1. Introduction
Lung cancer is a severe disease that predominantly affects heavy smokers or ex-smokers and represents with an estimated 1.8 million cases worldwide the leading cause of cancer death in 2020 [
1]. If lung cancer is detected at an early stage, the success of therapy can be significantly improved, so that early detection plays an important role.
Due to technological progress in recent years, low-dose computed tomography (LDCT) offers a promising perspective for the early detection of lung cancer in asymptomatic (ex-)smokers. A meta-analysis of randomized controlled trials (RCTs) recently presented by our group [
2] underlines that LDCT screening can significantly reduce lung cancer mortality in heavy (ex-)smokers by about 20% compared to a non-screened group. However, this benefit must be carefully weighed against adverse health effects associated with this screening approach.
This applies in particular for radiation risks unavoidably related to CT screening examinations of the chest. In this context, it is essential to consider that only a small portion of screening participants will benefit from the examinations due to the low prevalence of lung cancer in the target population, while all participants will be subjected to the risks associated with the test [
3]. The European Directive 2013/59/Euratom [
4] underlines this dilemma and thus requires a specific justification for each medical radiological procedure performed on asymptomatic individuals for early detection of disease (article 55 para. 2 letters f, h).
It is the aim of the present article (i) to give an overview of current radiation risk models with respect to lung cancer LDCT screening, including some that consider possible interactions between radiation and smoking, and (ii) to provide conservative estimates, for different screening scenarios, addressing both lifetime radiation risks and benefit–risk ratios associated with the LDCT examinations. The focus of this study is to obtain appropriate and up-to-date estimates of radiation risk (vs. benefit) for a Western European population using current German baseline data. Due to the relatively low radiation exposure of LDCT, only stochastic radiation effects are relevant in this context and thus only these will be dealt with. This especially includes the radiation-induced development of malignant diseases that can occur years or even decades after radiation exposure (latency period). Since various aspects of analyzing and modelling stochastic radiation risks are still subject of scientific debate, radiation risks in this study will be estimated using conservative assumptions that are consistent with radio-epidemiological data obtained over many decades.
2. Theoretical Background
2.1. Basic Assumptions and Study Cohorts of Persons Exposed to Ionizing Radiation
The risk of radiation-induced cancers and leukaemias is derived from radio-epidemiological studies of large cohorts of persons who were exposed to ionizing radiation and appropriate control groups of non-exposed or low-exposed persons. Data on the type and level of radiation exposure, age at exposure, age attained during follow-up, gender as well as type and time of disease and/or disease-specific deaths occurring during observation are recorded over a long period of time. The cancer rates observed in the exposed cohort are compared with those of the control group to derive dose-dependent risk estimates. In addition, other parameters that may influence radiation risk, such as age and gender, can also be accounted for in these risk analyses (e.g., [
5]).
By means of radio-epidemiological studies, however, significant increases in cancer risk can only be detected at dose levels that are much higher than the radiation doses associated with most X-ray examinations. Even for a large cohort of persons exposed to low doses of only a few millisieverts (mSv), stochastic radiation effects cannot be observed with statistical significance [
6]. For the low dose range, therefore, no reliable information on the dose–effect relation is available, so that one has to rely on an extrapolation of the effects observed in the higher dose range towards lower doses. For this purpose, radiation protection assumes a proportional relationship between cancer risk and dose without assuming a threshold dose (linear-non-threshold, LNT hypothesis). According to the International Commission on Radiological Protection (ICRP), the use of the LNT model represents “the best practical approach to managing risk from radiation exposure and commensurate with the ‘precautionary principle’” [
7]. Furthermore, for solid cancers, the ICRP assumes a dose and dose rate effectiveness factor (DDREF) of 2 for the low dose range. In fact, the DDREF is a reduction factor that is intended to account for the lower biological effectiveness (per dose unit) of radiation exposure assumed by the ICRP at low doses and low dose rates compared with an exposure at high doses and high dose rates. Other commissions adopt a DDREF of 1.5 (BEIR VII Committee [
5]) or of 1 (German Radiation Protection Commission, SSK [
8]).
The assessment of radiation risks at higher doses can rely on a solid database of epidemiological studies. The so-called Life Span Study (LSS) of survivors of the atomic bombings in Hiroshima and Nagasaki exposed to acute gamma (and to some extent neutron) radiation is the main source of current knowledge on radiation risks due to its size (more than 85,000 persons of both sexes and all ages with dose estimates), the long observation period (>50 years) and the large internal control group of low- or non-exposed survivors. It is thus the most important basis for quantitative risk assessments [
5,
9]. For the Japanese LSS, data are available on both cancer incidence (follow-up from 1958, the start of cancer registries in Hiroshima and Nagasaki) and cancer mortality (follow-up from 1950). In addition to the Japanese LSS, there are also numerous study cohorts of persons who were exposed to X-ray or other radiation for diagnostic or therapeutic reasons, e.g., the study by Boice et al. [
10] in which women received pneumothorax therapy for tuberculosis and had repeated chest X-rays for control. As with breast cancer, the evidence on radiation risk in lung cancer has been derived not only from the Japanese LSS but also from studies of patient populations undergoing radiotherapy for malignant or benign diseases, such as Hodgkin’s lymphoma [
11] or ankylosing spondylitis [
12]. For an overview of relevant studies, see [
5] or [
13].
2.2. Risk Model Approaches: Absolute and Relative Risk Models
In the following, the terminology primarily refers to cancer incidence. But the terms introduced for this purpose apply accordingly to cancer mortality. For reasons of simplicity, a separate parameter for gender is omitted.
The age-specific baseline (or background) rate
r0(
a) is the rate of a specific type of cancer occurring in a normal population at a given age,
a. The excess absolute rate,
ear(
a,
e,
D), is the age-specific absolute rate of cancer caused by radiation exposure at a certain age,
e, to an organ equivalent dose,
D. In one approach, the radiation-related increased cancer rate,
r(
a,
e,
D), is given by the sum:
In this so-called absolute (or additive) risk model it is assumed that the excess absolute rate is independent of the background rate. An alternative approach, the so-called relative (or multiplicative) risk model, is based on the assumption that the excess rate depends on the background rate, i.e., is a certain multiple of the background rate:
with
the so-called excess relative rate, i.e., the age-specific relative cancer rate caused by radiation exposure at age
e to an organ equivalent dose,
D. For example, an excess relative rate of 1 means that the cancer rate observed in the exposure group is twice as high as expected (
r = 2
r0), i.e., compared to the baseline rate.
Several measures of lifetime risk have been used to express radiation risks [
13,
14,
15,
16]. The BEIR VII committee has chosen to use what Kellerer et al. refer to as the lifetime attributable risk,
LAR [
16]. Denoting the minimum latency time to clinical manifestation of radiation-induced cancer by
lag, LAR is the radiation-related excess risk of developing cancer from
e +
lag to the end of life,
amax. Correspondingly, the
LAR is the excess absolute cancer rate,
ear, integrated over time from
e +
lag. To account for competing (life-shortening) risks,
ear is usually multiplied by the age-specific conditional survival probability
S(
a,
e) of a person who has already reached age
e [
5]:
2.3. Risk Transfer between Populations with Different Cancer Rates
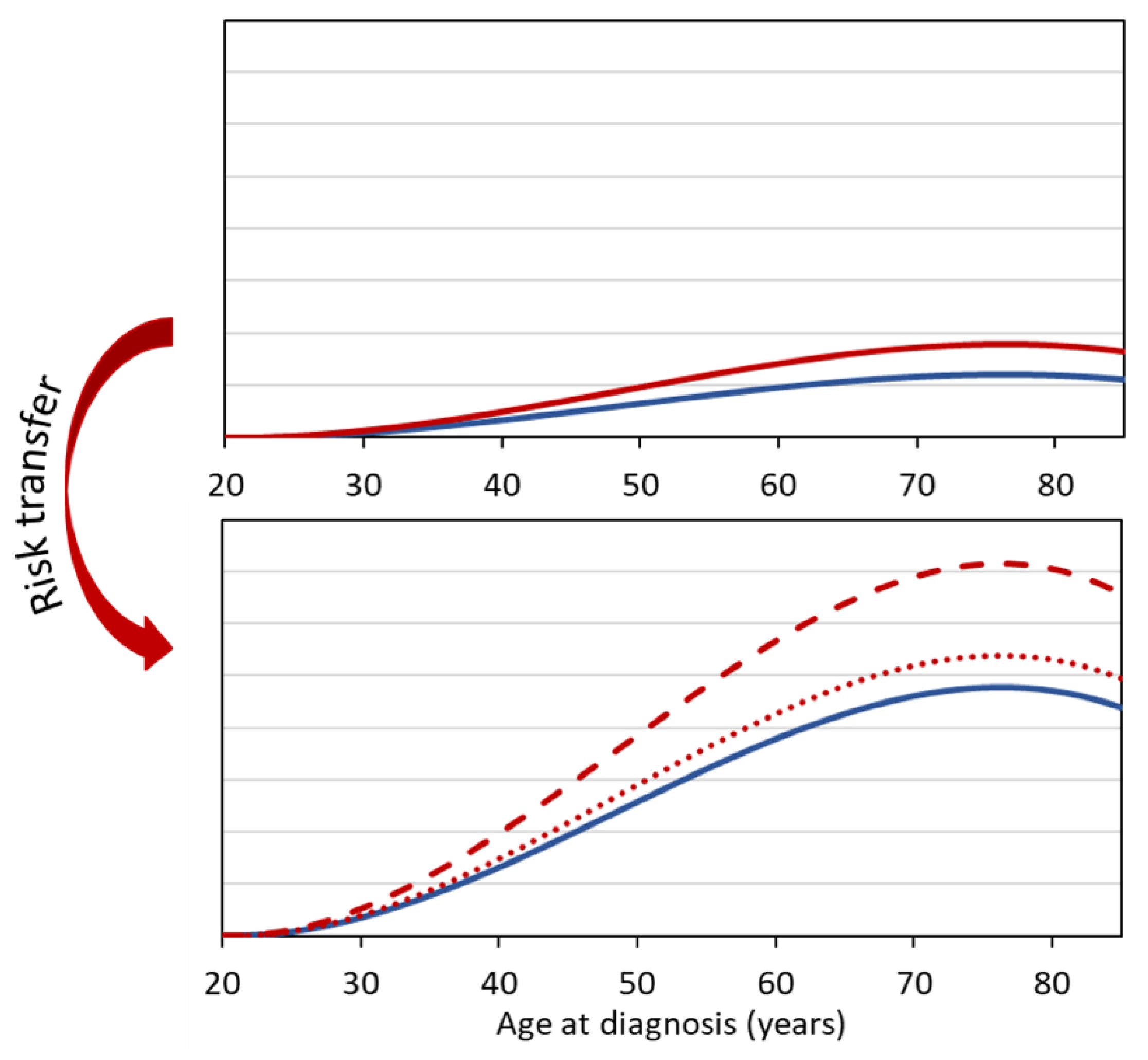
For gamma radiation, and thus also for X-rays, risk estimates or risk models are mostly based on analyses of data of the LSS of Japanese atomic bomb survivors. When transferring risk estimates to Western populations, the question arises whether the relative risk model (multiplicative transfer model) can be used to determine the excess risk in the Western population, or whether the absolute risk model (additive transfer model) is more appropriate. In the first case, in Equation (2) the baseline rate of the Western population is to be used, while in the second case only the excess rate of the exposed (Japanese) study cohort is accounted for (Equation (1)). The same problem arises if the normal rates of two cohorts differ due to different non-radiation risk factors, e.g., due to strongly differing smoking behavior.
For cancers for which baseline rates vary substantially between different populations, the method of risk transfer is crucial. For example, the baseline rates for breast cancer in Germany are considerably higher than the breast cancer rates in Japan. Similarly, lung cancer rates are significantly higher in heavy smokers than in moderate or non-smokers.
Figure 1 illustrates that in such cases the choice of the transfer model (multiplicative or additive approach) leads to significantly different results for the estimated radiation risk.
Besides the purely additive or multiplicative model, there are also more complex models such as the models of the BEIR VII Committee [
5] or models that consider a possible interaction between radiation and smoking [
17].
2.4. The Risk Models of the BEIR VII Committee
In 2006, the most recent report of the BEIR Committee was published [
5]. It is the seventh report in a series of contributions of the National Research Council of the USA, dealing with the effects of ionizing radiation in the low dose range. On the one hand, the BEIR VII report is a comprehensive review of the most recent radiation biological, biophysical, and radiation epidemiological literature at the time of evaluation. On the other hand, the report also gives comprehensive radiation risk models for cancer incidence and mortality. These are based on data from the LSS of Japanese atomic bomb survivors as well as on meta-analyses of data from persons exposed for diagnostic or therapeutic reasons.
The BEIR VII Committee considered both relative and absolute risk models to model the radiation-related excess rates defined in Equations (1) and (2). The models depend not only on organ equivalent dose, D, and gender, but also on age attained, a, and, in the case of leukemia, time since exposure, t. The risk models include linear dose response relationships for all solid tumors and a linear quadratic dose relationship for leukemias. Again, a separate parameter to account for the influence of gender is omitted.
For persons exposed after age 30, the models are:
• For solid malignant tumors (except breast cancer and thyroid cancer)
The parameters denoted by Greek letters are the result of maximum likelihood analyses to the Japanese LSS data. These parameters differ for the different organs and for the relative and absolute risk models. The parameter β differs also for male and female persons.
For breast and thyroid cancer, the risk models used by the BEIR VII Committee deviate from those in Equations (4) and (5). For thyroid cancer, no absolute risk model was used, but only a relative risk model with an age at exposure dependence based on a meta-analysis by Ron et al. [
18]:
This meta-analysis demonstrated that the excess risk was significantly affected by age at exposure, e, with a strong decrease in risk with increasing age at exposure.
For breast cancer, an absolute risk model with dependencies on age at exposure and attained age based on a meta-analysis by Preston et al. [
19] was considered:
In the study by Preston et al., a pronounced dependence of the excess absolute risk per dose was observed both on age at exposure and on age attained, with a decrease with age of exposure and an increase with attained age. For the Japanese LSS data on breast cancer, the BEIR VII Committee also performed risk modeling for a relative risk model. However, because Preston et al. [
19] also included data from Western cohorts in their modeling, the BEIR VII Committee favored the absolute risk model (Equation (7)).
With the exception of thyroid and breast cancer, a mixed risk transfer approach was used for other cancers. Accounting for both models, the
LAR (Equation (3)) estimates resulting from the relative risk model,
LARrel, and the
LAR estimates resulting from the absolute risk model,
LARabs, were combined by calculating the geometric mean with different weights:
where
wrel = 0.7 and
wabs = 0.3 for cancers other than breast, thyroid and lung cancer and
wrel = 0.3 and
wabs = 0.7 for lung cancer. The BEIR VII Committee thus adopted a predominantly additive approach for lung cancer and a predominantly multiplicative approach for other cancers (except for thyroid and breast cancer). The higher weighting of the relative risk model for cancers other than breast, thyroid, and lung cancer was due to the observation that relative risk models often provide a slightly better fit to the data. In addition, the BEIR VII Committee assumed that relative risk models are less susceptible to potential bias from underreporting of cancer cases.
The BEIR VII committee estimated 95% “subjective confidence intervals (CI)” including not only random errors but also “judgmental uncertainties”. These reflect the most important sources of uncertainty, namely, statistical variation, uncertainty in the DDREF, and uncertainty in the method of risk transfer from the Japanese LSS to the U.S. population. The resulting subjective CI of the estimated LAR values for all solid cancers can roughly be given by the interval [0.5 LAR, 2 LAR].
2.5. Interaction between Radiation and Smoking for Lung Cancer Risk
The question of a possible synergistic or antagonistic interaction of the two noxious agents radiation and smoking plays an important role in the assessment of the radiation risks of lung cancer. Depending on the model, the risk of radiation-related lung cancer may be overestimated or underestimated. Four models that assume either independent or interacting risks between radiation and smoking are explained below. The additional risk caused by smoking alone is denoted by ears(a), which is 0 for non-smokers.
• Simple additive model: The radiation-related excess absolute rate,
ear(
a,
e,
D), for smokers and non-smokers is equal.
• Generalized additive model:
ear(
a,
e,
D) for smokers and for non-smokers differ.
If ρ is smaller than 1, the radiation-associated risk for smokers is smaller than that for non-smokers; if it is larger than 1, the radiation risk for smokers is higher than that for non-smokers.
• Simple multiplicative model: The radiation-related excess relative rate
err(
a, e, D) for smokers and non-smokers is equal.
• Generalized multiplicative model:
err(
e,
a,
D) for smokers and for non-smokers differ.
with
ρ as defined in Equation (10).
The BEIR VII Committee’s favored risk model for lung cancer gives more weight to the additive approach for the interaction between radiation and smoking than to the multiplicative approach. This was motivated by the results of a former analysis by Pierce et al. [
20], which found an additive relationship between radiation and smoking. Preston et al. [
21] analyzed the incidence data of solid cancers in the Japanese LSS, which was the same data set used for modeling in the BEIR VII report. Although smoking could not be explicitly accounted for in these analyses, the authors concluded that smoking and radiation may have independent (additive) effects on lung cancer risk in the Japanese LSS. In a recent study, Cahoon et al. analyzed the incidence data of the Japanese LSS (follow-up 1958–2009) for cancer in lung and other respiratory organs [
22]. To characterize the combined effect of radiation and smoking, they considered both (generalized) additive and (generalized) multiplicative models. The generalized multiplicative model yields the best fit to the data. Similar to an older study of the same study population with follow-up until 1999 [
17], the analyses showed a significantly higher excess relative risk per dose for lung cancer at low to moderate compared to high tobacco use. No radiation-associated excess risk was observed in heavy smokers (from one pack per day). In an analysis by Grant et al. [
23], the Japanese LSS incidence data were analyzed for the sum of all solid cancers (follow-up 1958–2009). Smoking as a factor influencing the radiation-associated risk was also examined. Only the simple additive and multiplicative models were considered. The authors opted for the simple multiplicative model, although the additive model achieved a better fit of the data. However, this was for practical reasons, as it allowed comparison with previous analyses of the Japanese LSS data, where cigarette consumption was not accounted for. Overall, it was concluded that the choice of model—multiplicative or additive—had little impact on the shape of the dose–effect curve or the age dependencies of the radiation-related risk, suggesting that smoking only slightly modifies radiation risk estimates. In a study of mortality data from the Japanese LSS with follow-up until 2003 by Ozasa et al. [
24], models similar to the BEIR VII models were applied, i.e., multiplicative and absolute models. No information on smoking behavior was included in the analysis. However, the radiation risk estimates are consistent with those estimated by Cahoon et al. [
22] using the generalized multiplicative model for non-smokers.
In summary, there is currently no consensus on a possible interaction of smoking and radiation. Therefore, the BEIR VII method of LAR weighting for lung cancer is used in the following estimation of the radiation risk resulting from LDCT screening.
4. Results
Representative organ-specific dose coefficients used for the estimation of the required organ doses are summarized in
Table 2. Multiplying these coefficients by the
CTDIvol of any LDCT scan used for lung cancer screening provides representative organ doses.
Figure 2 gives the organ-specific
LAR for cancer incidence for organ equivalent doses of 10 mSv for the German general population separately for women and men as a function of age at exposure from 50 years on. For technical reasons (over-scanning), radiation-sensitive organs adjacent to the actual scan region, the lung, are also exposed with multi-slice CT systems. As the
LARs for these organs contribute to the total
LAR when considering lung cancer screening, they are included in
Figure 2. The organ equivalent dose of the heart is comparable to the lung dose but is of no relevance for the radiation-related cancer risk, as cancers of the heart are extremely rare. For women and exposure age up to about 60 years, the highest radiation risks are those for lung and breast cancer, while at higher ages the radiation risks for lung cancer and leukemia dominate. It is striking that the radiation risk for lung cancer is significantly higher for women than for men.
Figure 3 shows for a representative LDCT protocol with a
CTDIvol of 1 mGy the
LAR for females and males as a function of age at the start of screening, assuming annual LDCT screening until the age of 75 years. The
LAR estimates for women are 0.23%, 0.11%, and 0.03% if screening starts at age 50, 60, and 70 years, respectively. The estimates in
Figure 4 are based on the assumption that LDCT screening examinations are performed annually only over a ten-year period. In this case,
LAR estimates for women are 0.11% and 0.08% at starting ages 50 and 60, respectively. In all screening scenarios, the risk estimates for women are slightly twice as high as those for men. The reasons for this are that (i) the radiation-related lung cancer risk according to BEIR VII is higher for women than for men and, (ii) for women the excess breast cancer risk must also be accounted for. The
LAR values given in
Figure 3 and
Figure 4 are halved when LDCTs are performed biannually rather than annually.
Assuming annual screening and a 20% overall benefit for both sexes,
Figure 5 gives estimates of the benefit–risk ratio for the considered screening scenarios. For men, the ratio is about 25 and above, but for females it is below 15 in three of the four screening scenarios. It should be noted that the benefit assumed to be constant for the scenarios considered was derived from screening studies in which LDCTs were performed over much shorter screening periods (8 to 10 years) and in some cases with longer intervals between LDCT examinations (up to 2.5 years). Under the assumption, which can be considered realistic, that the benefit increases with both longer screening periods and the frequency of examinations, the benefit–risk ratios given in the figure for the four scenarios are underestimated and can thus be regarded as conservative estimates.
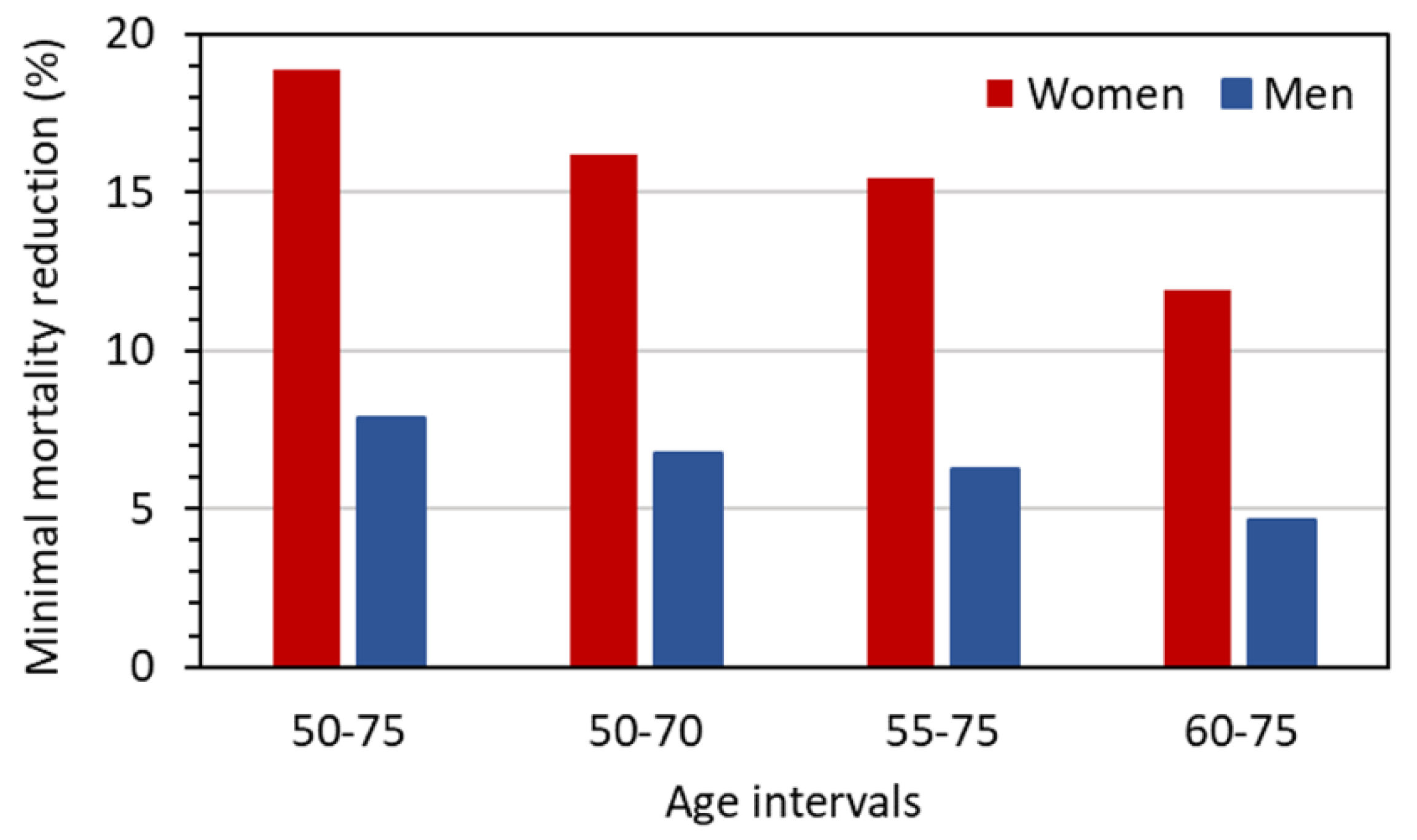
The problem of uncertainty in the benefit can be addressed by considering the values for the required reduction of lung cancer mortality,
MR10, by LDCT screening to achieve at least a pre-defined benefit–risk ratio for the considered screening scenarios. These
MR10 values are given in
Figure 6. While the values for men range between 5% and 8%, those for women are considerably higher and reach almost 20% for a screening with annual LDCT examinations from 50 to 75 years of age.
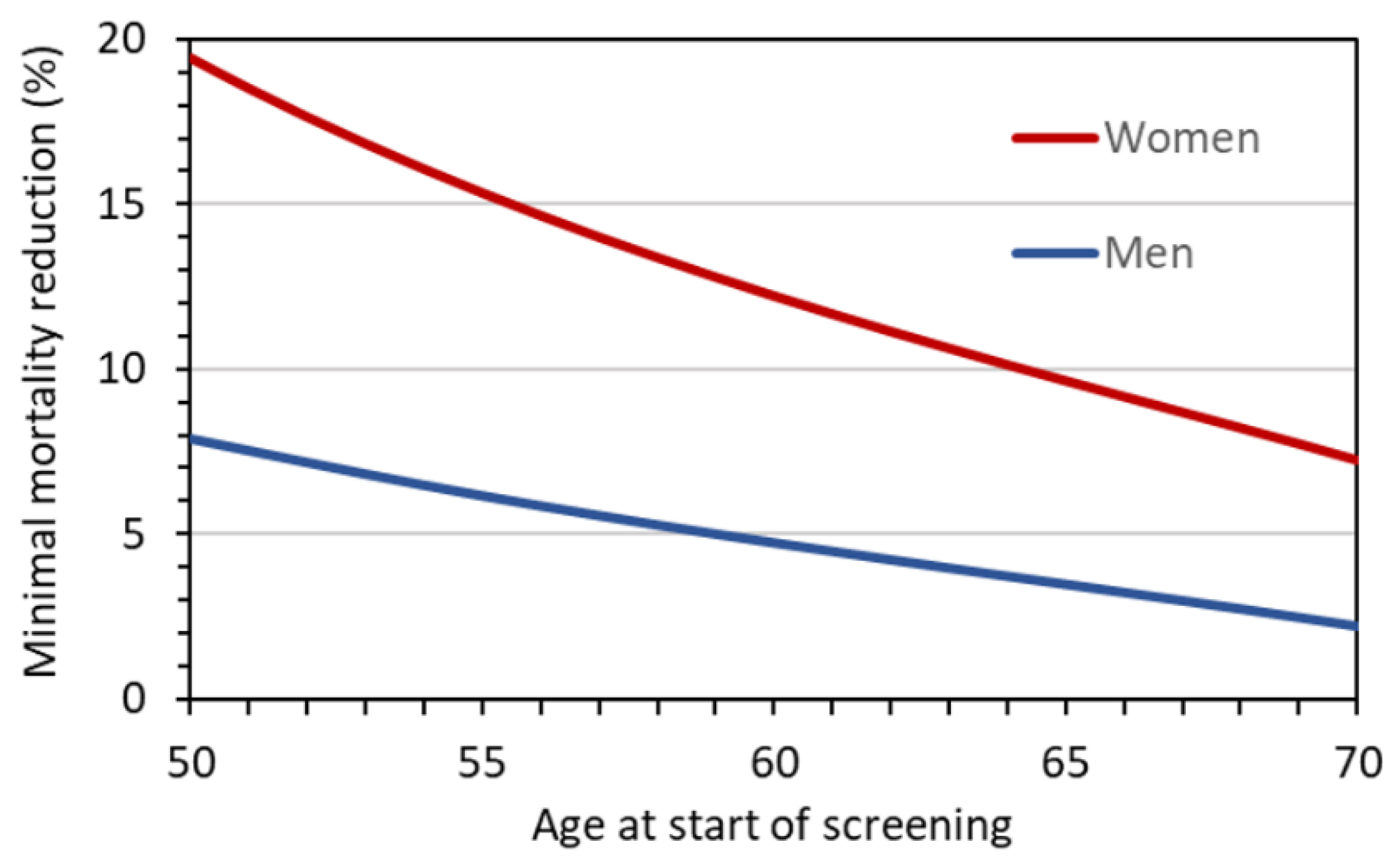
Figure 7 gives
MR10 as a function of age at the start of screening, assuming annual LDCT screening until the age of 75 years. For men, the minimum required reduction decreases from about 8 to 2% with increasing age at first screening examination. For women, the required reduction is 20% if screening starts at age 50 and takes values below 10% only if screening starts at age 64 or later.
5. Discussion
The estimated lifetime attributable cancer risks resulting from repeated LDCT screening tests were estimated for a German population of heavy smokers or ex-smokers. The values can be considered representative for a Western European population. As demonstrated, the estimated LAR are not negligible, especially for women if screening starts at the age of 50 years. From a radiation protection perspective, they must be taken into consideration in any decision-making, since all screening participants are affected, whereas the benefit only applies to those with screening-detected lung cancer. However, it has to be underlined that the assumptions for the above risk estimates and risk-benefit analyses are mostly conservative, for example by using a DDREF of 1 and by assuming that screening will be performed annually for the full screening period.
Moreover, it should be considered that the potential benefit for patients with a screening-detected lung cancer would be immediate, whereas a radiation-associated cancer is hypothetical and would only occur after a latency time of several years or even decades. The latency period is an important factor, especially for participants who only take part in screening at an older age.
The estimates presented in
Figure 3 and
Figure 4 refer to organ equivalent dose values for an LDCT protocol with
CTDIvol = 1 mGy. Since the risk is linearly dependent on
CTDIvol, the estimates can easily be converted for other protocols. In a similar line of reasoning, the benefit–risk ratios given in
Figure 5 can be adapted to other protocols because they are inversely proportional to the risk. Moreover, the ratios in
Figure 5, based on the assumption of a 20% reduction in lung cancer mortality due to LDCT screening, can easily be transferred to screening scenarios with other values for the benefit as the benefit–risk ratio is proportional to the benefit. This is particularly useful when considering women participating in an LDCT screening, since there is evidence that the benefit for women is higher than that for men [
29].
There is a controversial discussion in the literature about which baseline risks should be assumed for female smokers. For the estimates presented in this paper, it was assumed that the lung cancer risk for smoking women corresponds to that for smoking men. If female heavy smokers had a different (presumably higher) smoking-related lung cancer risk, this would affect the radiation risk estimates for women as well as the corresponding benefit–risk ratio. The higher weighting of the additive model in the BEIR VII approach for lung cancer implies that in this case the benefit–risk ratio would be more affected than the radiation risk.
Various publications have addressed the radiation risk of LDCT screening. Some authors (e.g., [
30]) estimate the radiation risk by applying the effective dose and the nominal risk coefficients of the ICRP. The ICRP’s risk estimates are called ‘nominal’ because “they relate to the exposure of a nominal population of females and males with a typical age distribution and are computed by averaging over age groups and both sexes”. The ICRP thus explicitly states that “for the estimation of the likely consequences of an exposure of an individual or a known population, it is necessary to use specific data relating to the exposed individual” [
7].
In the Italian COSMOS study (a non-randomized screening study of approximately 3400 men and 1800 women aged 50 years and older and up to 10 annual screening rounds), organ doses were estimated for both annual LDCT screening and recall (PET/CT) examinations in individuals with suspicious pulmonary foci [
31]. Based on these data, the excess lifetime risks (incidence) for all major cancer types were determined using the original BEIR VII estimates of
LAR for a US population in 1999.
LARs were approximately 4 per 10,000 for men and 8 per 10,000 for women, assuming a screening start at age 50. Considering the differences between the risk analysis used by the authors and our approach (e.g.,
DDREF = 1.5 in Rampinelli et al. [
31]) and different doses, the estimates are compatible.
Radiological LDCT examinations to clarify suspicious findings during screening increase the dose and thus also the radiation risk for some participants. Becker et al. [
32] give the frequency of recall LDCT for the first 5 screening rounds (T0 to T4) for the LUSI study. In round T0 about 20% of the participants had a recall LDCT after 3 or 6 months and in the following rounds on average about 3.5%. Under these assumptions, the lifetime risks after annual LDCT screening from age 50 until age 75 increase by about 4%.
No uncertainty figures for the risk and benefit–risk estimates are provided in our study. Radiation risk estimates are subject to numerous uncertainty factors because of the inherent limitations of (radio-)epidemiological data. In addition to statistical uncertainty, the populations and exposures for which a risk estimate is to be made differ from those in radio-epidemiological studies. This means that different “educated guesses” have to be made, which are inevitably subject to uncertainties. Despite the large amount of epidemiological and experimental data on radiation risks, these data are not sufficient to give a realistic uncertainty for these guesses [
5]. In a paper by Zhang et al. [
33], a sensitivity analysis was performed to investigate the impact of 12 different parameters or methodological assumptions on the radiation risk estimate for solid tumors. This sensitivity analysis indicated that DDREF, age at exposure, sex, and lethality strongly influence radiation risk and that the risk transfer model also has a noticeable impact. In particular, our assumptions on the DDREF and the transfer model thus contribute to the uncertainty of our risk estimates, but they were chosen in such a way that the estimated values can be assumed to be conservative.
Radiation-associated noncancer diseases were outside the scope of our study. Nevertheless, they might be a matter of concern when it comes to an LDCT screening. An association between radiation exposure and noncancer respiratory diseases (NCRD) has been reported in the LSS of atomic bomb survivors [
34]. However, the corresponding radiation-related risk was not increased in the period up to 1980, i.e., for up to 35 years after the bombings. Moreover, the majority of NCRDs were pneumonia or influenza, and it was only in this category that a significant radiation risk was found. As mentioned, the organ with a comparably high organ equivalent dose as that of the lung is the heart. Radiation-related heart diseases, however, such as ischemic heart disease, do not play a role in the low dose range. In the LSS, the lowest dose above which a significant increase in the risk of heart disease was observed is given as 0.7 Gy [
35]. This is in line with the view of the ICRP, which has proposed a “practical” threshold dose of 0.5 Gy for radiation-related cardiovascular diseases that manifest late after irradiation [
36].