Prenatal Sonographic Features of Cri-du-Chat Syndrome: A Case Report and Analytical Literature Review
Abstract
1. Introduction
2. Case Presentation
3. Literature Review and Analysis
3.1. Methods
3.2. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lejeune, J.; Lafourcade, J.; Berger, R.; Vialatte, J.; Boeswillwald, M.; Seringe, P.; Turpin, R. 3 Cases of Partial Deletion of the Short Arm of a 5 Chromosome. C. R. Hebd Seances Acad. Sci. 1963, 257, 3098–3102. [Google Scholar] [PubMed]
- Cerruti Mainardi, P. Cri du Chat syndrome. Orphanet. J. Rare Dis. 2006, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Shalev, S.; Weiner, E.; Shneor, Y.; Shalev, E. Prenatal diagnosis of 5p deletion syndrome following abnormally low maternal serum human chorionic gonadotrophin. Prenat. Diagn. 2003, 23, 572–574. [Google Scholar] [CrossRef]
- Mainardi, P.C.; Perfumo, C.; Cali, A.; Coucourde, G.; Pastore, G.; Cavani, S.; Zara, F.; Overhauser, J.; Pierluigi, M.; Bricarelli, F.D. Clinical and molecular characterisation of 80 patients with 5p deletion: Genotype-phenotype correlation. J. Med. Genet. 2001, 38, 151–158. [Google Scholar] [CrossRef]
- Bakkum, J.N.; Watson, W.J.; Johansen, K.L.; Brost, B.C. Prenatal diagnosis of cri du chat syndrome with encephalocele. Am. J. Perinatol. 2005, 22, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, L.; Brundler, A.M.; Dahoun, S. Cri-du-chat syndrome diagnosed by amniocentesis performed due to abnormal maternal serum test. Prenat. Diagn. 1998, 18, 1099–1100. [Google Scholar] [CrossRef]
- Muller, F.; Aegerter, P.; Boue, A. Prospective maternal serum human chorionic gonadotropin screening for the risk of fetal chromosome anomalies and of subsequent fetal and neonatal deaths. Prenat. Diagn. 1993, 13, 29–43. [Google Scholar] [CrossRef]
- Peng, Y.; Pang, J.; Hu, J.; Jia, Z.; Xi, H.; Ma, N.; Yang, S.; Liu, J.; Huang, X.; Tang, C.; et al. Clinical and molecular characterization of 12 prenatal cases of Cri-du-chat syndrome. Mol. Genet. Genom. Med. 2020, 8, e1312. [Google Scholar] [CrossRef]
- Stefanou, E.G.; Hanna, G.; Foakes, A.; Crocker, M.; Fitchett, M. Prenatal diagnosis of cri du chat (5p-) syndrome in association with isolated moderate bilateral ventriculomegaly. Prenat. Diagn. 2002, 22, 64–66. [Google Scholar] [CrossRef]
- Torun, D.; Bahce, M.; Alanbay, I.; Guran, S.; Baser, I. Prenatal diagnosis of Cri-du chat syndrome following high maternal serum human chorionic gonodotrophin and choroid plexus cysts. Prenat. Diagn. 2009, 29, 536–537. [Google Scholar] [CrossRef]
- Vialard, F.; Robyr, R.; Hillion, Y.; Molina Gomes, D.; Selva, J.; Ville, Y. Dandy-Walker syndrome and corpus callosum agenesis in 5p deletion. Prenat. Diagn. 2005, 25, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z. Prenatal diagnosis of Cri-du chat syndrome following high maternal serum human chorionic gonodotrophin and ventricular septal defect. Prenat. Diagn. 2009, 29, 914. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Hata, T.; Hata, K.; Miyazaki, K. Antenatal sonographic features of cri-du-chat syndrome. Ultrasound Obs. Gynecol. 1999, 13, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Tullu, M.S.; Muranjan, M.N.; Sharma, S.V.; Sahu, D.R.; Swami, S.R.; Deshmukh, C.T.; Bharucha, B.A. Cri-du-chat syndrome: Clinical profile and prenatal diagnosis. J. Postgrad. Med. 1998, 44, 101–104. [Google Scholar] [PubMed]
- Teoh, X.H.; Tan, T.Y.; Chow, K.K.; Lee, I.W. Prenatal diagnosis of cri-du-chat syndrome: Importance of ultrasonographical markers. Singap. Med. J. 2009, 50, e181–e184. [Google Scholar]
- Cardoso, M.C.; Raposo, M.I.; Ormonde, M.; Monteiro, R.; Sampaio, A.; Cosme, P.; Mota-Vieira, L. Prenatal sonographic diagnosis of isolated fetal ascites in cri-du-chat (5p-) syndrome: A case report. J. Clin. Ultrasound 2019, 47, 232–234. [Google Scholar] [CrossRef]
- Han, Y.J.; Kwak, D.W. Prenatal diagnosis of 5p deletion syndrome: A case series report. J. Genet. Med. 2017, 14, 34–37. [Google Scholar] [CrossRef][Green Version]
- Kaymak, D.; Alpay, V.; Erenel, H.; Adaletli, I.; Comunoglu, N.; Madazli, R. Prenatal Diagnosis of 5p Deletion Syndrome with Brain Abnormalities by Ultrasonography and Fetal Magnetic Resonance Imaging: A Case Report. Fetal. Pediatr. Pathol. 2020, 39, 446–451. [Google Scholar] [CrossRef]
- Mak, A.S.L.; Ma, T.W.L.; Chan, K.Y.K.; Kan, A.S.Y.; Tang, M.H.Y.; Leung, K.Y. Prenatal diagnosis of 5p deletion syndrome: Report of five cases. J. Obs. Gynaecol. Res. 2019, 45, 923–926. [Google Scholar] [CrossRef]
- Sarno, A.P., Jr.; Polzin, W.J.; Kalish, V.B. Fetal choroid plexus cysts in association with cri du chat (5p-) syndrome. Am. J. Obs. Gynecol. 1993, 169, 1614–1615. [Google Scholar] [CrossRef]
- Sherer, D.M.; Eugene, P.; Dalloul, M.; Khoury-Collado, F.; Abdelmalek, E.; Kheyman, M.; Osho, J.A.; Abulafia, O. Second-trimester diagnosis of cri du chat (5p-) syndrome following sonographic depiction of an absent fetal nasal bone. J. Ultrasound Med. 2006, 25, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Fu, H.; Xie, B.; Lu, W.; Li, W.; Wei, Y.; Zhang, Q.; Wei, S.; Chen, Q.; Lu, Y.; et al. Prenatal diagnosis of cri-du-chat syndrome by SNP array: Report of twelve cases and review of the literature. Mol. Cytogenet. 2019, 12, 49. [Google Scholar] [CrossRef]
- Hills, C.; Moller, J.H.; Finkelstein, M.; Lohr, J.; Schimmenti, L. Cri du chat syndrome and congenital heart disease: A review of previously reported cases and presentation of an additional 21 cases from the Pediatric Cardiac Care Consortium. Pediatrics 2006, 117, e924–e927. [Google Scholar] [CrossRef]
- Petersen, A.K.; Cheung, S.W.; Smith, J.L.; Bi, W.; Ward, P.A.; Peacock, S.; Braxton, A.; Van Den Veyver, I.B.; Breman, A.M. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am. J. Obs. Gynecol 2017, 217, 691.e1–691.e6. [Google Scholar] [CrossRef] [PubMed]
- Church, D.M.; Bengtsson, U.; Nielsen, K.V.; Wasmuth, J.J.; Niebuhr, E. Molecular definition of deletions of different segments of distal 5p that result in distinct phenotypic features. Am. J. Hum. Genet. 1995, 56, 1162–1172. [Google Scholar] [PubMed]
- Overhauser, J.; McMahon, J.; Oberlender, S.; Carlin, M.E.; Niebuhr, E.; Wasmuth, J.J.; Lee-Chen, J. Parental origin of chromosome 5 deletions in the cri-du-chat syndrome. Am. J. Med. Genet. 1990, 37, 83–86. [Google Scholar] [CrossRef]
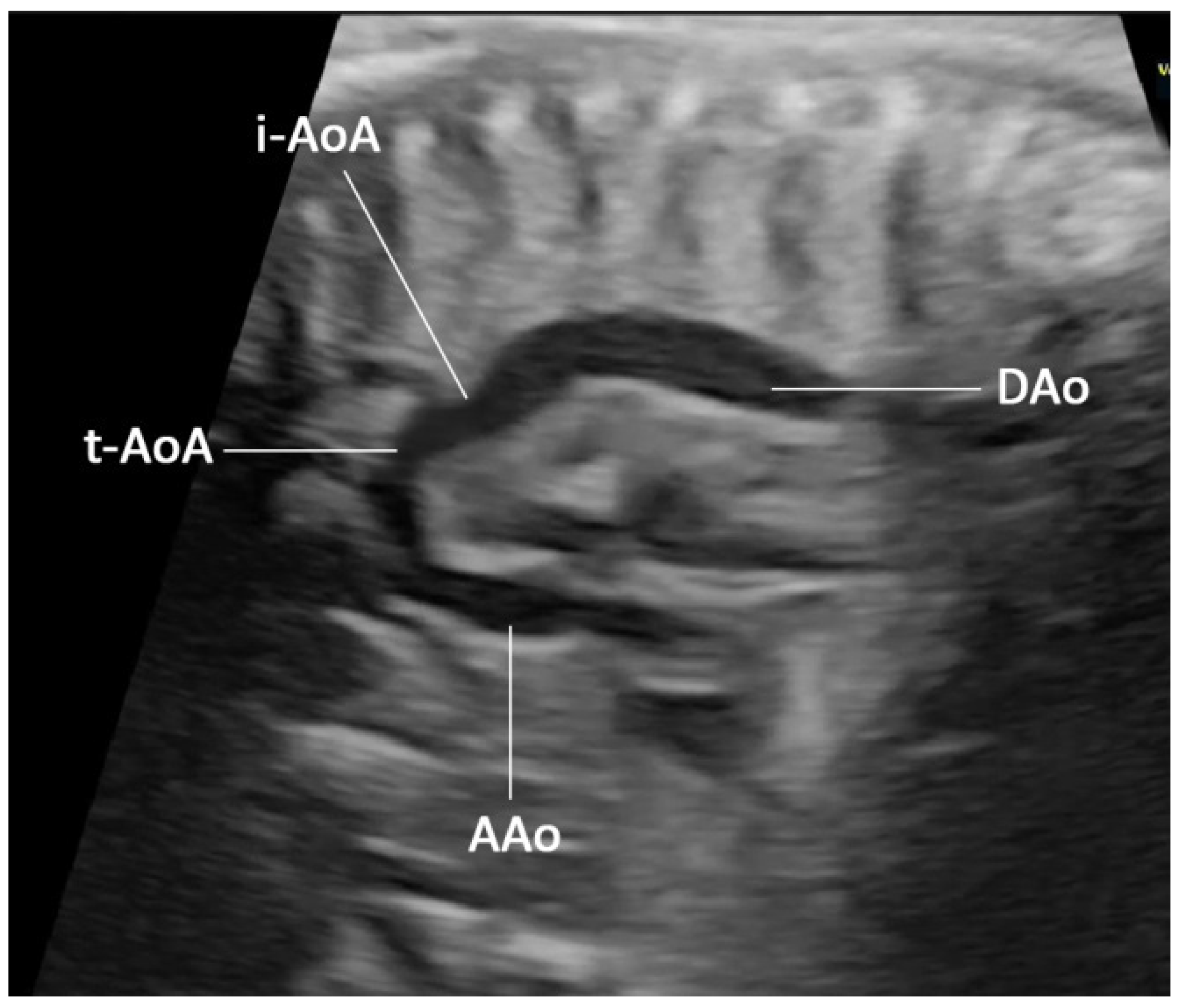
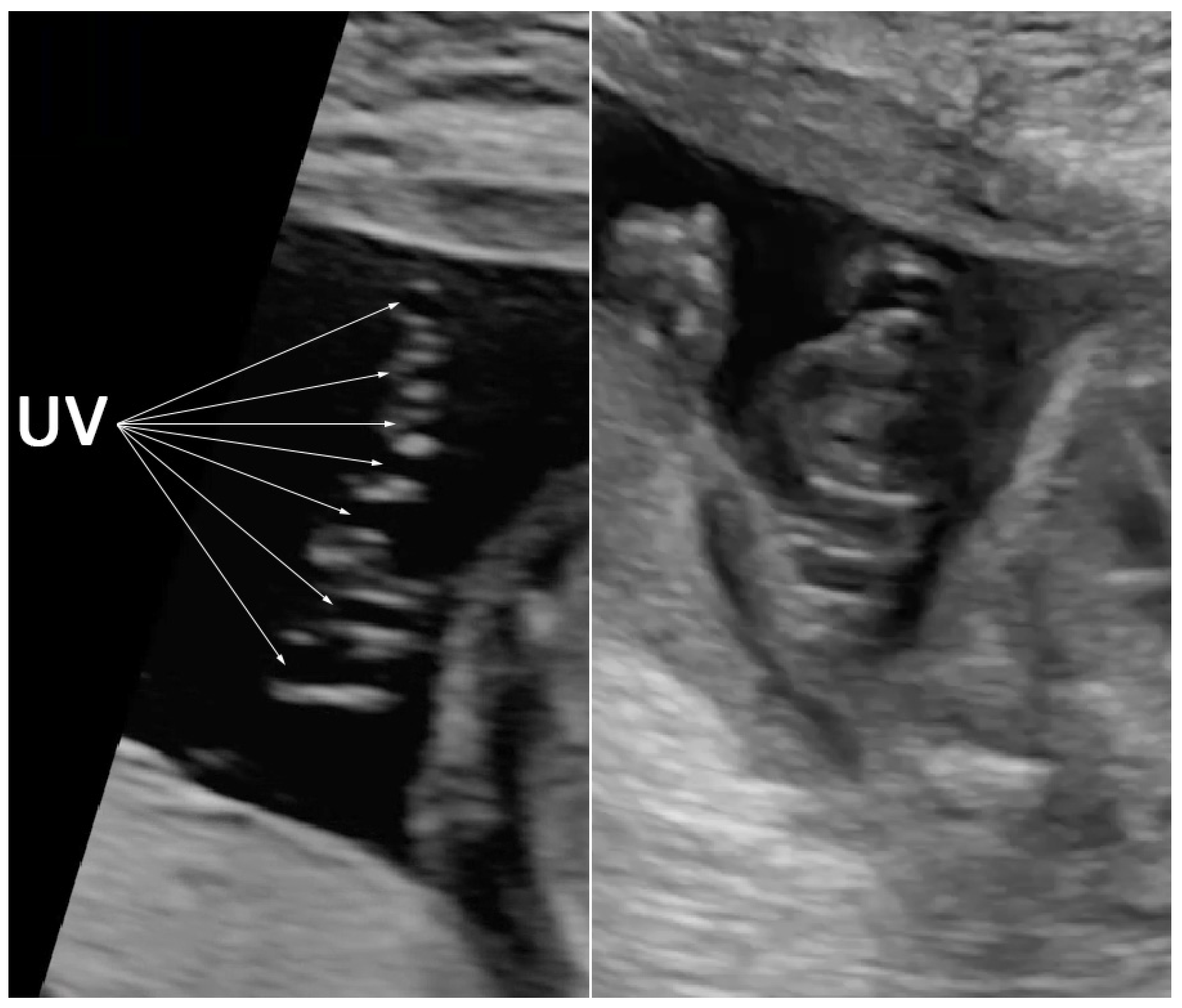
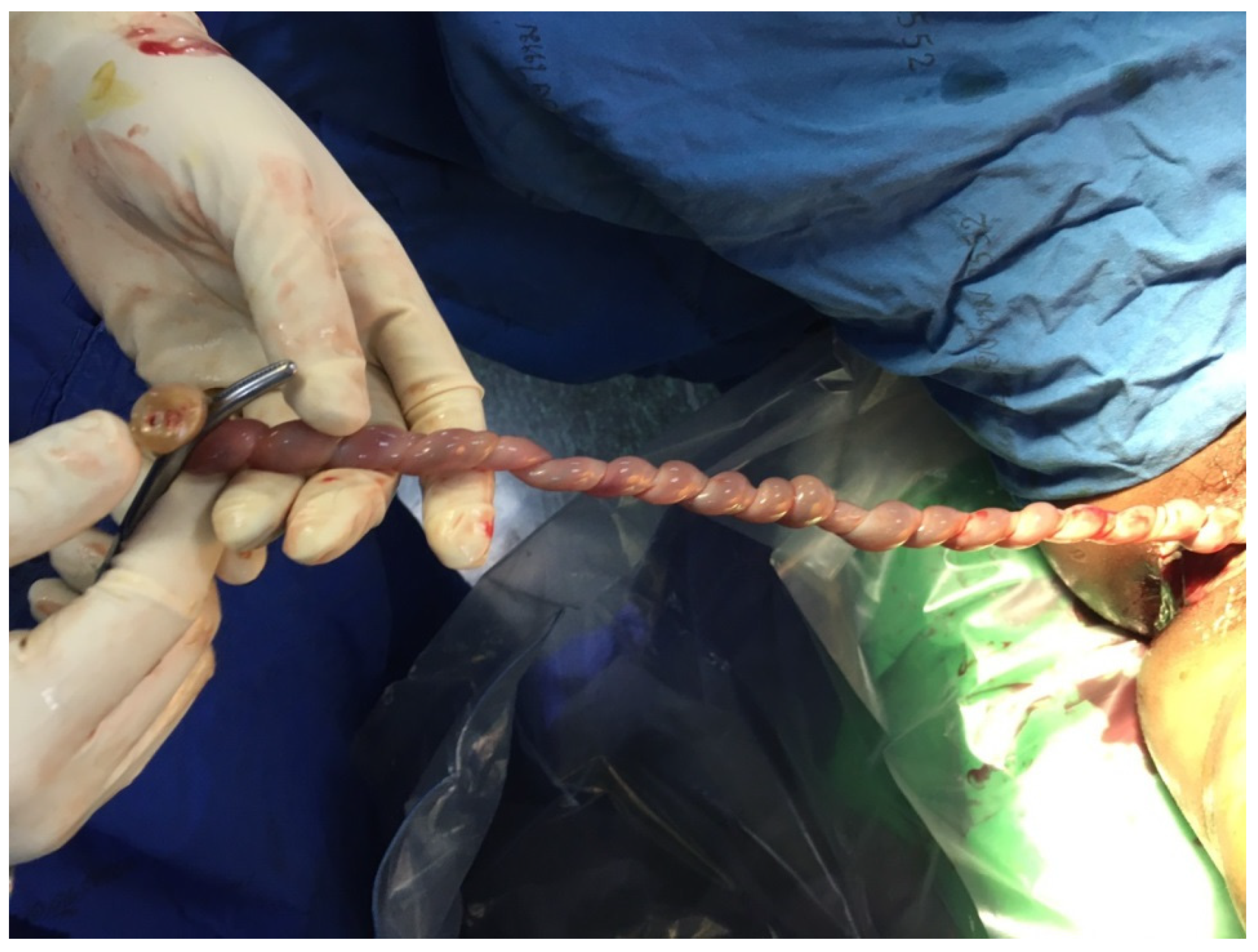
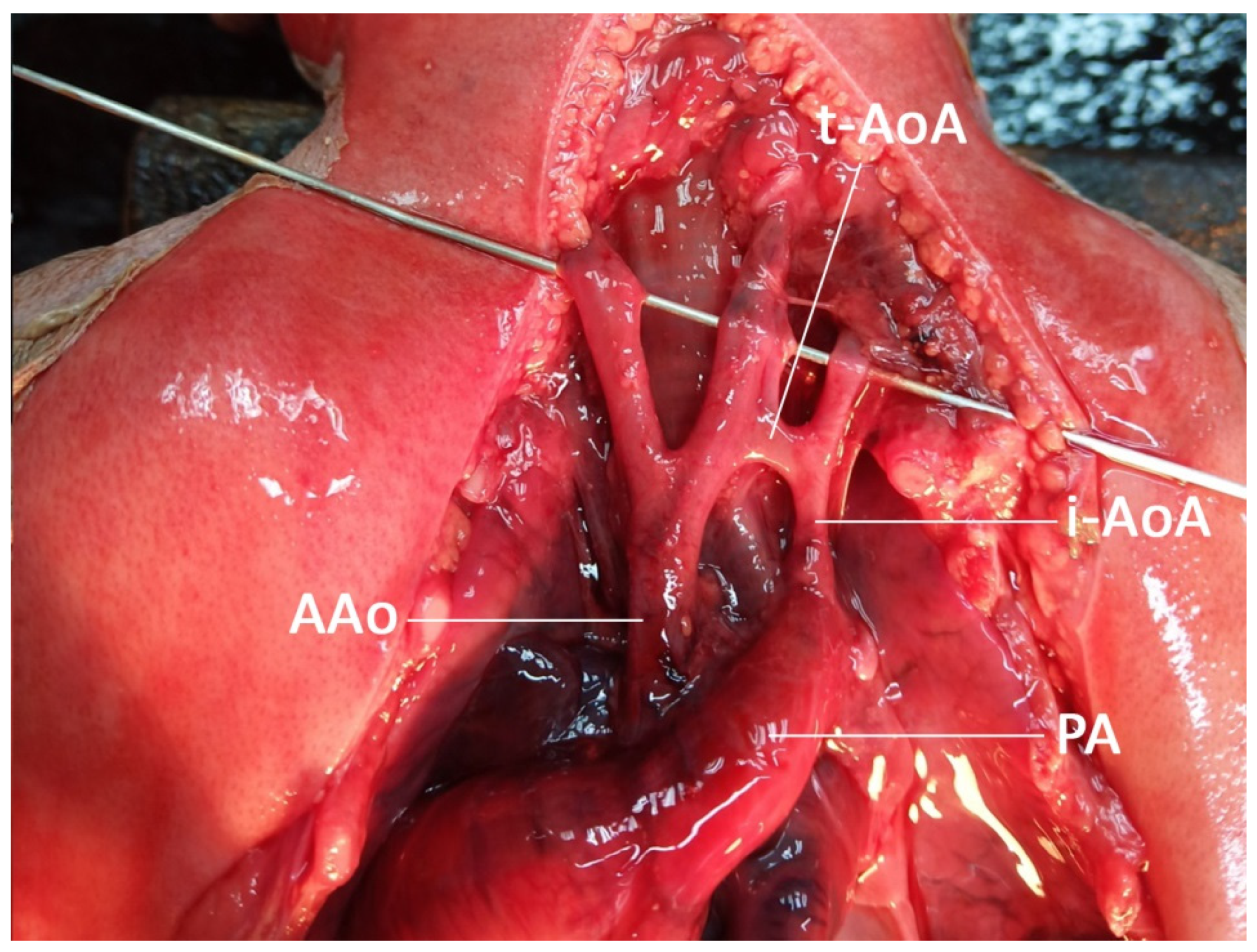
| No. | Findings | N (47) | % |
|---|---|---|---|
| 1 | Cerebellar hypoplasia | 14 | 29.8 |
| 2 | Cardiac anomaly (VSD, others) | 9 | 19.1 |
| 3 | Hydrops fetalis (fluid collection) | 8 | 17.0 |
| 4 | Ventriculomegaly | 7 | 14.9 |
| 5 | Choroid plexus cyst (CPC) | 6 | 12.8 |
| 6 | Nasal bone hypoplasia | 6 | 12.8 |
| 7 | Abnormal growth | 4 | 8.5 |
| 8 | Increased nuchal fold thickness (INF) | 4 | 8.5 |
| 9 | Increased nuchal translucency thickness (INT) | 4 | 8.5 |
| 10 | Dilated cisterna magna | 3 | 6.4 |
| 11 | Clubfoot | 2 | 4.3 |
| 12 | Hypospadias | 2 | 4.3 |
| 13 | Neural tube defect (NTD) | 2 | 4.3 |
| 14 | Pyelectasis | 2 | 4.3 |
| 15 | Single umbilical artery (SUA) | 2 | 4.3 |
| 16 | Agenesis of corpus callosum | 1 | 2.1 |
| 17 | Cystic adenomatoid malformation | 1 | 2.1 |
| 18 | Cystic hygroma | 1 | 2.1 |
| 19 | Dandy-Walker syndrome | 1 | 2.1 |
| 20 | Facial cleft | 1 | 2.1 |
| 21 | Interrupted inferior vena cava | 1 | 2.1 |
| 22 | Microretrognathia | 1 | 2.1 |
| 23 | Negative | 6 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traisrisilp, K.; Yanase, Y.; Ake-sittipaisarn, S.; Tongsong, T. Prenatal Sonographic Features of Cri-du-Chat Syndrome: A Case Report and Analytical Literature Review. Diagnostics 2022, 12, 421. https://doi.org/10.3390/diagnostics12020421
Traisrisilp K, Yanase Y, Ake-sittipaisarn S, Tongsong T. Prenatal Sonographic Features of Cri-du-Chat Syndrome: A Case Report and Analytical Literature Review. Diagnostics. 2022; 12(2):421. https://doi.org/10.3390/diagnostics12020421
Chicago/Turabian StyleTraisrisilp, Kuntharee, Yuri Yanase, Srimeunwai Ake-sittipaisarn, and Theera Tongsong. 2022. "Prenatal Sonographic Features of Cri-du-Chat Syndrome: A Case Report and Analytical Literature Review" Diagnostics 12, no. 2: 421. https://doi.org/10.3390/diagnostics12020421
APA StyleTraisrisilp, K., Yanase, Y., Ake-sittipaisarn, S., & Tongsong, T. (2022). Prenatal Sonographic Features of Cri-du-Chat Syndrome: A Case Report and Analytical Literature Review. Diagnostics, 12(2), 421. https://doi.org/10.3390/diagnostics12020421






