Prenatal Diagnosis of Bovine Aortic Arch Anatomic Variant
Abstract
:1. Introduction
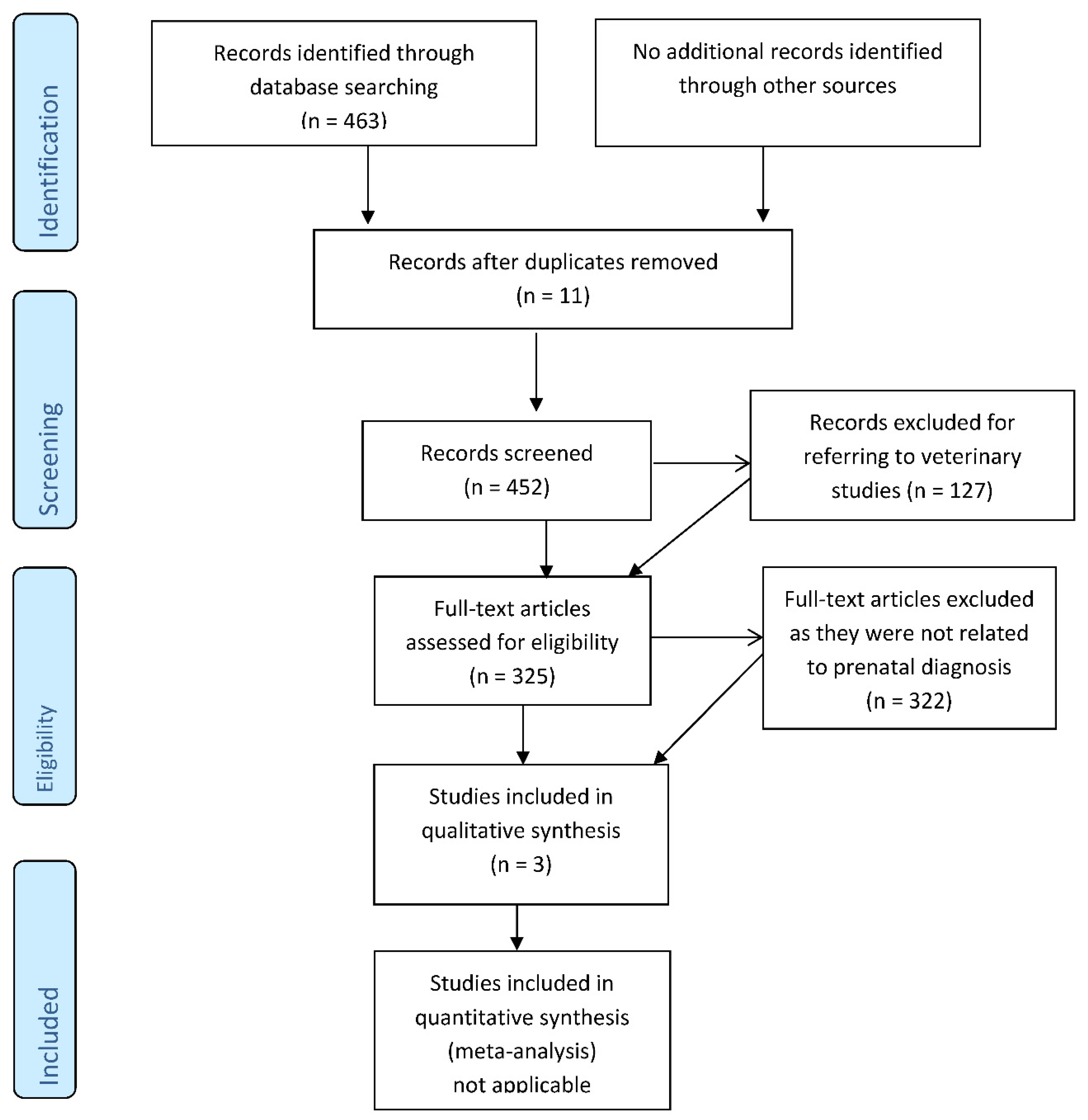
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAAV | anatomic aortic arch variant |
| IA | innominate artery |
| BCT | brachiocephalic trunk |
| RCCa | common right carotid |
| RSCa | right subclavian artery |
| LCCa | common left carotid artery |
| LSCa | left subclavian artery |
References
- Effman, E.; Whitman, S.; Smith, B. Aortic arch development. Radiographics 1986, 6, 1056–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liechty, J.D.; Shields, T.W.; Anson, B.J. Variations pertaining to the aortic arches and their branches. Q. Bull. Northwest Univ. Med. Sch. 1957, 31, 136. [Google Scholar] [PubMed]
- Zidere, V.; Tsapakis, E.G.; Huggon, I.C.; Allan, L. Right aortic arch in the fetus. Ultrasound Obstet. Gynecol. 2006, 28, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Sotiriadis, A.; Girardelli, S.; Spinillo, S.; Candiani, M.; Amodeo, S.; Farina, A.; Fesslova, V. Postnatal Outcome and Associated Anomalies of Prenatally Diagnosed Right Aortic Arch with Concomitant Right Ductal Arch: A Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Khalil, A.; Zidere, V.; Carvalho, J.S. Fetuses with right aortic arch Multicentre cohort study and meta-analysis. Ultrasound Obstet. Gynecol. 2016, 47, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Chaoui, R.; Heling, K.S.; Sarioglu, N.; Schwabe, M.; Dankof, A.; Bollmann, R. Aberrant right subclavian artery as a new cardiac sign in second- and third-trimester fetuses with Down syndrome. Am. J. Obstet. Gynecol. 2005, 192, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Scala, C.; Leone Roberti Maggiore, U.; Candiani, M.; Venturini, P.L.; Ferrero, S.; Greco, T.; Cavoretto, P. Aberrant right subclavian artery in fetuses with Down syndrome: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Babu, C.S.; Sharma, V. Two Common Trunks Arising from Arch of Aorta: Case Report and Literature Review of a Very Rare Variation. J. Clin. Diagn. Res. 2015, 9, AD05-7. [Google Scholar] [CrossRef] [PubMed]
- Jakanani, G.C.; Adair, W. Frequency of variations in aortic arch anatomy depicted on multidetector CT. Clin. Radiol. 2010, 65, 481. [Google Scholar] [CrossRef]
- Angouras, D.C.; Boudoulas, K.D.; Boudoulas, H. Bovine aortic arch: Normal variant or a marker of aortopathy? Cardiology 2012, 123, 113. [Google Scholar] [CrossRef] [PubMed]
- Layton, K.F.; Kallmes, D.F.; Cloft, H.J.; Lindell, E.P.; Cox, V.S. Bovine aortic arch variant in humans: Clarification of a common misnomer. Am. J. Neuroradiol. 2006, 27, 1541. [Google Scholar] [PubMed]
- Williams, G.D.; Aff, H.M.; Schmeckebier, M.; Edmonds, H.W.; Graul, E.G. Variations in the arrangement of the branches arising from the aortic arch in American whites and negroes. Anat. Rec. 1932, 54, 247. [Google Scholar] [CrossRef]
- Reinshagen, L.; Vodiskar, J.; Mühler, E.; Hövels-Gürich, H.H.; Vazquez-Jimenez, J.F. Bicarotid trunk: How much is “not uncommon”? Ann. Thorac. Surg. 2014, 97, 945. [Google Scholar] [CrossRef] [PubMed]
- Popieluszko, P.; Henry, B.M.; Sanna, B.; Hsieh, W.C.; Saganiak, K.; Pękala, P.A.; Walocha, J.A.; Tomaszewski, K.A. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J. Vasc. Surg. 2018, 68, 298–306.e10. [Google Scholar] [CrossRef]
- Goldsher, Y.W.; Salem, Y.; Weisz, B.; Achiron, R.; Jacobson, J.M.; Gindes, L. Bovine aortic arch: Prevalence in human fetuses. J. Clin. Ultrasound 2019, 48, 198–203. [Google Scholar] [CrossRef]
- Alghamdi, M.H.; Sanatani, S. Bovine aortic arch in association with double inlet left ventricle and pulmonary atresia: A novel association. Congenit. Heart Dis. 2009, 4, 295. [Google Scholar] [CrossRef] [PubMed]
- Clerici, G.; Giulietti, E.; Babucci, G.; Chaoui, R. Bovine aortic arch: Clinical significance and h hemodynamic evaluation. J. Matern. Fetal Neonatal Med. 2017, 31, 2381. [Google Scholar] [CrossRef]
- Pinto, A.; Luise, C.; Stella, N.; Di Prisco, L. Menditto A Clinica Mediterranea, Naples, Italy Bovine aortic arch variant T2BA: A novel association with cardiovascular pathologies and fetal structural abnormalities. In Proceedings of the 17th Eorld Congres Fetal Medicine, Athens, Greece, 24–28 June 2018; Available online: https://fetalmedicine.org/abstracts/2018/var/pdf/abstracts/2018/2912.pdf (accessed on 10 December 2021).
- Natsis, K.; Piagkou, M.; Lazaridis, N.; Kalamatianos, T.; Chytas, D.; Manatakis, D.; Anastasopoulos, N.; Loukas, M. A systematic classification of the left-sided aortic arch variants based on cadaveric studies’ prevalence. Surg. Radiol. Anat. 2021, 43, 327–345. [Google Scholar] [CrossRef]
- Marrocco-Trischitta, M.M.; Alaidroos, M.; Romarowski, R.M.; Milani, V.; Ambrogi, F.; Secchi, F.; Glauber, M.; Nano, G. Aortic arch variant with a common origin of the innominate and left carotid artery as a determinant of thoracic aortic disease: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2020, 57, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.; Piagkou, M.; Loukas, M.; Piperaki, E.T.; Totlis, T.; Noussios, G.; Natsis, K. A systematic classification of the vertebral artery variable origin: Clinical and surgical implications. Surg. Radiol. Anat. 2018, 40, 779–797. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Bartelings, M.M.; Deruiter, M.C.; Poelmann, R.E. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr. Res. 2005, 57, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, S.J.; Min, J.Y.; Lee, Y.H.; Roman, K.; Jaeggi, E.; Smallhorn, J. Fetal sonographic diagnosis of aortic arch anomalies. Ultrasound Obstet. Gynecol. 2003, 22, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y. Hoxa3 and signaling molecules involved in aortic arch patterning and remodeling. Cell Tissue Res. 2009, 336, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yang, N.; Lu, T.; Wu, T.; Wang, L.; Pan, Y.H.; Cao, X.; Yuan, X.; Wisniewski, T.; Dang, S.; et al. Adamts18 modulates the development of the aortic arch and common carotid artery. iScience 2021, 24, 102672. [Google Scholar] [CrossRef] [PubMed]
- Hornick, M.; Moomiaie, R.; Mojibian, H.; Ziganshin, B.; Almuwaqqat, Z.; Lee, E.S.; Rizzo, J.A.; Tranquilli, M.; Elefteriades, J.A. ‘Bovine’ aortic arch–a marker for thoracic aortic disease. Cardiology 2012, 123, 116. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.D.; Urbania, T.H.; Crook, S.E.; Hope, M.D. Bovine aortic arch: A novel association with thoracic aortic dilation. Clin. Radiol. 2012, 67, 28–31. [Google Scholar] [CrossRef]
- Wanamaker, K.M.; Amadi, C.C.; Mueller, J.S.; Moraca, R.J. Incidence of aortic arch anomalies in patients with thoracic aortic dissections. J. Card. Surg. 2013, 28, 151. [Google Scholar] [CrossRef]
- Amir, S.K.; Shamsuddin, A.M.; Mamat, A.Z.; Zain, M.R.M.; Ramli, N.; Mokhtar, A.M.; Ali, S. Right-sided patent ductus arteriosus with bovine aortic arch, tetralogy of Fallot, dextrocardia and situs inversus. J. Surg. Case Rep. 2021, 2021, rjab307. [Google Scholar] [CrossRef]
- Turek, J.W.; Conway, B.D.; Cavanaugh, N.B.; Meyer, A.M.; Aldoss, O.; Reinking, B.E.; El-Hattab, A.; Rossi, N.P. Bovine arch anatomy influences recoarctation rates in the era of the extended end-to-end anastomosis. J. Thorac. Cardiovasc. Surg. 2018, 155, 1178. [Google Scholar] [CrossRef] [Green Version]
- Mazzanti, L.; Lovato, L.; Prandstraller, D.; Scarano, E.; Tamburrino, F.; Montanari, F.; Mineo, G.G.; Perri, A.; Vestrucci, B.; Giardini, A. Turner syndrome strategies to improve care outcomes--cardiac evaluation using new imaging techniques. Pediatr. Endocrinol. Rev. 2012, 9 (Suppl. 2), 701–709. [Google Scholar]
- Granger, A.; Zurada, A.; Zurada-Zielińska, A.; Gielecki, J.; Loukas, M. Anatomy of turner syndrome. Clin. Anat. 2016, 29, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.A. Finding value in a common medical misnomer: The importance of identifying a bovine arch in patients with aortic coarctation. J. Thorac. Cardiovasc. Surg. 2018, 155, 1176–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celikyay, Z.R.Y.; Koner, A.E.; Celikyay, F.; Denız, C.; Acu, B.; Firat, M.M. Frequency and imaging findings of variations in human aortic arch anatomy based on multidetector computed tomography data. Clin. Imaging 2013, 37, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Uchino, A.; Saito, N.; Okada, Y.; Kozawa, E.; Nishi, N.; Mizukoshi, W.; Nakajima, R.; Takahashi, M.; Watanabe, Y. Variation of the origin of the left common carotid artery diagnosed by CT angiography. Surg. Radiol. Anat. 2013, 35, 339–342. [Google Scholar] [CrossRef]
- Ergun, O.; Tatar, İ.G.; Birgi, E.; Durmaz, H.A.; Akçalar, S.; Kurt, A.; Hekimoğlu, B. Angiographic evaluation of branching pattern and anatomy of the aortic arch. Turk. Kardiyol. Dern. Ars. 2015, 43, 219–226. [Google Scholar] [CrossRef]
- Werner, M.; Bausback, Y.; Braunlich, S.; Ulrich, M.; Piorkowski, M.; Friedenberger, J.; Schuster, J.; Botsios, S.; Scheinert, D.; Schmidt, A. Anatomic variables contributing to a higher periprocedural incidence of stroke and TIA in carotid artery stenting: Single center experience of 833 consecutive cases. Catheter. Cardiovasc. Interv. 2012, 80, 32. [Google Scholar] [CrossRef]
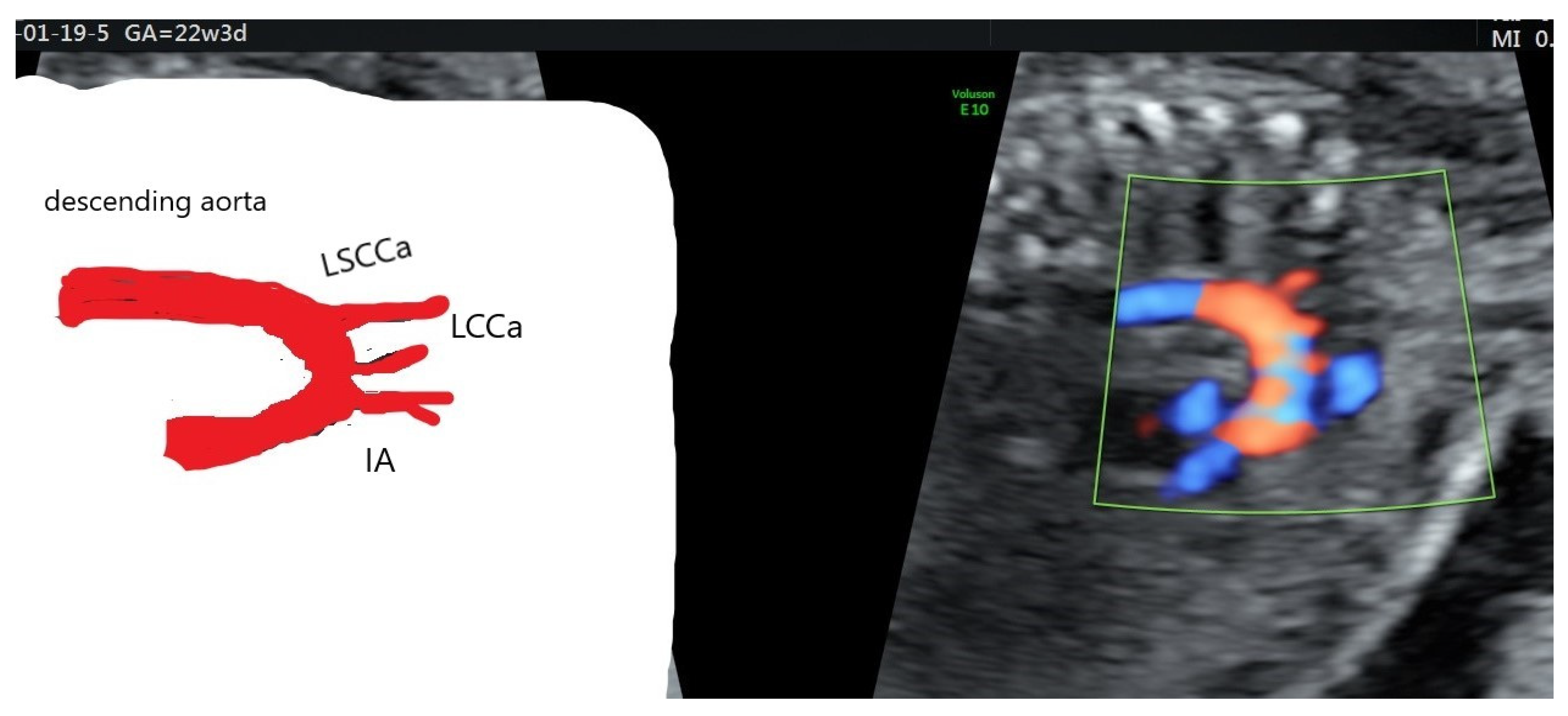
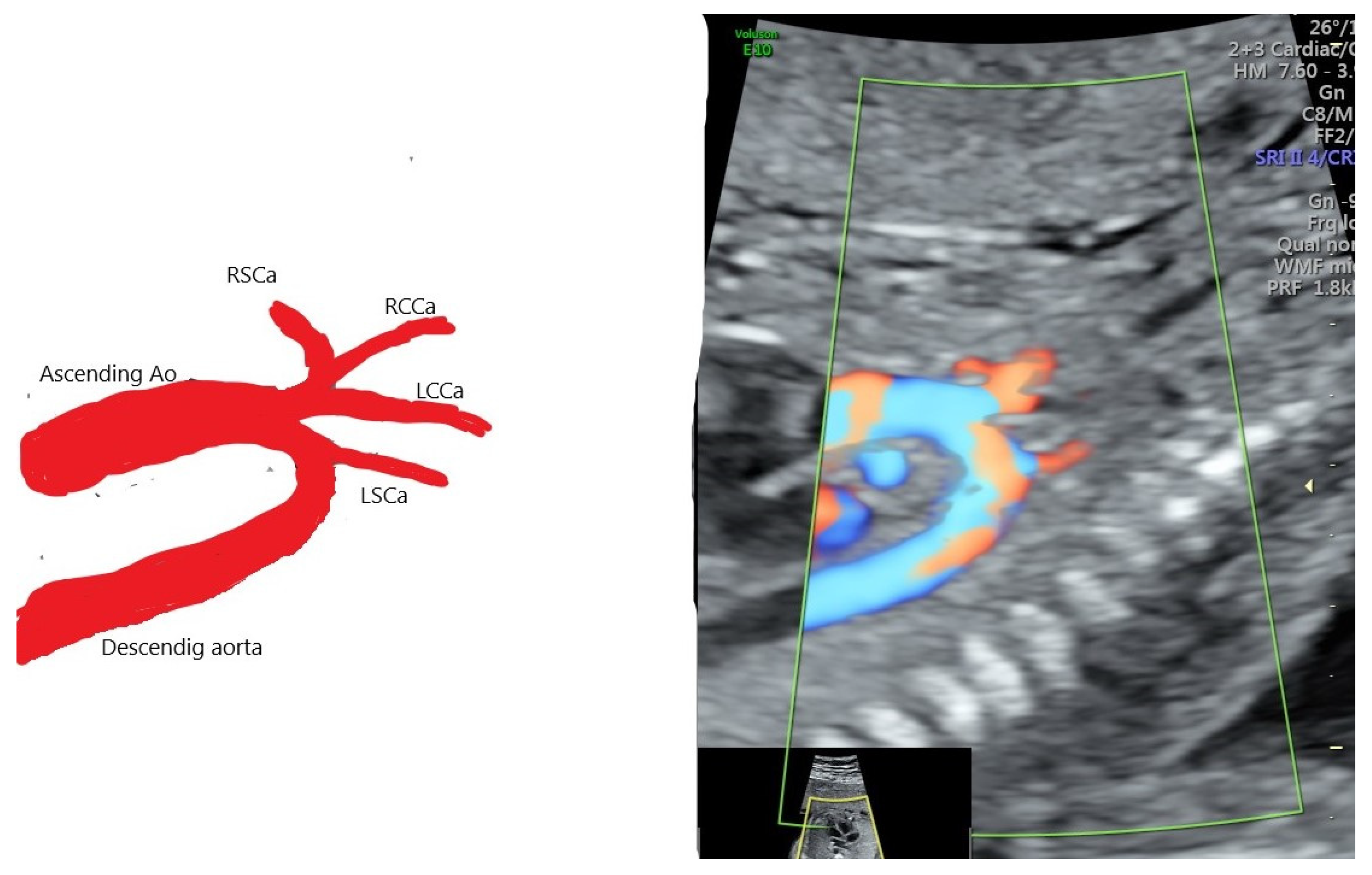


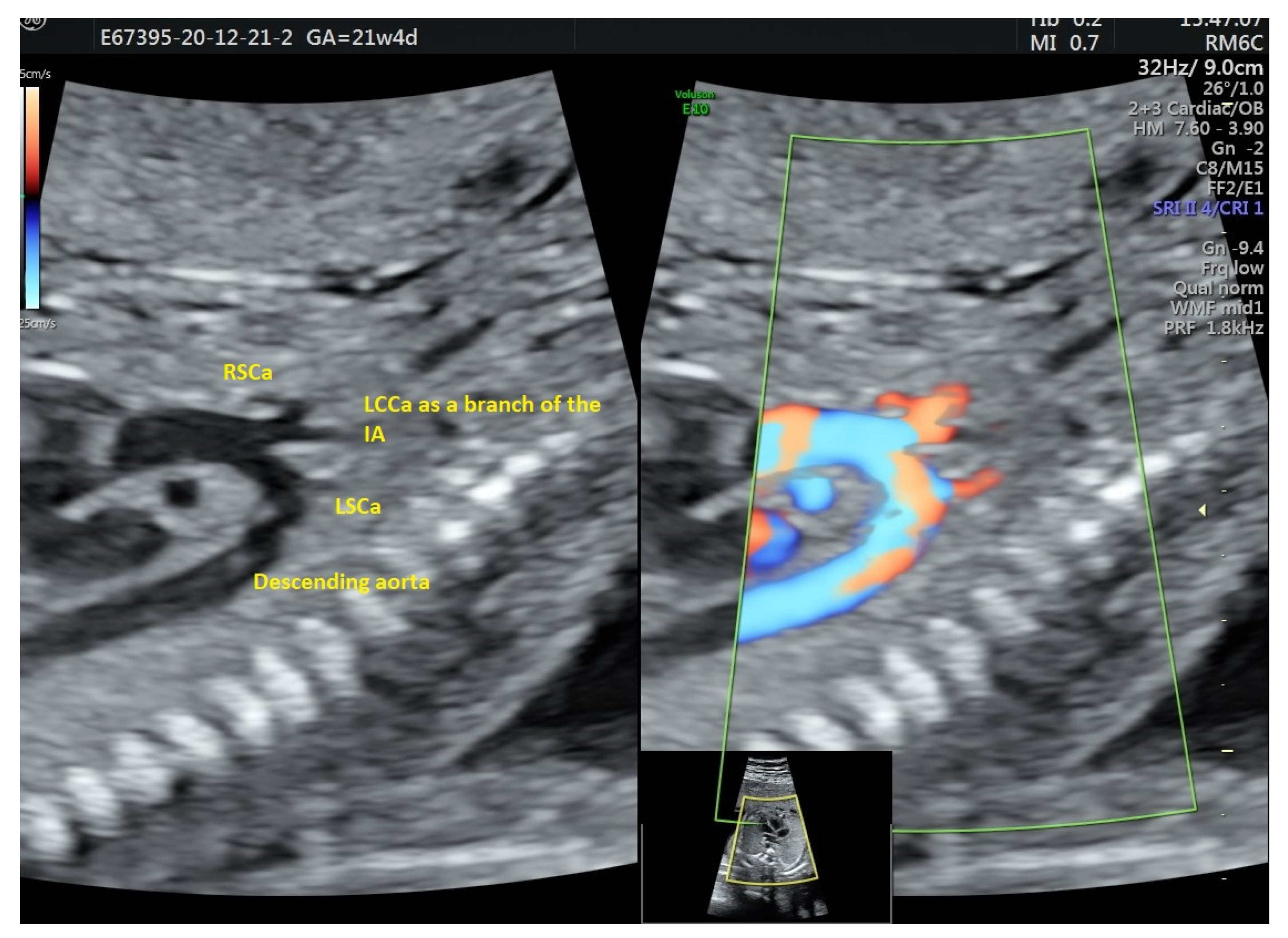
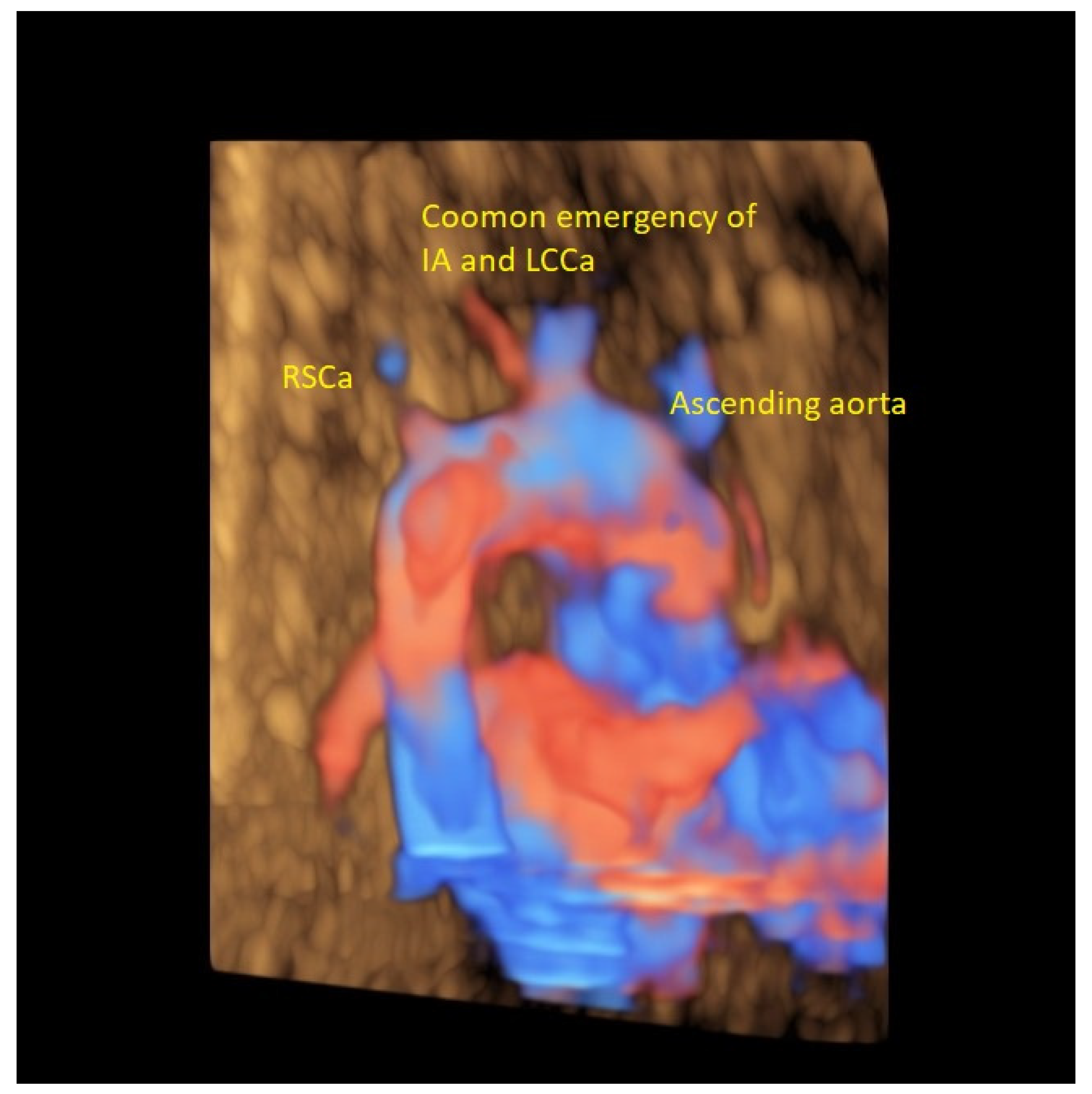
| Case Maternal Age | Gestational Age at Diagnosis | Parity | Type of AAAV | Significant Maternal Conditions | Associations | Genetic Testing | Outcome at Birth |
|---|---|---|---|---|---|---|---|
| 31 | 21 + 4 | 1 | type B | none | none | ND | Normal neonatal cardiac scan confirmed AAAV |
| 43 | 23 + 2 | 1 | type B | IVF pregnancy | FGR | NIPT (low risk) | Normal small-outlet VSD postnatal scan confirmed AAAV |
| 28 | 30 + 2 | 3 | type B | Previous fetus with Noonan sdr | Megacysterna magna, weird profile with long philtrum | Amnio 46XY | Normal neonatal cardiac scan confirmed AAAV |
| 32 | 24 + 1 | 2 | type B | none | Bilateral hydronephrosis | ND | Normal neonatal cardiac scan confirmed AAAV |
| 36 | 32 + 3 | 1 | type A | MBI 36.5 | Polyhydramnios | NIPT (low risk) | Premature birth neonatal cardiac scan confirmed AAAV |
| Study, Author Type Year | Number of Reported Patients | Gestational Age at Diagnosis (Wks.) | Associated Anomalies | Fetal Gender | Genetic Assessment | Follow-Up Image Findings | Pregnancy Outcome | Neonatal/Post Termination Diagnosis |
|---|---|---|---|---|---|---|---|---|
| Goldsher YW 2019 Retrospective | 20 4.8% | 15–40 weks | No | NR | NP | NR | Normal | NR |
| Pinto A2018 Retrosepective | 13 2% | Average 23 wks | 7 53.8% VSD, HB, ARSA, DIRV, D-TGA Polidactily | NR | 9 normal 4 NP | NR | Nr | 4 neonate with major cardiac anomalies requiered surgery |
| Clerici G 2018 Prospective descriptive | 45 6.06% | 21–39 wks | NR | 66.7% males | Not performed | Velocimetry Differences from Type I AA | Normal | Non reported |
| Alghamadi MH 2009 | Case report | No specifid | DILV Sigle coronary artery Bovine aortic arch B | Male | Normal | NR | Normal | Anomalies confirmed, surgery after DA patency mentained by prostaglandin infusion Scheduled for Fontan palliation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleș, L.; Cîrstoveanu, C.; Sima, R.-M.; Gorecki, G.-P.; Chicea, R.; Haj Hamoud, B. Prenatal Diagnosis of Bovine Aortic Arch Anatomic Variant. Diagnostics 2022, 12, 624. https://doi.org/10.3390/diagnostics12030624
Pleș L, Cîrstoveanu C, Sima R-M, Gorecki G-P, Chicea R, Haj Hamoud B. Prenatal Diagnosis of Bovine Aortic Arch Anatomic Variant. Diagnostics. 2022; 12(3):624. https://doi.org/10.3390/diagnostics12030624
Chicago/Turabian StylePleș, Liana, Cătălin Cîrstoveanu, Romina-Marina Sima, Gabriel-Petre Gorecki, Radu Chicea, and Bashar Haj Hamoud. 2022. "Prenatal Diagnosis of Bovine Aortic Arch Anatomic Variant" Diagnostics 12, no. 3: 624. https://doi.org/10.3390/diagnostics12030624
APA StylePleș, L., Cîrstoveanu, C., Sima, R.-M., Gorecki, G.-P., Chicea, R., & Haj Hamoud, B. (2022). Prenatal Diagnosis of Bovine Aortic Arch Anatomic Variant. Diagnostics, 12(3), 624. https://doi.org/10.3390/diagnostics12030624







