Parenchymal Insults in Abuse—A Potential Key to Diagnosis
Abstract
:1. Introduction
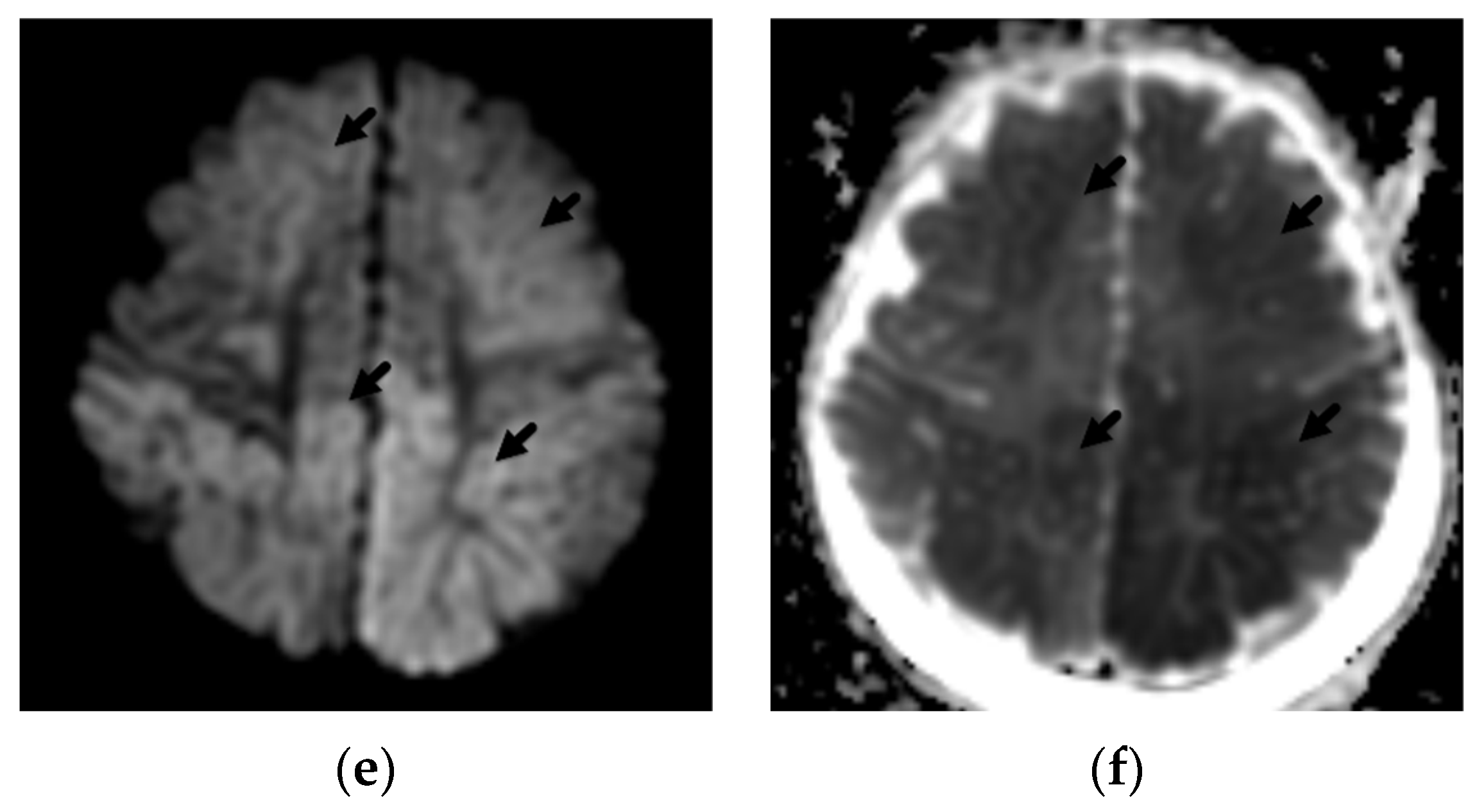
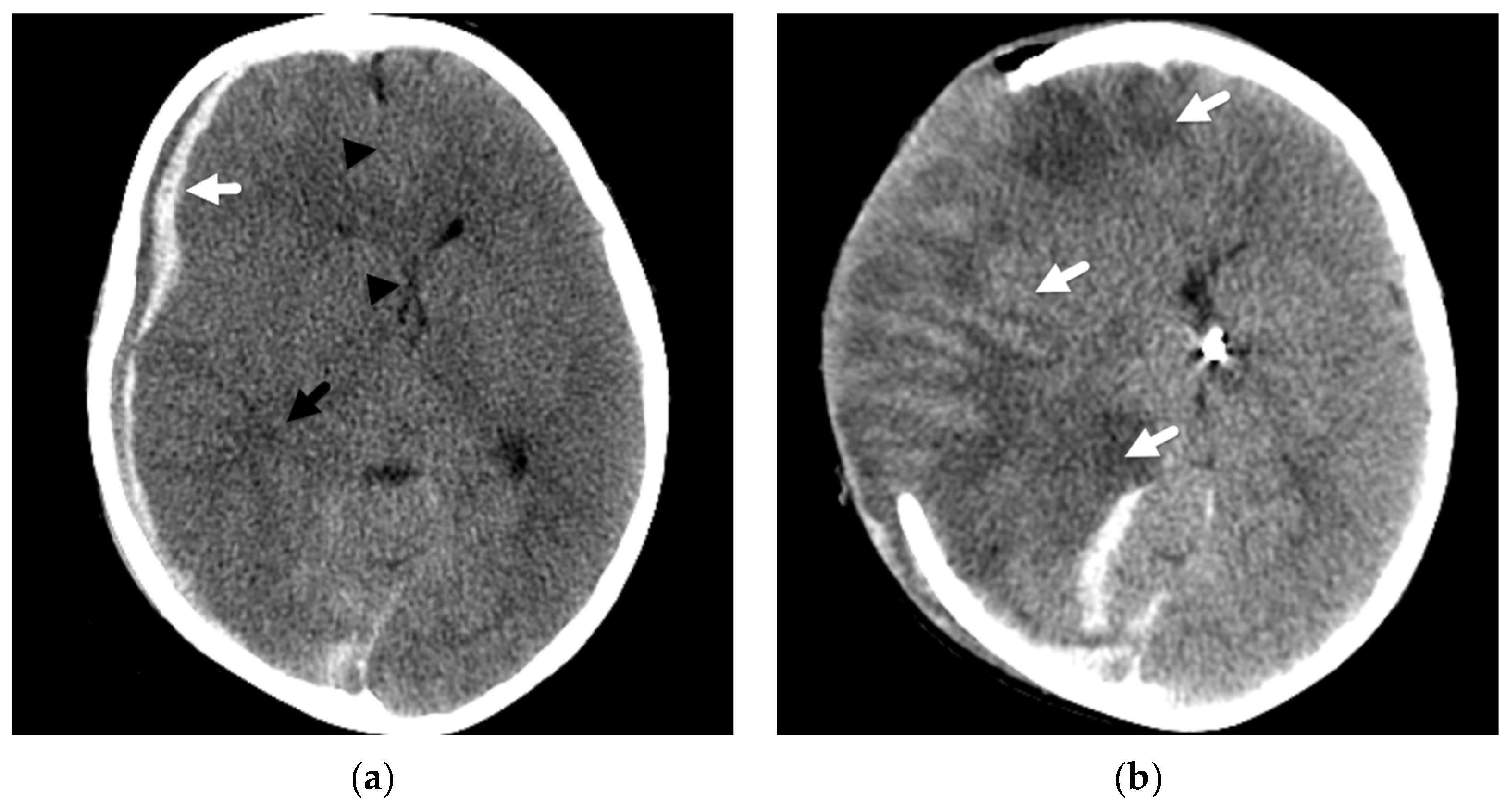
2. Patterns of Diffuse Parenchymal Insults
3. Imaging of Diffuse Insults
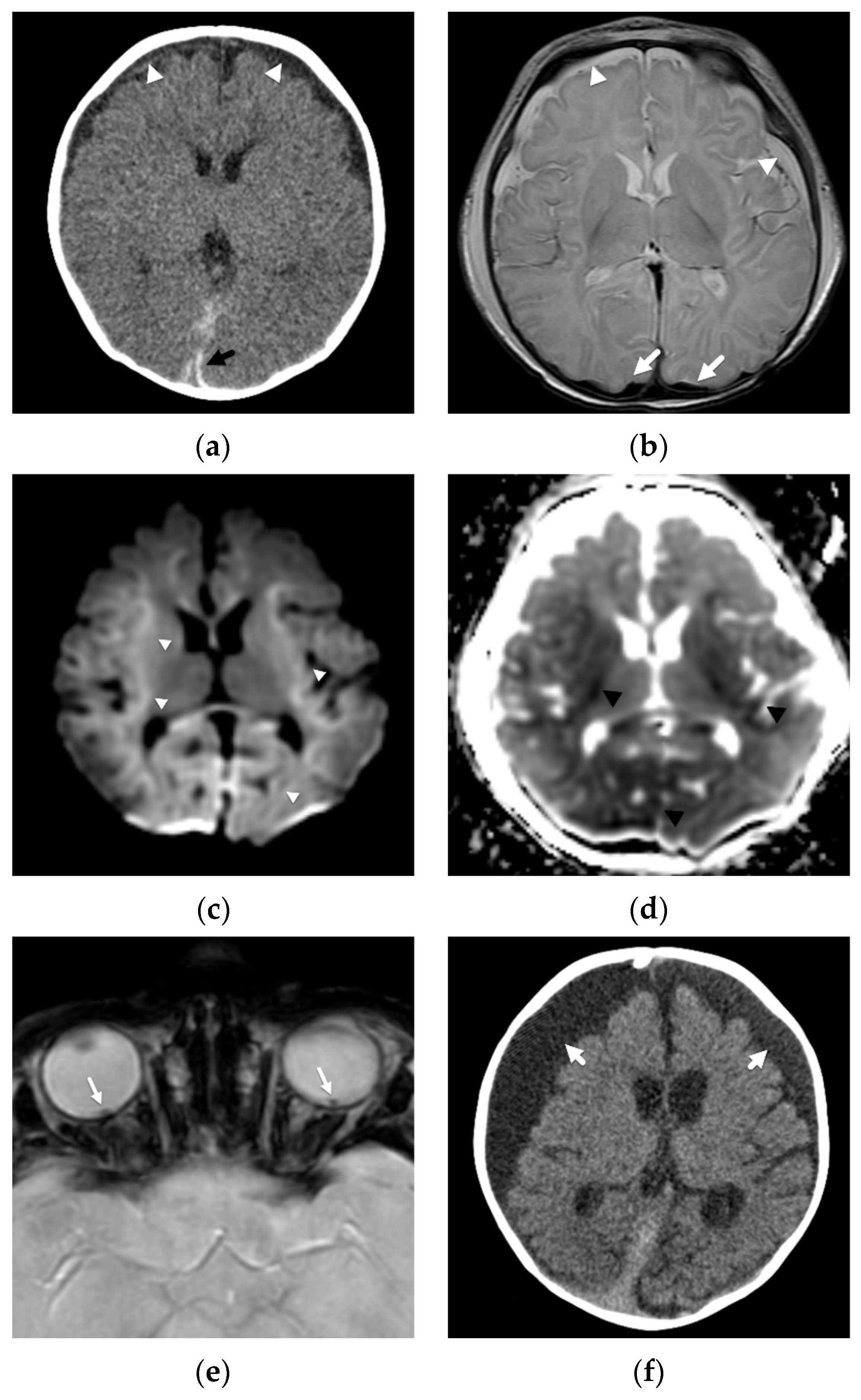
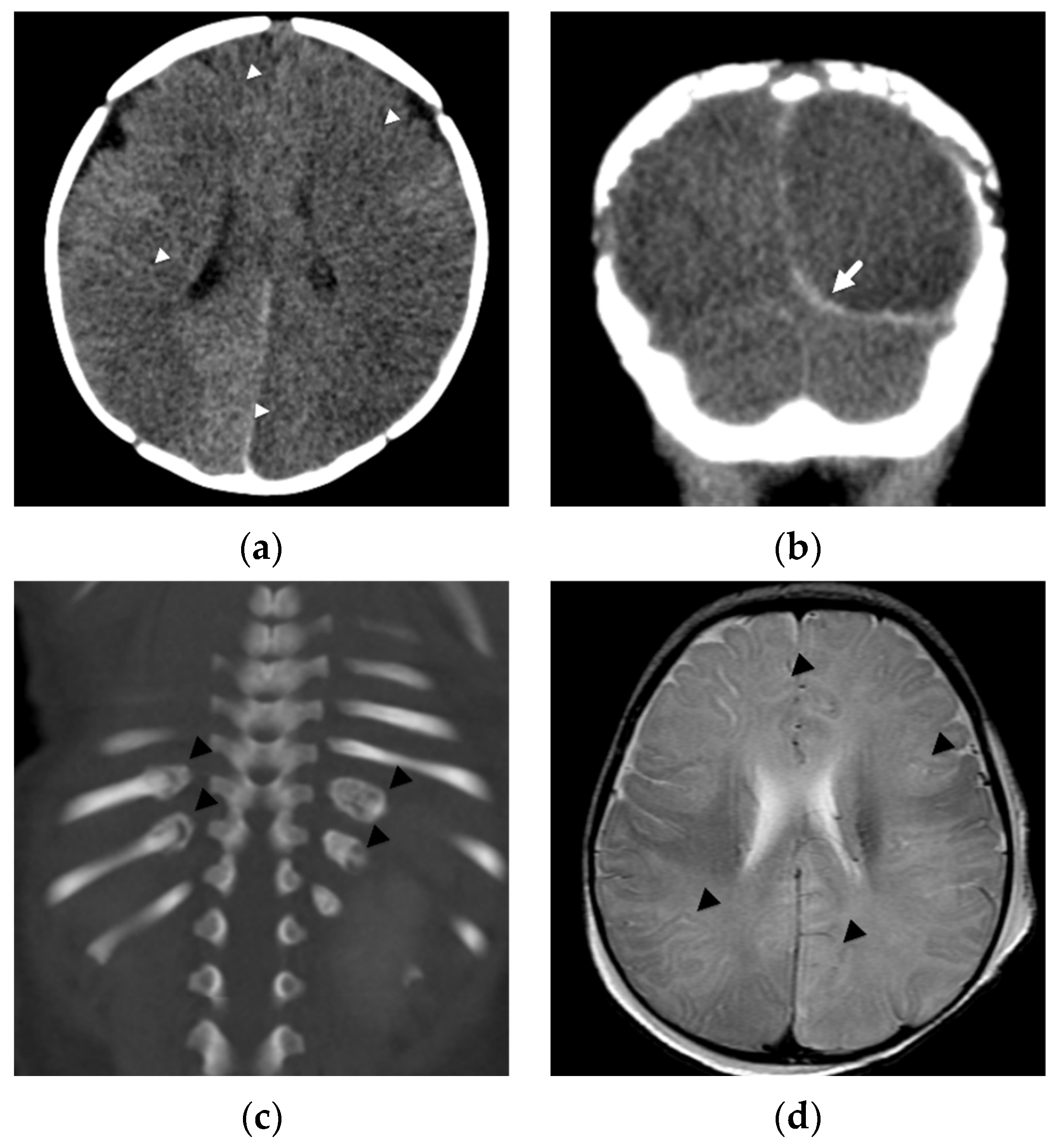
4. Focal Parenchymal Insults
5. Imaging Evaluation
6. Clinical and Surgical Considerations and Outcomes
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Department of Health and Human Services, AfCaF. Child Welfare Information Gateway. Child Abuse and Neglect Fatalities 2019: Statistics and Interventions. 2021. Available online: https://www.childwelfare.gov/pubs/factsheets/fatality/ (accessed on 28 February 2022).
- Palusci, V.J.; Covington, T.M. Child maltreatment deaths in the U.S. National Child Death Review Case Reporting System. Child Abus. Negl. 2014, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.E.; Beres, A.L.; Theodorou, C.M.; Ugiliweneza, B.; Boakye, M.; Nuño, M. Long-term impact of abusive head trauma in young children: Outcomes at 5 and 11 years old. J. Pediatr. Surg. 2021, 56, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Chevignard, M.P.; Lind, K. Long-term outcome of abusive head trauma. Pediatr. Radiol. 2014, 44 (Suppl. S4), S548–S558. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Servaes, S.; Slovis, T.L.; Palusci, V.J.; Hedlund, G.L.; Narang, S.K.; Moreno, J.A.; Dias, M.S.; Christian, C.W.; Nelson, M.D., Jr.; et al. Consensus statement on abusive head trauma in infants and young children. Pediatr. Radiol. 2018, 48, 1048–1065. [Google Scholar] [CrossRef] [Green Version]
- Committee on Child Abuse and Neglect. Policy statement—Child abuse, confidentiality, and the health insurance portability and accountability act. Pediatrics 2010, 125, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boos, S.C.; Wang, M.; Karst, W.A.; Hymel, K.P. Traumatic Head Injury and the Diagnosis of Abuse: A Cluster Analysis. Pediatrics 2022, 149, e2021051742. [Google Scholar] [CrossRef]
- Zimmerman, R.A.; Bilaniuk, L.T.; Bruce, D.; Schut, L.; Uzzell, B.; Goldberg, H.I. Computed tomography of craniocerebral injury in the abused child. Radiology 1979, 130, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Merten, D.F.; Osborne, D.R.; Radkowski, M.A.; Leonidas, J.C. Craniocerebral trauma in the child abuse syndrome: Radiological observations. Pediatr. Radiol. 1984, 14, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.S.; Backstrom, J.; Falk, M.; Li, V. Serial radiography in the infant shaken impact syndrome. Pediatr. Neurosurg. 1998, 29, 77–85. [Google Scholar] [CrossRef]
- Bradford, R.; Choudhary, A.K.; Dias, M.S. Serial neuroimaging in infants with abusive head trauma: Timing abusive injuries. J. Neurosurg. Pediatr. 2013, 12, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.; John, S.; Vincent, A.L.; Reed, P. Abusive head trauma and accidental head injury: A 20-year comparative study of referrals to a hospital child protection team. Arch. Dis. Child. 2015, 100, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matschke, J.; Voss, J.; Obi, N.; Görndt, J.; Sperhake, J.P.; Püschel, K.; Glatzel, M. Nonaccidental head injury is the most common cause of subdural bleeding in infants <1 year of age. Pediatrics 2009, 124, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Duhaime, A.C.; Alario, A.J.; Lewander, W.J.; Schut, L.; Sutton, L.N.; Seidl, T.S.; Nudelman, S.; Budenz, D.; Hertle, R.; Tsiaras, W.; et al. Head injury in very young children: Mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics 1992, 90, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hymel, K.P.; Rumack, C.M.; Hay, T.C.; Strain, J.D.; Jenny, C. Comparison of intracranial computed tomographic (CT) findings in pediatric abusive and accidental head trauma. Pediatr. Radiol. 1997, 27, 743–747. [Google Scholar] [CrossRef]
- Reece, R.M.; Sege, R. Childhood head injuries: Accidental or inflicted? Arch. Pediatr. Adolesc. Med. 2000, 154, 11–15. [Google Scholar]
- Feldman, K.W.; Bethel, R.; Shugerman, R.P.; Grossman, D.C.; Grady, M.S.; Ellenbogen, R.G. The cause of infant and toddler subdural hemorrhage: A prospective study. Pediatrics 2001, 108, 636–646. [Google Scholar] [CrossRef]
- Myhre, M.C.; Grogaard, J.B.; Dyb, G.A.; Sandvik, L.; Nordhov, M. Traumatic head injury in infants and toddlers. Acta Paediatr. 2007, 96, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Ewing-Cobbs, L.; Kramer, L.; Prasad, M.; Canales, D.N.; Louis, P.T.; Fletcher, J.M.; Vollero, H.; Landry, S.H.; Cheung, K. Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics 1998, 102, 300–307. [Google Scholar] [CrossRef]
- Ewing-Cobbs, L.; Prasad, M.; Kramer, L.; Louis, P.T.; Baumgartner, J.; Fletcher, J.M.; Alpert, B. Acute neuroradiologic findings in young children with inflicted or noninflicted traumatic brain injury. Childs Nerv. Syst. 2000, 16, 25–33; discussion 34. [Google Scholar] [CrossRef]
- Piteau, S.J.; Ward, M.G.; Barrowman, N.J.; Plint, A.C. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: A systematic review. Pediatrics 2012, 130, 315–323. [Google Scholar] [CrossRef]
- Kemp, A.M.; Jaspan, T.; Griffiths, J.; Stoodley, N.; Mann, M.K.; Tempest, V.; Maguire, S.A. Neuroimaging: What neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch. Dis. Child. 2011, 96, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Recker, M.J.; Lee, P.S.; Bell, M.J.; Tyler-Kabara, E.C. Factors associated with hemispheric hypodensity after subdural hematoma following abusive head trauma in children. J. Neurotrauma 2014, 31, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichord, R.N.; Naim, M.; Pollock, A.N.; Nance, M.L.; Margulies, S.S.; Christian, C.W. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: The role of diffusion-weighted imaging. J. Neurotrauma 2007, 24, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.A.; Bilaniuk, L.T.; Farina, L. Non-accidental brain trauma in infants: Diffusion imaging, contributions to understanding the injury process. J. Neuroradiol. 2007, 34, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.Y.; Davis, P.C.; Hopkins, K.L.; Fajman, N.N.; Mapstone, T.B. Nonaccidental pediatric head injury: Diffusion-weighted imaging findings. Neurosurgery 2001, 49, 309–318; discussion 318–320. [Google Scholar] [CrossRef]
- Orru, E.; Huisman, T.; Izbudak, I. Prevalence, Patterns, and Clinical Relevance of Hypoxic-Ischemic Injuries in Children Exposed to Abusive Head Trauma. J. Neuroimaging 2018, 28, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Suh, D.Y.; Newman, N.J.; Davis, P.C.; Mapstone, T.; Lambert, S.R. Diffusion-weighted magnetic resonance imaging in Shaken Baby Syndrome. Am. J. Ophthalmol. 2002, 133, 249–255. [Google Scholar] [CrossRef]
- Kadom, N.; Khademian, Z.; Vezina, G.; Shalaby-Rana, E.; Rice, A.; Hinds, T. Usefulness of MRI detection of cervical spine and brain injuries in the evaluation of abusive head trauma. Pediatr. Radiol. 2014, 44, 839–848. [Google Scholar] [CrossRef]
- Silverman, L.B.; Lindberg, D.M.; O’Neill, B.R.; Orru, E.; Huisman, T.; Izbudak, I. Cytotoxic Edema in Pediatric Abusive Head Trauma: Adopting a Common Nomenclature. J. Neuroimaging 2019, 29, 272–273. [Google Scholar] [CrossRef]
- Geddes, J.F.; Hackshaw, A.K.; Vowles, G.H.; Nickols, C.D.; Whitwell, H.L. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain 2001, 124, 1290–1298. [Google Scholar] [CrossRef]
- Geddes, J.F.; Vowles, G.H.; Hackshaw, A.K.; Nickols, C.D.; Scott, I.S.; Whitwell, H.L. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain 2001, 124, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Büttner, A.; Bergmann, M.; Hagel, C.; Püschel, K.; Glatzel, M. Encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int. J. Legal Med. 2015, 129, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Hegde, S.V.; Dineen, R.A.; Jaspan, T. Patterns of accidental craniocerebral injury occurring in early childhood. Arch. Dis. Child. 2013, 98, 787–792. [Google Scholar] [CrossRef]
- Karmazyn, B.; Reher, T.A.; Supakul, N.; Streicher, D.A.; Kiros, N.; Diggins, N.; Jennings, S.G.; Eckert, G.J.; Hibbard, R.A.; Radhakrishnan, R. Whole spine MRI in children with suspected child abuse. AJR Am. J. Roentgenol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Bradford, R.K.; Dias, M.S.; Moore, G.J.; Boal, D.K. Spinal subdural hemorrhage in abusive head trauma: A retrospective study. Radiology 2012, 262, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Ishak, R.; Zacharia, T.T.; Dias, M.S. Imaging of spinal injury in abusive head trauma: A retrospective study. Pediatr. Radiol. 2014, 44, 1130–1140. [Google Scholar] [CrossRef]
- Rabbitt, A.L.; Kelly, T.G.; Yan, K.; Zhang, J.; Bretl, D.A.; Quijano, C.V. Characteristics associated with spine injury on magnetic resonance imaging in children evaluated for abusive head trauma. Pediatr. Radiol. 2020, 50, 83–97. [Google Scholar] [CrossRef]
- McKinney, A.M.; Thompson, L.R.; Truwit, C.L.; Velders, S.; Karagulle, A.; Kiragu, A. Unilateral hypoxic-ischemic injury in young children from abusive head trauma, lacking craniocervical vascular dissection or cord injury. Pediatr. Radiol. 2008, 38, 164–174. [Google Scholar] [CrossRef]
- Saunders, R.L.; Harbaugh, R.E. The second impact in catastrophic contact-sports head trauma. JAMA 1984, 252, 538–539. [Google Scholar] [CrossRef]
- Cantu, R.C.; Gean, A.D. Second-impact syndrome and a small subdural hematoma: An uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. J. Neurotrauma 2010, 27, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- McCrory, P. Does second impact syndrome exist? Clin. J. Sport Med. 2001, 11, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Boal, D.; Baule, R. Role of apnea in nonaccidental head injury. Pediatr. Neurosurg. 1995, 23, 305–310. [Google Scholar] [CrossRef]
- Nikam, R.M.; Yue, X.; Kandula, V.V.; Paudyal, B.; Langhans, S.A.; Averill, L.W.; Choudhary, A.K. Unravelling neuroinflammation in abusive head trauma with radiotracer imaging. Pediatr. Radiol. 2021, 51, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, R.A.; Kochanek, P.M.; Adelson, P.D.; Rose, M.E.; Wisniewski, S.R.; Bell, M.J.; Clark, R.S.; Marion, D.W.; Graham, S.H. Excitatory amino acid concentrations in ventricular cerebrospinal fluid after severe traumatic brain injury in infants and children: The role of child abuse. J. Pediatr. 2001, 138, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, R.A.; Clark, R.S.; Bayir, H.; Satchell, M.A.; Kochanek, P.M. Critical mechanisms of secondary damage after inflicted head injury in infants and children. Neurosurg. Clin. N. Am. 2002, 13, 169–182. [Google Scholar] [CrossRef]
- Case, M.E. Inflicted traumatic brain injury in infants and young children. Brain Pathol. 2008, 18, 571–582. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Jaspan, T.; Narborough, G.; Punt, J.A.; Lowe, J. Cerebral contusional tears as a marker of child abuse–detection by cranial sonography. Pediatr. Radiol. 1992, 22, 237–245. [Google Scholar] [CrossRef]
- Palifka, L.A.; Frasier, L.D.; Metzger, R.R.; Hedlund, G.L. Parenchymal Brain Laceration as a Predictor of Abusive Head Trauma. AJNR Am. J. Neuroradiol. 2016, 37, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Au-Yong, I.T.; Wardle, S.P.; McConachie, N.S.; Jaspan, T. Isolated cerebral cortical tears in children: Aetiology, characterisation and differentiation from non-accidental head injury. Br. J. Radiol. 2009, 82, 735–741. [Google Scholar] [CrossRef]
- Larsen, K.B.; Barber, Z.; Squier, W. The pathology and aetiology of subcortical clefts in infants. Forensic Sci. Int. 2019, 296, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, A.; Triquenot-Bagan, A.; Andriuta, D.; Wallon, D.; Guegan-Massardier, E.; Leclercq, C.; Martinaud, O.; Castier-Amouyel, M.; Godefroy, O.; Bugnicourt, J.M. Imaging Characteristics of Venous Parenchymal Abnormalities. Stroke 2017, 48, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Hymel, K.P.; Abshire, T.C.; Luckey, D.W.; Jenny, C. Coagulopathy in pediatric abusive head trauma. Pediatrics 1997, 99, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.M.; Rajaram, S.; Mann, M.; Tempest, V.; Farewell, D.; Gawne-Cain, M.L.; Jaspan, T.; Maguire, S. What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected? A systematic review. Clin. Radiol. 2009, 64, 473–483. [Google Scholar] [CrossRef]
- Wootton-Gorges, S.; Soares, B.; Alazraki, A.; Anupindi, S.; Blount, J.; Booth, T.; Dempsey, M.; Falcone, R.; Hayes, L.; Kulkarni, A.; et al. ACR Appropriateness Criteria® Suspected Physical Abuse—Child. J. Am. Coll. Radiol. 2017, 14, S338–S349. [Google Scholar] [CrossRef] [Green Version]
- Slovis, T.L.; Strouse, P.J.; Strauss, K.J. Radiation Exposure in Imaging of Suspected Child Abuse: Benefits versus Risks. J. Pediatr. 2015, 167, 963–968. [Google Scholar] [CrossRef]
- Silvera, V.M.; Danehy, A.R.; Newton, A.W.; Stamoulis, C.; Carducci, C.; Grant, P.E.; Wilson, C.R.; Kleinman, P.K. Retroclival collections associated with abusive head trauma in children. Pediatr. Radiol. 2014, 44 (Suppl. S4), S621–S631. [Google Scholar] [CrossRef]
- Langford, S.; Panigrahy, A.; Narayanan, S.; Hwang, M.; Fitz, C.; Flom, L.; Lee, V.K.; Zuccoli, G. Multiplanar reconstructed CT images increased depiction of intracranial hemorrhages in pediatric head trauma. Neuroradiology 2015, 57, 1263–1268. [Google Scholar] [CrossRef]
- Hahnemann, M.L.; Kinner, S.; Schweiger, B.; Bajanowski, T.; Karger, B.; Pfeiffer, H.; Wittschieber, D. Imaging of bridging vein thrombosis in infants with abusive head trauma: The “Tadpole Sign”. Eur. Radiol. 2015, 25, 299–305. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Bradford, R.; Dias, M.S.; Thamburaj, K.; Boal, D.K. Venous injury in abusive head trauma. Pediatr. Radiol. 2015, 45, 1803–1813. [Google Scholar] [CrossRef]
- Prabhu, S.P.; Newton, A.W.; Perez-Rossello, J.M.; Kleinman, P.K. Three-dimensional skull models as a problem-solving tool in suspected child abuse. Pediatr. Radiol. 2013, 43, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.T.; Wiester, R.T.; Done, S.L.; Sugar, N.F.; Feldman, K.W. Three-Dimensional Computed Tomography Skull Reconstructions as an Aid to Child Abuse Evaluations. Pediatr. Emerg. Care 2015, 31, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Tosaka, M.; Sato, N.; Fujimaki, H.; Tanaka, Y.; Kagoshima, K.; Takahashi, A.; Saito, N.; Yoshimoto, Y. Diffuse pachymeningeal hyperintensity and subdural effusion/hematoma detected by fluid-attenuated inversion recovery MR imaging in patients with spontaneous intracranial hypotension. AJNR Am. J. Neuroradiol. 2008, 29, 1164–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jevtovic-Todorovic, V.; Absalom, A.R.; Blomgren, K.; Brambrink, A.; Crosby, G.; Culley, D.J.; Fiskum, G.; Giffard, R.G.; Herold, K.F.; Loepke, A.W.; et al. Anaesthetic neurotoxicity and neuroplasticity: An expert group report and statement based on the BJA Salzburg Seminar. Br. J. Anaesth. 2013, 111, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Barton, K.; Nickerson, J.P.; Higgins, T.; Williams, R.K. Pediatric anesthesia and neurotoxicity: What the radiologist needs to know. Pediatr. Radiol. 2018, 48, 31–36. [Google Scholar] [CrossRef]
- Berger, R.P.; Furtado, A.D.; Flom, L.L.; Fromkin, J.B.; Panigrahy, A. Implementation of a brain injury screen MRI for infants at risk for abusive head trauma. Pediatr. Radiol. 2020, 50, 75–82. [Google Scholar] [CrossRef]
- Flom, L.; Fromkin, J.; Panigrahy, A.; Tyler-Kabara, E.; Berger, R.P. Development of a screening MRI for infants at risk for abusive head trauma. Pediatr. Radiol. 2016, 46, 519–526. [Google Scholar] [CrossRef]
- Lindberg, D.M.; Stence, N.V.; Grubenhoff, J.A.; Lewis, T.; Mirsky, D.M.; Miller, A.L.; O’Neill, B.R.; Grice, K.; Mourani, P.M.; Runyan, D.K. Feasibility and Accuracy of Fast MRI Versus CT for Traumatic Brain Injury in Young Children. Pediatrics 2019, 144, e20190419. [Google Scholar] [CrossRef]
- Rozovsky, K.; Ventureyra, E.C.; Miller, E. Fast-brain MRI in children is quick, without sedation, and radiation-free, but beware of limitations. J. Clin. Neurosci. 2013, 20, 400–405. [Google Scholar] [CrossRef]
- Kralik, S.F.; Yasrebi, M.; Supakul, N.; Lin, C.; Netter, L.G.; Hicks, R.A.; Hibbard, R.A.; Ackerman, L.L.; Harris, M.L.; Ho, C.Y. Diagnostic Performance of Ultrafast Brain MRI for Evaluation of Abusive Head Trauma. AJNR Am. J. Neuroradiol. 2017, 38, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.S.; Thamburaj, K. Neuroradiologic timing of intracranial hemorrhage in abusive head trauma. Pediatr. Radiol. 2021, 51, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hettler, J.; Greenes, D.S. Can the initial history predict whether a child with a head injury has been abused? Pediatrics 2003, 111, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Hymel, K.P.; Lee, G.; Boos, S.; Karst, W.A.; Sirotnak, A.; Haney, S.B.; Laskey, A.; Wang, M. Estimating the Relevance of Historical Red Flags in the Diagnosis of Abusive Head Trauma. J. Pediatr. 2020, 218, 178–183.e172. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.A.; Drazin, D.; Smith, C.; Waldman, J.B. Comparison of accidental and nonaccidental traumatic brain injuries in infants and toddlers: Demographics, neurosurgical interventions, and outcomes. J. Neurosurg. Pediatr. 2009, 4, 414–419. [Google Scholar] [CrossRef]
- Oluigbo, C.O.; Wilkinson, C.C.; Stence, N.V.; Fenton, L.Z.; McNatt, S.A.; Handler, M.H. Comparison of outcomes following decompressive craniectomy in children with accidental and nonaccidental blunt cranial trauma. J. Neurosurg. Pediatr. 2012, 9, 125–132. [Google Scholar] [CrossRef]
- Boop, S.; Axente, M.; Weatherford, B.; Klimo, P., Jr. Abusive head trauma: An epidemiological and cost analysis. J. Neurosurg. Pediatr. 2016, 18, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Makaroff, K.L.; Putnam, F.W. Outcomes of infants and children with inflicted traumatic brain injury. Dev. Med. Child Neurol. 2003, 45, 497–502. [Google Scholar] [CrossRef]
- Risen, S.R.; Suskauer, S.J.; Dematt, E.J.; Slomine, B.S.; Salorio, C.F. Functional outcomes in children with abusive head trauma receiving inpatient rehabilitation compared with children with nonabusive head trauma. J. Pediatr. 2014, 164, 613–619.e611–e612. [Google Scholar] [CrossRef] [Green Version]
- Barlow, K.M.; Spowart, J.J.; Minns, R.A. Early posttraumatic seizures in non-accidental head injury: Relation to outcome. Dev. Med. Child Neurol. 2000, 42, 591–594. [Google Scholar] [CrossRef]
- Greiner, M.V.; Lawrence, A.P.; Horn, P.; Newmeyer, A.J.; Makoroff, K.L. Early clinical indicators of developmental outcome in abusive head trauma. Childs Nerv. Syst. 2012, 28, 889–896. [Google Scholar] [CrossRef]
- Gencturk, M.; Tore, H.G.; Nascene, D.R.; Zhang, L.; Koksel, Y.; McKinney, A.M. Various Cranial and Orbital Imaging Findings in Pediatric Abusive and Non-abusive Head trauma, and Relation to Outcomes. Clin. Neuroradiol. 2019, 29, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.V.; Greiner, H.M.; Caré, M.M.; Owens, D.; Shapiro, R.; Holland, K. Adding Insult to Injury: Nonconvulsive Seizures in Abusive Head Trauma. J. Child Neurol. 2015, 30, 1778–1784. [Google Scholar] [CrossRef]
- Dingman, A.L.; Stence, N.V.; O’Neill, B.R.; Sillau, S.H.; Chapman, K.E. Seizure Severity Is Correlated With Severity of Hypoxic-Ischemic Injury in Abusive Head Trauma. Pediatr. Neurol. 2018, 82, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Painter, S.; Kodagali, S.S.; Jones, N.R.; Roalfe, A.; Jayawant, S.; Elston, J.; Anand, G. Disability and visual outcomes following suspected abusive head trauma in children under 2 years. Arch. Dis. Child. 2021, 106, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.A.; Fouzdar Jain, S.; Svec, A.; Svec, C.; Haney, S.B.; Allbery, S.; High, R.; Suh, D.W. Clinical comparison of ocular and systemic findings in diagnosed cases of abusive and non-abusive head trauma. Clin. Ophthalmol. 2018, 12, 1505–1510. [Google Scholar] [CrossRef] [Green Version]
- Eismann, E.A.; Theuerling, J.; Cassedy, A.; Curry, P.A.; Colliers, T.; Makoroff, K.L. Early developmental, behavioral, and quality of life outcomes following abusive head trauma in infants. Child Abus. Negl. 2020, 108, 104643. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caré, M.M. Parenchymal Insults in Abuse—A Potential Key to Diagnosis. Diagnostics 2022, 12, 955. https://doi.org/10.3390/diagnostics12040955
Caré MM. Parenchymal Insults in Abuse—A Potential Key to Diagnosis. Diagnostics. 2022; 12(4):955. https://doi.org/10.3390/diagnostics12040955
Chicago/Turabian StyleCaré, Marguerite M. 2022. "Parenchymal Insults in Abuse—A Potential Key to Diagnosis" Diagnostics 12, no. 4: 955. https://doi.org/10.3390/diagnostics12040955
APA StyleCaré, M. M. (2022). Parenchymal Insults in Abuse—A Potential Key to Diagnosis. Diagnostics, 12(4), 955. https://doi.org/10.3390/diagnostics12040955




