; 1.5) | NS |
| Albumin, g/dL | 4.1 (3.8; 4.4) | 4.3 (4.1; 4.5) | 4 (3.7; 4.3) | NS |
| Platelet Count, ×103 cell/mm3 | 123 (98; 171) | 171 (131; 215) | 109 (81; 132) | p = 0.003 |
| Creatinine, mg/dL | 0.83 (0.74; 0.93) | 0.83 (0.66; 0.91) | 0.85 (0.76; 0.97) | NS |
| INR | 1.1 (1.03; 1.12) | 1.04 (1.01; 1.07) | 1.09 (1.03; 1.14) | NS |
| AFP ng/mL | 4.4 (2.6; 6.9) | 4.8 (3; 23) | 4.1 (2.3; 5.3) | NS |
| PLR | 98.5 (86; 133.5) | 101 (87.5; 139) | 96 (85; 127) | NS |
| LMR | 2.9 (2.1; 3.8) | 2.9 (2.5; 4.3) | 2.8 (1.95; 3.4) | NS |
| NLR | 2.3 (1.8; 3.2) | 2.3 (1.7; 3.2) | 2.3 (1.85; 3.4) | NS |
| Child–Pugh | ||||
| A5 A6 A7 | 44 (75.9%) 13 (22.4%) 1 (1.7%) | 18 (78.3%) 5 (21.7%) 0 (0%) | 26 (74.3%) 8 (22.9%) 1 (2.8) | NS |
| MELD | 7 (7; 9) | 7 (7; 7) | 8 (7; 10) | NS |
| Patients with HCC Recurrence | 24 (41.4%) | 8 (34.8%) | 16 (45.7%) | NS |
| Recurrence Time from Treatment, months | 6 (1; 9) | 10.5 (4.75; 12) | 2 (1; 6) | p = 0.016 |
| Variables | Altogether N = 58 | Without HCC Recurrence N = 34 | HCC Recurrence N = 24 | Significance |
|---|---|---|---|---|
| Gender, Male | 48 (82.8%) | 26 (76.5%) | 22 (91.7%) | p = 0.032 |
| Age, years | 70 (65; 77) | 70 (65; 78) | 70 (65; 76) | NS |
| Etiology, Viral | 27 (46.6%) | 14 (41.2%) | 13 (54.2%) | NS |
| Presence of Liver Cirrhosis | 51 (87.9%) | 28 (82.4%) | 23 (67.6%) | NS |
| Number of Nodules, number | 1 (55, 94.2%) 2 (3, 6.8%) | 1 (33, 97%) 2 (1, 3%) | 1 (22, 91.7%) 2 (2, 8.3%) | NS |
| Nodule Maximum Diameter, mm | 22 (15; 30) | 18 (13; 23.5) | 33 (24; 48) | p < 0.0001 |
| BCLC | ||||
| Stage 0 Stage A | 28 (48.3%) 30 (51.7%) | 17 (50%) 17 (50%) | 11 (45.8%) 13 (54.2%) | NS |
| AST, IU/L | 25.5 (22; 37) | 25 (22; 36) | 28 (21; 65) | NS |
| ALT, IU/L | 22 (17; 35) | 20.5 (15; 33) | 25 (18; 59) | NS |
| ALP, IU/L | 92 (75; 112) | 94 (77; 114) | 84 (75; 101) | NS |
| GGT, IU/L | 56 (38; 112) | 50 (30; 111) | 60 (48.5; 128) | NS |
| Total Bilirubin, mg/dL | 0.89 (0.62; 1.20) | 0.75 (0.58; 1.15) | 0.93 (0.75; 1.21) | NS |
| Albumin, g/dL | 4.1 (3.8; 4.4) | 4.2 (3.82; 4.4) | 4.1 (3.8; 4.5) | NS |
| Platelet Count, × 103 cell/mm3 | 123 (98; 171) | 143 (107; 205) | 111.5 (92; 129) | p = 0.03 |
| Creatinine, mg/dL | 0.83 (0.74; 0.93) | 0.87 (0.78; 0.94) | 0.80 (0.71; 0.92) | NS |
| INR | 1.1 (1.03; 1.12) | 1.05 (1; 1.11) | 1.08 (1.03; 1.13) | NS |
| AFP, ng/mL | 4.4 (2.6; 6.9) | 4 (2.5; 6.35) | 4.7 (3.55; 7.37) | NS |
| PLR | 98.5 (86; 133.5) | 103.5 (89.5; 148) | 92 (78.5; 114) | p = 0.026 |
| LMR | 2.9 (2.1; 3.8) | 2.6 (2; 3.7) | 3.2 (2.7; 4.2) | p = 0.041 |
| NLR | 2.3 (1.8; 3.2) | 2.6 (1.9; 3.4) | 1.9 (1.5; 2.8) | p = 0.043 |
| Child–Pugh | ||||
| A5 A6 A7 | 44 (75.9%) 13 (22.4%) 1 (1.7%) | 24 (70.6%) 10 (29.4%) 0 (0%) | 20 (83.3%) 3 (12.5%) 1 (4.2%) | NA |
| MELD | 7 (7; 9) | 7 (7; 10) | 7 (7; 9) | NS |
| Variable of Interest | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | Significance | Hazard Ratio (95% Confidence Interval) | Significance | |
| Gender, Male | 2.5 (1.9–3.1) | p = 0.001 | 3.1 (2.1–3.2) | p = 0.001 |
| Nodule Max Diameter > 20 mm | 4.5 (3.9–5.1) | p < 0.001 | 5.9 (2.8–6.9) | p = 0.001 |
| Nodule Max Diameter > 30 mm | 5.1 (4.5–5.5) | p < 0.001 | ||
| Nodule Max Diameter > 40 mm | 6.1 (4.9–6.7) | p < 0.001 | ||
| Platelet Count < 125 × 103 cell/mm3 | 1.6 (1.2–1.9) | p = 0.030 | ||
| PLR < 95 | 2.1 (1.7–2.6) | p = 0.022 | ||
| LMR < 2.5 | 1.9 (1.4–2.5) | p = 0.019 | ||
| NLR > 2 | 2.7 (2.2–3.3) | p = 0.002 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuffrè, M.; Zuliani, E.; Visintin, A.; Tarchi, P.; Martingano, P.; Pizzolato, R.; Bonazza, D.; Masutti, F.; Moretti, R.; Crocè, L.S., on behalf of the Liver Multidisciplinary Group of Trieste. Predictors of Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A Single-Center Experience. Diagnostics 2022, 12, 2517. https://doi.org/10.3390/diagnostics12102517
Giuffrè M, Zuliani E, Visintin A, Tarchi P, Martingano P, Pizzolato R, Bonazza D, Masutti F, Moretti R, Crocè LS on behalf of the Liver Multidisciplinary Group of Trieste. Predictors of Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A Single-Center Experience. Diagnostics. 2022; 12(10):2517. https://doi.org/10.3390/diagnostics12102517
Chicago/Turabian StyleGiuffrè, Mauro, Enrico Zuliani, Alessia Visintin, Paola Tarchi, Paola Martingano, Riccardo Pizzolato, Deborah Bonazza, Flora Masutti, Rita Moretti, and Lory Saveria Crocè on behalf of the Liver Multidisciplinary Group of Trieste. 2022. "Predictors of Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A Single-Center Experience" Diagnostics 12, no. 10: 2517. https://doi.org/10.3390/diagnostics12102517
APA StyleGiuffrè, M., Zuliani, E., Visintin, A., Tarchi, P., Martingano, P., Pizzolato, R., Bonazza, D., Masutti, F., Moretti, R., & Crocè, L. S., on behalf of the Liver Multidisciplinary Group of Trieste. (2022). Predictors of Hepatocellular Carcinoma Early Recurrence in Patients Treated with Surgical Resection or Ablation Treatment: A Single-Center Experience. Diagnostics, 12(10), 2517. https://doi.org/10.3390/diagnostics12102517







