Postoperative Quality Assessment Score Can Select Patients with High Risk for Locoregional Recurrence in Colon Cancer
Abstract
:1. Introduction
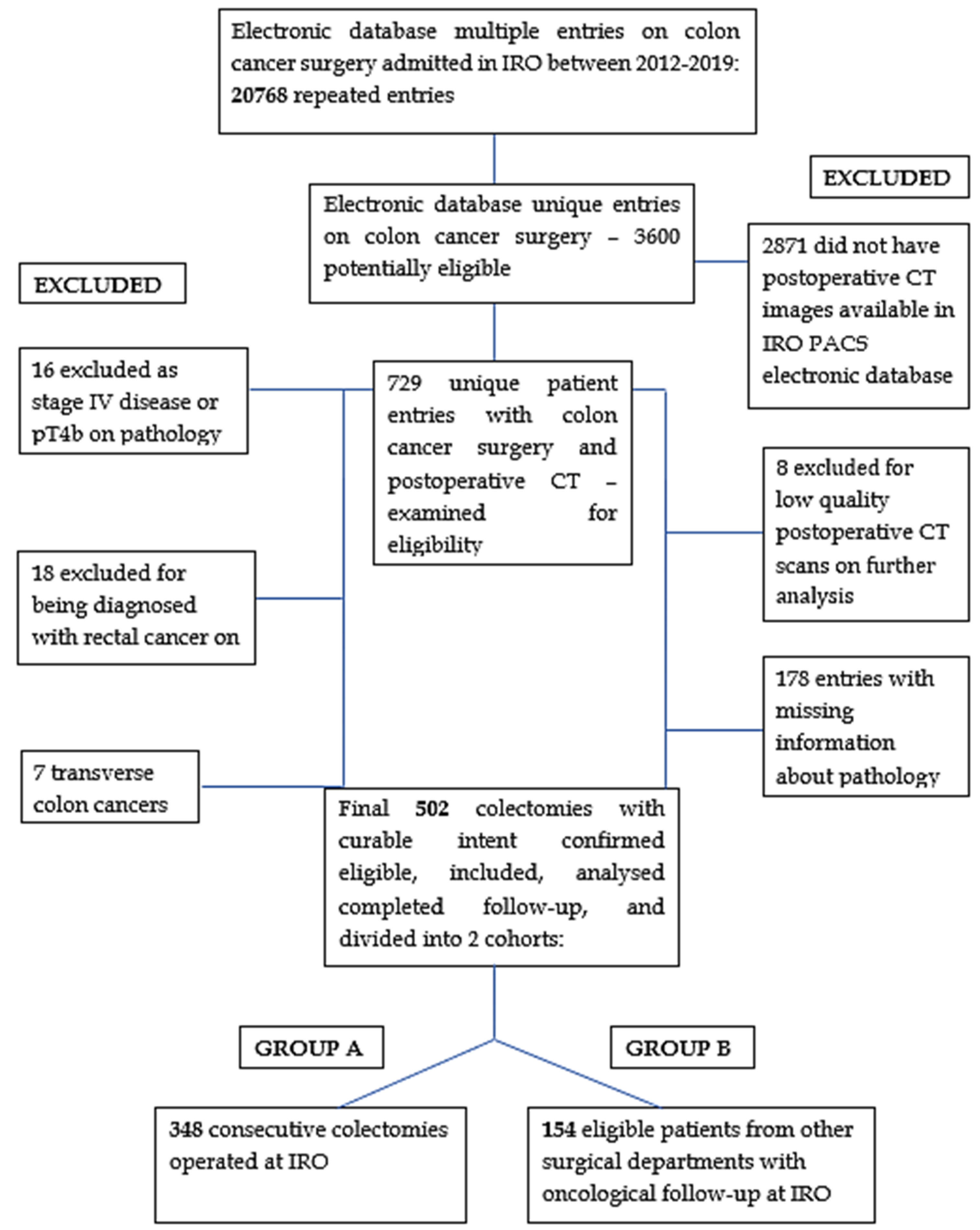
2. Materials and Methods
2.1. Design and Setting
- high ligation of the ileocolic artery (ICA) adjacent to the lateral wall of the superior mesenteric vein (commonly referred to as SMV) in right colectomies (i.e., D2 lymphadenectomy);
- high ligation of the inferior mesenteric artery (IMA), at approximately 10 mm from the aorta in left colectomies;
- low ligation of the IMA in segmental colectomies for sigmoid cancer, just below the emergence of the left colic artery (LCA), but with further dissection around the IMA trunk up to its origin from the aorta (i.e., D3 lymphadenectomy).
2.2. Patients and Selection Criteria
2.3. Data Collection and Database Architecture
2.4. Image Acquisition Protocol
2.5. CT Angiometry Protocol
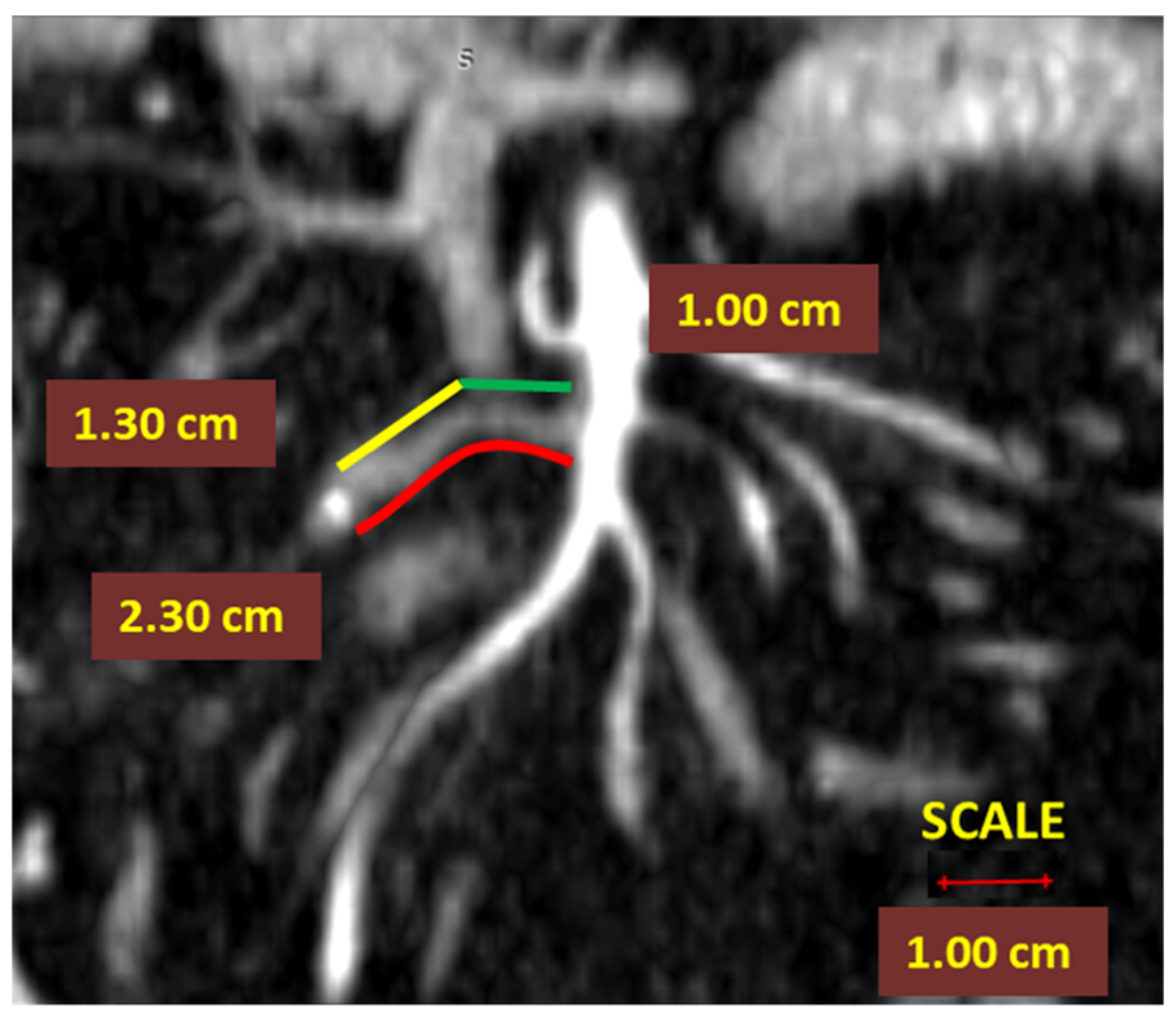
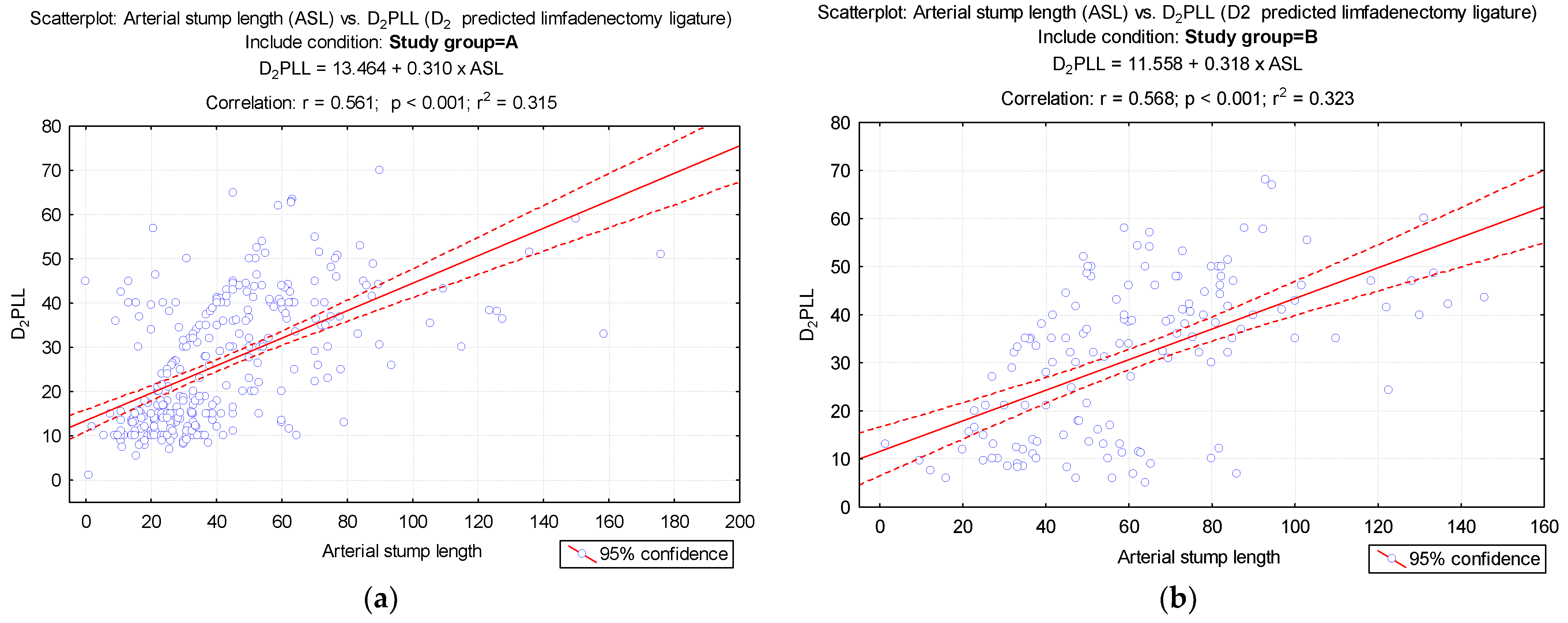
- In right colectomies, the ASL was defined as the length from the starting point of the ICA from the superior mesenteric artery to the ligation point. The presumed arterial length was defined as the length from the origin of ICA to the lateral side of the SMV, as benchmark length for D2 ligation, D2PLL (Figure 2);
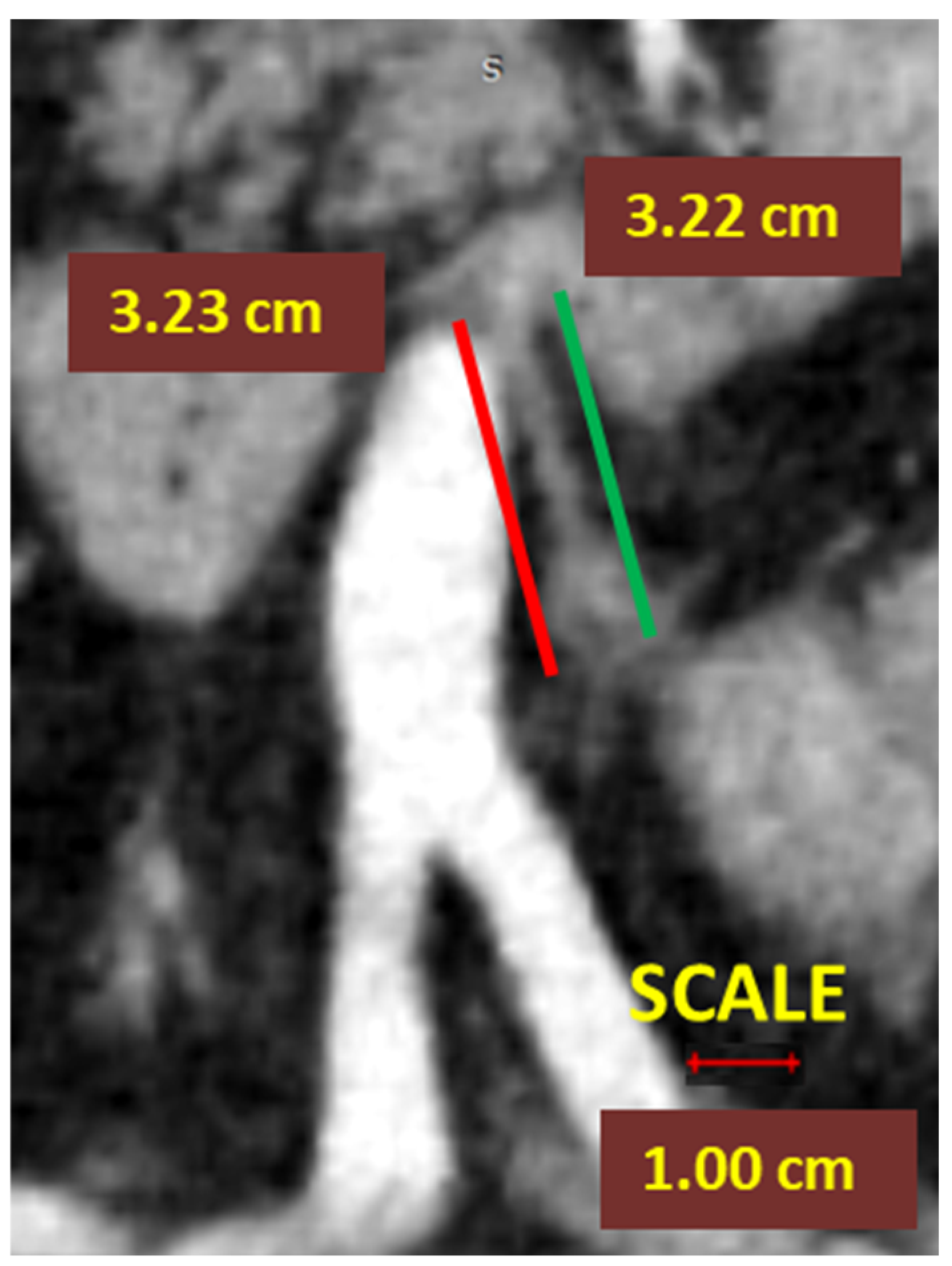
- In left-sided colectomies, the ligation point was analyzed in relation to the IMA and the LCA. The ASL was measured from the origin of the IMA to the ligation point, while the presumed stump length (D2PLL) was measured from the IMA origin to below the LCA emergence, explicitly as the norm D2 lymphadenectomy level with conservation of the LCA. The D2IP was defined as the subtraction between actual stump lengths and the D2PLL (Figure 3).
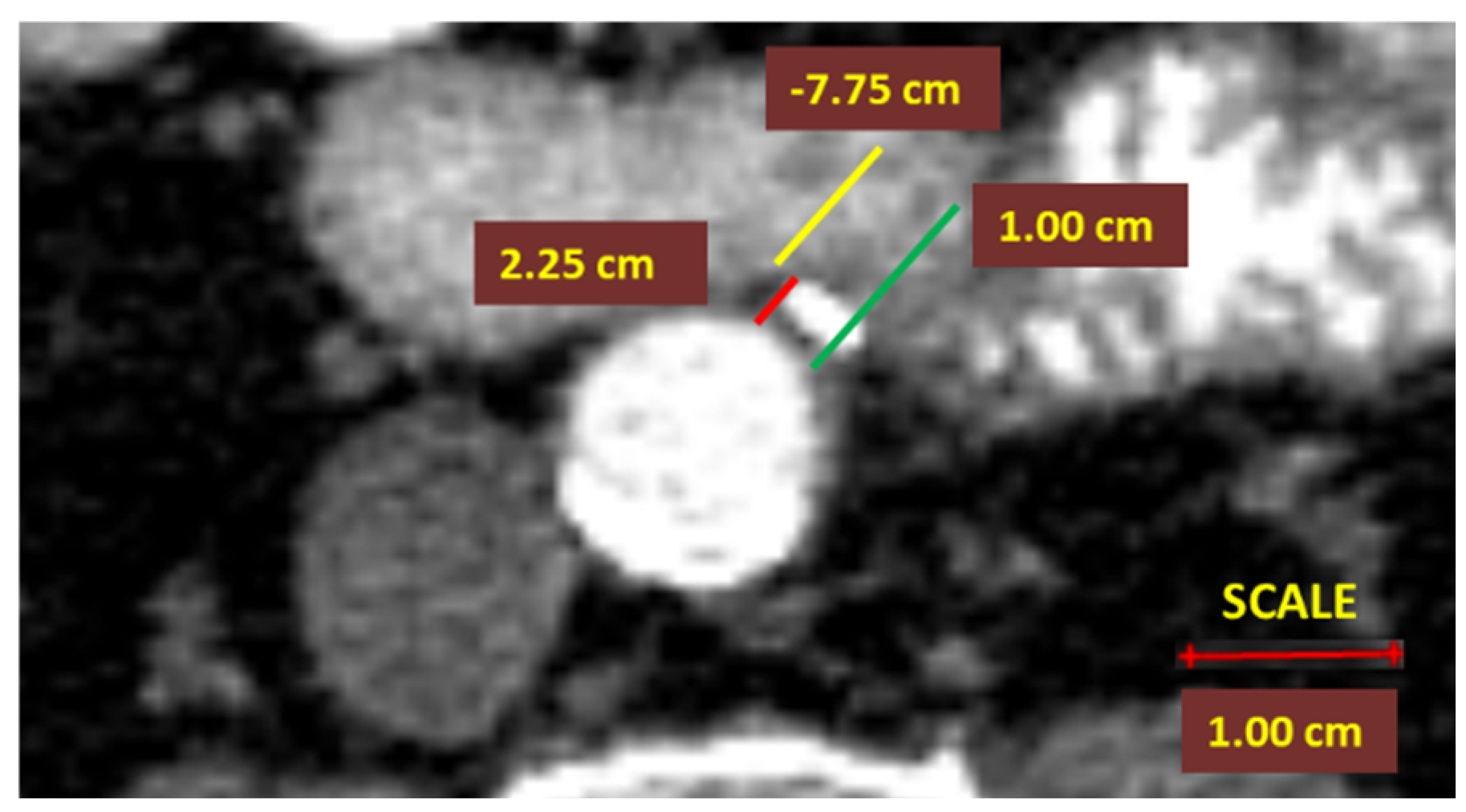
- Middle and right colic artery stumps were not analyzed in this study due to the small sample size. The right colic artery was inconsistent and presented multiple anatomical variants. The middle colic artery was not routinely ligated for a standard right hemicolectomy.
2.6. Statistical Analysis
3. Results
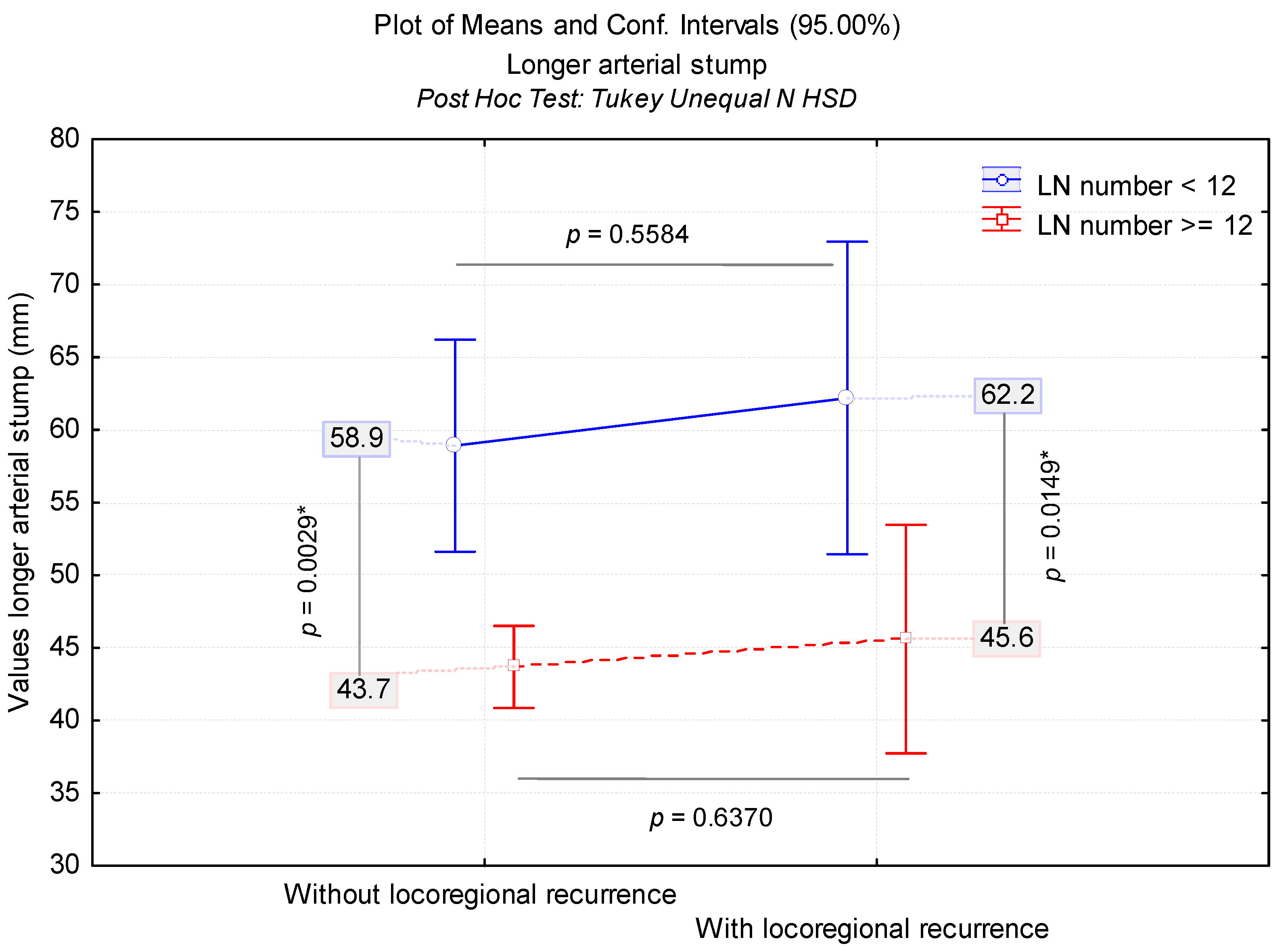
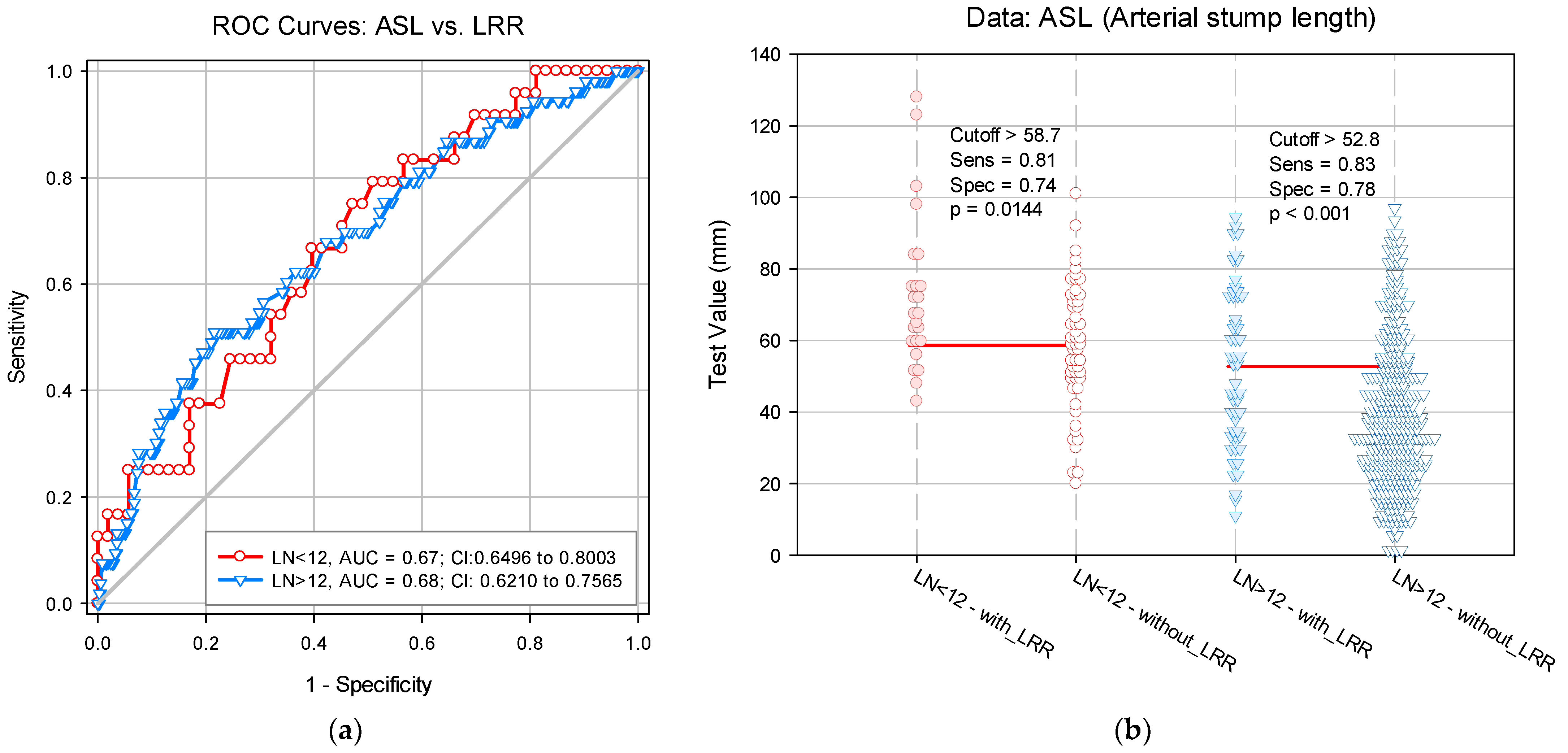
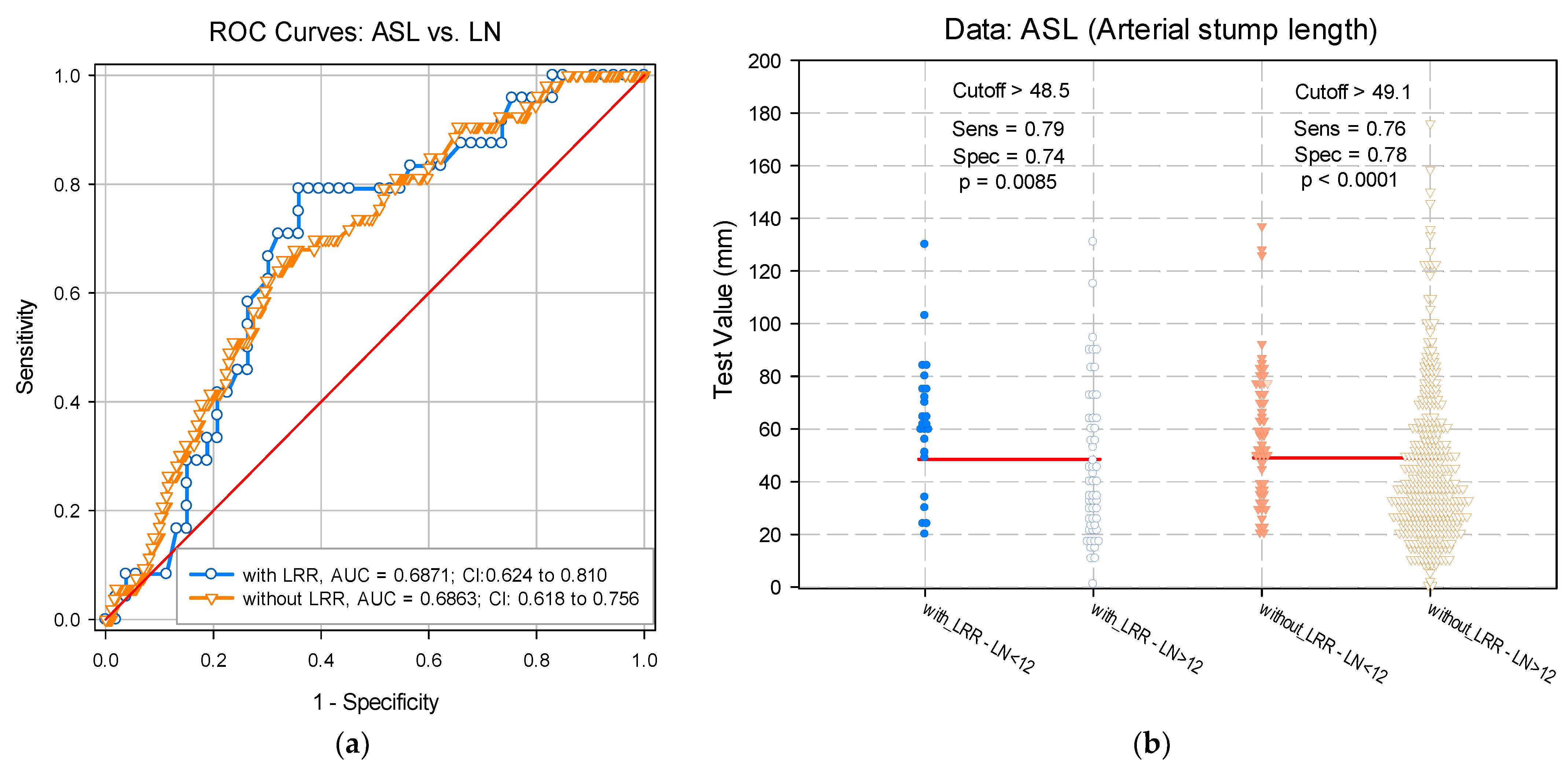
3.1. LN Number below 12 Correlates with LRR
3.2. Risk Score Calculation
4. Discussion
Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, C.A.; Neuenschwander, A.U.; Jansen, J.E.; Wilhelmsen, M.; Kirkegaard-Klitbo, A.; Tenma, J.R.; Bols, B.; Ingeholm, P.; Rasmussen, L.A.; Jepsen, L.V.; et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: A retrospective, population-based study. Lancet Oncol. 2015, 16, 161–168. [Google Scholar] [CrossRef]
- Slim, K.; Blay, J.Y.; Brouquet, A.; Chatelain, D.; Comy, M.; Delpero, J.R.; Denet, C.; Elias, D.; Fléjou, J.F.; Fourquier, P.; et al. Digestive oncology: Surgical practices. J. Chir. 2009, 146 (Suppl. 2), S11–S80. [Google Scholar] [CrossRef]
- Otchy, D.; Hyman, N.H.; Simmang, C.; Anthony, T.; Buie, W.D.; Cataldo, P.; Church, J.; Cohen, J.; Dentsman, F.; Ellis, C.N.; et al. Practice parameters for colon cancer. Dis. Colon Rectum 2004, 47, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.J.; Kaiser, A.M.; Mills, S.; Rafferty, J.F.; Buie, W.D. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the Management of Colon Cancer. Dis. Colon Rectum 2012, 55, 831–843. [Google Scholar] [CrossRef]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S.; et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, S1–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helsedirektoratet. National Action Plan with Guidelines for Diagnosis, Treatment and Follow up of Cancer in the Colon and Rectum, 5th ed.; Norwegian Helsedirektoratet: Oslo, Norway, 2017. Available online: http://www.helsedirektoratet.no/vp/multimedia/archive/00287/Nasjonalt_handlings_287789a.pdf (accessed on 24 January 2021).
- Ratnayake, I.; Park, J.; Biswanger, N.; Feely, A.; Musto, G.; Decker, K. Colorectal Cancer Surgery Quality in Manitoba: A Population-Based Descriptive Analysis. Curr. Oncol. 2021, 28, 30206. [Google Scholar] [CrossRef]
- Livadaru, C.; Morarasu, S.; Frunza, T.C.; Ghitun, F.A.; Paiu-Spiridon, E.F.; Sava, F.; Terinte, C.; Ferariu, D.; Lunca, S.; Dimofte, G.M. Post-operative computed tomography scan—Reliable tool for quality assessment of complete mesocolic excision. World J. Gastrointest. Oncol. 2019, 11, 208–226. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; West, N. Quality of surgery: Has the time come for colon cancer? Lancet Oncol. 2015, 16, 121–122. [Google Scholar] [CrossRef]
- Osterman, E.; Hammarström, K.; Imam, I.; Osterlund, E.; Sjöblom, T.; Glimelius, B. Recurrence Risk after Radical Colorectal Cancer Surgery-Less Than before, But How High Is It? Cancers 2020, 12, 3308. [Google Scholar] [CrossRef]
- Osterman, E.; Glimelius, B. Recurrence Risk after Up-to-Date Colon Cancer Staging, Surgery, and Pathology: Analysis of the Entire Swedish Population. Dis. Colon Rectum 2018, 61, 1016–1025. [Google Scholar] [CrossRef]
- Siani, L.M.; Pulica, C. Stage I-IIIC right colonic cancer treated with complete mesocolic excision and central vascular ligation: Quality of surgical specimen and long term oncologic outcome according to the plane of surgery. Minerva Chir. 2014, 69, 199–208. [Google Scholar] [PubMed]
- Procházka, V.; Zetelová, A.; Grolich, T.; Frola, L.; Kala, Z. Complete mesocolic excision during right hemicolectomy. Rozhl. Chir. 2016, 95, 359–364. [Google Scholar] [PubMed]
- Culligan, K.; Coffey, J.C.; Kiran, R.P.; Kalady, M.; Lavery, I.C.; Remzi, F.H. The mesocolon: A prospective observational study. Colorectal Dis. 2012, 14, 421–428. [Google Scholar] [CrossRef]
- Heald, R.J. The ‘Holy Plane’ of rectal surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.; Tepper, J.E.; Cohen, A.; Orlow, E.; Welch, C.; Donaldson, G. Local failure following curative resection of colonic adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 645–651. [Google Scholar] [CrossRef]
- Read, T.E.; Mutch, M.G.; Chang, B.W.; McNevin, M.S.; Fleshman, J.W.; Birnbaum, E.H.; Fry, R.D.; Caushaj, P.F.; Kodner, I.J. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J. Am. Coll. Surg. 2002, 195, 33–40. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Sosin, H.; Levitt, S. Extrapelvic colon--areas of failure in a reoperation series: Implications for adjuvant therapy. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 731–741. [Google Scholar] [CrossRef]
- Harris, G.J.; Church, J.M.; Senagore, A.J.; Lavery, I.C.; Hull, T.L.; Strong, S.A.; Fazio, V.W. Factors affecting local recurrence of colonic adenocarcinoma. Dis. Colon Rectum 2002, 45, 1029–1034. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Shen, K.; Shen, Z.; Jiang, K.; Liang, B.; Yin, M.; Yang, X.; Wang, S.; Ye, Y. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: A systemic review and meta-analysis. Colorectal Dis. 2017, 19, 962–972. [Google Scholar] [CrossRef]
- Sjövall, A.; Granath, F.; Cedermark, B.; Glimelius, B.; Holm, T. Loco-regional recurrence from colon cancer: A population-based study. Ann. Surg. Oncol. 2007, 14, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Willaert, W.; Ceelen, W. Extent of surgery in cancer of the colon: Is more better? World J. Gastroenterol. 2015, 21, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmanuel, A.; Haji, A. Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: Is it worth that extra effort? A review of the literature. Int. J. Colorectal Dis. 2016, 31, 797–804. [Google Scholar] [CrossRef]
- Søndenaa, K.; Quirke, P.; Hohenberger, W.; Sugihara, K.; Kobayashi, H.; Kessler, H.; Brown, G.; Tudyka, V.; D’Hoore, A.; Kennedy, R.H.; et al. The rationale behind complete mesocolic excision (CME) and a central vascular ligation for colon cancer in open and laparoscopic surgery. Proceedings of a consensus conference. Int. J. Colorectal Dis. 2014, 29, 419–428. [Google Scholar] [CrossRef]
- Madoff, R.D. Defining quality in colon cancer surgery. J. Clin. Oncol. 2012, 30, 1738–1740. [Google Scholar] [CrossRef]
- West, N.P.; Morris, E.J.; Rotimi, O.; Cairns, A.; Finan, P.J.; Quirke, P. Pathology grading of colon cancer surgical resection and its association with survival: A retrospective observational study. Lancet Oncol. 2008, 9, 857–865. [Google Scholar] [CrossRef]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Su, X.; He, Z.; Zhang, C.; Lu, J.; Zhang, G.; Sun, Y.; Du, X.; Chi, P.; Wang, Z.; et al. Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): A randomised, controlled, phase 3, superiority trial. Lancet Oncol. 2021, 22, 391–401. [Google Scholar] [CrossRef]
- Makkai-Popa, S.T.; Lunca, S.; Tarcoveanu, E.; Carasevici, E.; Dimofte, G. Lymph node status assessed through the log odds ratio—A better tool in the prognosis of colorectal cancer relapse. Rom. J. Morphol. Embryol. 2014, 55, 97–102. [Google Scholar] [PubMed]
- Moldovanu, R.; Dimofte, G.; Stefan, I.; Filip, V.; Vlad, N.; Curca, G.; Crumpei, F.; Fotea, V.; Ferariu, D.; Danila, N.; et al. Right colon cancer—Clinicopathological findings. Chirurgia 2012, 107, 314–324. [Google Scholar] [PubMed]
- Dimofte, G.; Tarcoveanu, E.; Taraşi, M.; Panait, C.; Lozneanu, G.; Nicolescu, S.; Boboc, V.; Grigoraş, O. Mean number of lymph nodes in colonic cancer specimen: A possible quality control index for surgical accuracy. Chirurgia 2011, 106, 759–764. [Google Scholar] [PubMed]
- Jessup, J.M.; Goldberg, R.M.; Asare, E.A.; Benson, A.B., III; Brierley, J.D.; Chang, G.J.; Chen, V.; Compton, C.C.; De Nardi, P.; Goodman, K.A.; et al. Colon and Rectum. In The Eighth Edition AJCC Cancer Staging Manual; Amin, M.B., Greene, F.L., Edge, S.B., Compton, C.C., Gershenwald, J.E., Brookland, R.K., Meyer, L., Gress, D.M., Byrd, D.R., Winchester, D.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 251–274. [Google Scholar] [CrossRef]
- Hohenberger, P.; Schlag, P.; Kretzschmar, U.; Herfarth, C. Regional mesenteric recurrence of colorectal cancer after anterior resection or left hemicolectomy: Inadequate primary resection demonstrated by angiography of the remaining arterial supply. Int. J. Colorectal Dis. 1991, 6, 17–23. [Google Scholar] [CrossRef]
- Spasojevic, M.; Stimec, B.V.; Dyrbekk, A.P.; Tepavcevic, Z.; Edwin, B.; Bakka, A.; Ignjatovic, D. Lymph node distribution in the d3 area of the right mesocolon: Implications for an anatomically correct cancer resection. A postmortem study. Dis. Colon Rectum 2013, 56, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Kaye, T.L.; West, N.P.; Jayne, D.G.; Tolan, D.J.M. CT assessment of right colonic arterial anatomy pre and post cancer resection—a potential marker for quality and extent of surgery? Acta Radiol. 2016, 57, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Munkedal, D.L.E.; Rosenkilde, M.; Nielsen, D.T.; Sommer, T.; West, N.P.; Laurberg, S. Radiological and pathological evaluation of the level of arterial division after colon cancer surgery. Colorectal Dis. 2017, 19, O238–O245. [Google Scholar] [CrossRef] [Green Version]
- Rebuzzi, S.E.; Pesola, G.; Martelli, V.; Sobrero, A.F. Adjuvant Chemotherapy for Stage II Colon Cancer. Cancers 2020, 12, 2584. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.H.; Lee, J.; Kye, B.-H.; Lee, S.C.; Lee, I.K.; Kang, W.K.; Cho, H.-M.; Lee, Y.S. Addition of V-Stage to Conventional TNM Staging to Create the TNVM Staging System for Accurate Prediction of Prognosis in Colon Cancer: A Multi-Institutional Retrospective Cohort Study. Biomedicines 2021, 9, 888. [Google Scholar] [CrossRef]
- Leijssen, L.G.J.; Dinaux, A.M.; Taylor, M.S.; Deshpande, V.; Kunitake, H.; Bordeianou, L.G.; Berger, D.L. Perineural Invasion Is a Prognostic but not a Predictive Factor in Nonmetastatic Colon Cancer. Dis. Colon Rectum 2019, 62, 1212–1221. [Google Scholar] [CrossRef]
- Andrew, H.; Gossedge, G.; Croft, J.; Corrian, N.; Brown, J.M.; West, N.; Quirke, P.; Tolan, D.; Cahill, R.; Jayne, D.G. Next Generation intraoperative Lymph node staging for Stratified colon cancer surgery (GLiSten): A multicentre, multinational feasibility study of fluorescence in predicting lymph node-positive disease. Southampt. NIHR J. Libr. 2016, 3, 1–122. [Google Scholar] [CrossRef]
- Titu, L.V.; Tweedle, E.; Rooney, P.S. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: A systematic review. Dig. Surg. 2008, 25, 148–157. [Google Scholar] [CrossRef]
- Jung, H.-S.; Ryoo, S.-B.; Lim, H.-K.; Kim, M.J.; Moon, S.H.; Park, J.W.; Jeong, S.-Y.; Park, K.J. Tumor Size >5 cm and Harvested LNs <12 Are the Risk Factors for Recurrence in Stage I Colon and Rectal Cancer after Radical Resection. Cancers 2021, 13, 5294. [Google Scholar] [CrossRef]
- Bowne, W.B.; Lee, B.; Wong, W.D.; Ben-Porat, L.; Shia, J.; Cohen, A.M.; Enker, W.E.; Guillem, J.G.; Paty, P.B.; Weiser, M.R. Operative salvage for locoregional recurrent colon cancer after curative resection: An analysis of 100 cases. Dis. Colon Rectum 2005, 48, 897–909. [Google Scholar] [CrossRef]
- Fuzun, M.; Terzi, C.; Sokmen, S.; Unek, T.; Haciyanli, M. Potentially curative resection for locoregional recurrence of colorectal cancer. Surg. Today 2004, 34, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Koebrugge, B.; Bosscha, K.; Liefers, G.J.; Lips, D.J.; van de Velde, C. Can micrometastases be used to predict colon cancer prognosis? Hopes for the EnRoute+ study. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 559–561. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Country Profiles, Romania 2014. Available online: http://www.who.int/cancer/country-profiles/rou_en.pdf?ua=1 (accessed on 15 March 2021).
- Chen, S.L.; Bilchik, A.J. More extensive nodal dissection improves survival for stages I to III of colon cancer: A population-based study. Ann. Surg. 2006, 244, 602–610. [Google Scholar] [CrossRef]
- Johnson, P.M.; Porter, G.A.; Ricciardi, R.; Baxter, N.N. Increasing negative lymph node count is independently associated with improved long-term survival in stage IIIB and IIIC colon cancer. J. Clin. Oncol. 2006, 24, 3570–3575. [Google Scholar] [CrossRef]
- Le Voyer, T.E.; Sigurdson, E.R.; Hanlon, A.L.; Mayer, R.J.; Macdonald, J.S.; Catalano, P.J.; Haller, D.G. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J. Clin. Oncol. 2003, 21, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.L.; Schofield, J.B. Assessment of lymph node involvement in colorectal cancer. World J. Gastrointest. Surg. 2016, 8, 179–192. [Google Scholar] [CrossRef]
- Grinnell, R.S. Results of ligation of inferior mesenteric artery at the aorta in resections of carcinoma of the descending and sigmoid colon and rectum. Surg. Gynecol. Obstet. 1965, 120, 1031–1036. [Google Scholar]
- Rosi, P.A.; Cahill, W.J.; Carey, J. A ten year study of hemicolectomy in the treatment of carcinoma of the left half of the colon. Surg. Gynecol. Obstet. 1962, 114, 15–24. [Google Scholar] [PubMed]
- Rao, X.; Zhang, J.; Liu, T.; Wu, Y.; Jiang, Y.; Wang, P.; Chen, G.; Pan, Y.; Wu, T.; Liu, Y.; et al. Prognostic value of inferior mesenteric artery lymph node metastasis in cancer of the descending colon, sigmoid colon and rectum. Colorectal Dis. 2018, 20, O135–O142. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Reingruber, B.; Merkel, S. Surgery for colon cancer. Scand. J. Surg. 2003, 92, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lips, D.J.; Koebrugge, B.; Liefers, G.J.; van de Linden, J.C.; Smit, V.T.; Pruijt, H.F.; Putter, H.; van de Velde, C.J.; Bosscha, K. The influence of micrometastases on prognosis and survival in stage I–II colon cancer patients: The Enroute Study. BMC Surg. 2011, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Willaert, W.; Cosyns, S.; Ceelen, W. Biology-Based Surgery: The Extent of Lymphadenectomy in Cancer of the Colon. Eur. Surg. Res. 2018, 59, 371–379. [Google Scholar] [CrossRef]
- Kobayashi, H.; Mochizuki, H.; Morita, T.; Kotake, K.; Teramoto, T.; Kameoka, S.; Saito, Y.; Takahashi, K.; Hase, K.; Ohya, M.; et al. Timing of relapse and outcome after curative resection for colorectal cancer: A Japanese multicenter study. Dig. Surg. 2009, 26, 249–255. [Google Scholar] [CrossRef]
- Saad, E.D.; Katz, A.; Hoff, P.M.; Buyse, M. Progression-free survival as surrogate and as true end point: Insights from the breast and colorectal cancer literature. Ann. Oncol. 2009, 21, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sargent, D.J.; Wieand, H.S.; Haller, D.G.; Gray, R.; Benedetti, J.K.; Buyse, M.; Labianca, R.; Seitz, J.F.; O’Callaghan, C.J.; Francini, G.; et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. 2005, 23, 8664–8670. [Google Scholar] [CrossRef]
- De Gramont, A.; Hubbard, J.; Shi, Q.; O’Connell, M.J.; Buyse, M.; Benedetti, J.; Bot, B.; O’Callaghan, C.; Yothers, G.; Goldberg, R.M.; et al. Association between disease-free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: Simulations based on the 20,800 patient ACCENT data set. J. Clin. Oncol. 2010, 28, 460–465. [Google Scholar] [CrossRef]
- Weixler, B.; Warschkow, R.; Ramser, M.; Droeser, R.; von Holzen, U.; Oertli, D.; Kettelhack, C. Urgent surgery after emergency presentation for colorectal cancer has no impact on overall and disease-free survival: A propensity score analysis. BMC Cancer 2016, 16, 208. [Google Scholar] [CrossRef] [Green Version]
- Perron, L.; Daigle, J.M.; Vandal, N.; Guertin, M.H.; Brisson, J. Characteristics Affecting Survival after Locally Advanced Colorectal Cancer in Quebec. Curr. Oncol. 2015, 22, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antony, P.; Harnoss, J.C.; Warschkow, R.; Schmied, B.M.; Schneider, M.; Tarantino, I.; Ulrich, A. Urgent surgery in colon cancer has no impact on survival. J. Surg. Oncol. 2019, 119, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, K.; Withers, R.; Shariff, F.; Ashamalla, S.; Nadler, A. Quality of Colon Cancer Care in Patients Undergoing Emergency Surgery. Curr. Oncol. 2021, 28, 30192. [Google Scholar] [CrossRef] [PubMed]
- Quirt, J.S.; Nanji, S.; Wei, X.; Flemming, J.A.; Booth, C.M. Is There a Sex Effect in Colon Cancer? Disease Characteristics, Management, and Outcomes in Routine Clinical Practice. Curr. Oncol. 2017, 24, e15–e23. [Google Scholar] [CrossRef] [Green Version]
- Koper-Lenkiewicz, O.M.; Dymicka-Piekarska, V.; Milewska, A.J.; Zińczuk, J.; Kamińska, J. The Relationship between Inflammation Markers (CRP, IL-6, sCD40L) and Colorectal Cancer Stage, Grade, Size and Location. Diagnostics 2021, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Lungulescu, C.; Croitoru, V.M.; Volovat, S.R.; Cazacu, I.M.; Turcu-Stiolica, A.; Gheonea, D.I.; Sur, D.; Lungulescu, C.V. An Insight into Deficient Mismatch Repair Colorectal Cancer Screening in a Romanian Population—A Bi-Institutional Pilot Study. Medicina 2021, 57, 847. [Google Scholar] [CrossRef]
- Dijkstra, M.; Nieuwenhuizen, S.; Puijk, R.S.; Timmer, F.E.F.; Geboers, B.; Schouten, E.A.C.; Opperman, J.; Scheffer, H.J.; de Vries, J.J.J.; Versteeg, K.S.; et al. Primary Tumor Sidedness, RAS and BRAF Mutations and MSI Status as Prognostic Factors in Patients with Colorectal Liver Metastases Treated with Surgery and Thermal Ablation: Results from the Amsterdam Colorectal Liver Met Registry (AmCORE). Biomedicines 2021, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Maak, M.; Nitsche, U.; Gotoh, K.; Rosenberg, R.; Janssen, K.-P. Prediction of Metastasis and Recurrence in Colorectal Cancer Based on Gene Expression Analysis: Ready for the Clinic? Cancers 2011, 3, 2858–2869. [Google Scholar] [CrossRef]
- Martin, B.; Gonçalves, J.P.L.; Bollwein, C.; Sommer, F.; Schenkirsch, G.; Jacob, A.; Seibert, A.; Weichert, W.; Märkl, B.; Schwamborn, K. A Mass Spectrometry Imaging Based Approach for Prognosis Prediction in UICC Stage I/II Colon Cancer. Cancers 2021, 13, 5371. [Google Scholar] [CrossRef]
- Kang, G.; Oh, I.; Pyo, J.; Kang, D.; Son, B. Clinicopathological Significance and Prognostic Implications of REG4 Immunohistochemical Expression in Colorectal Cancer. Medicina 2021, 57, 938. [Google Scholar] [CrossRef] [PubMed]
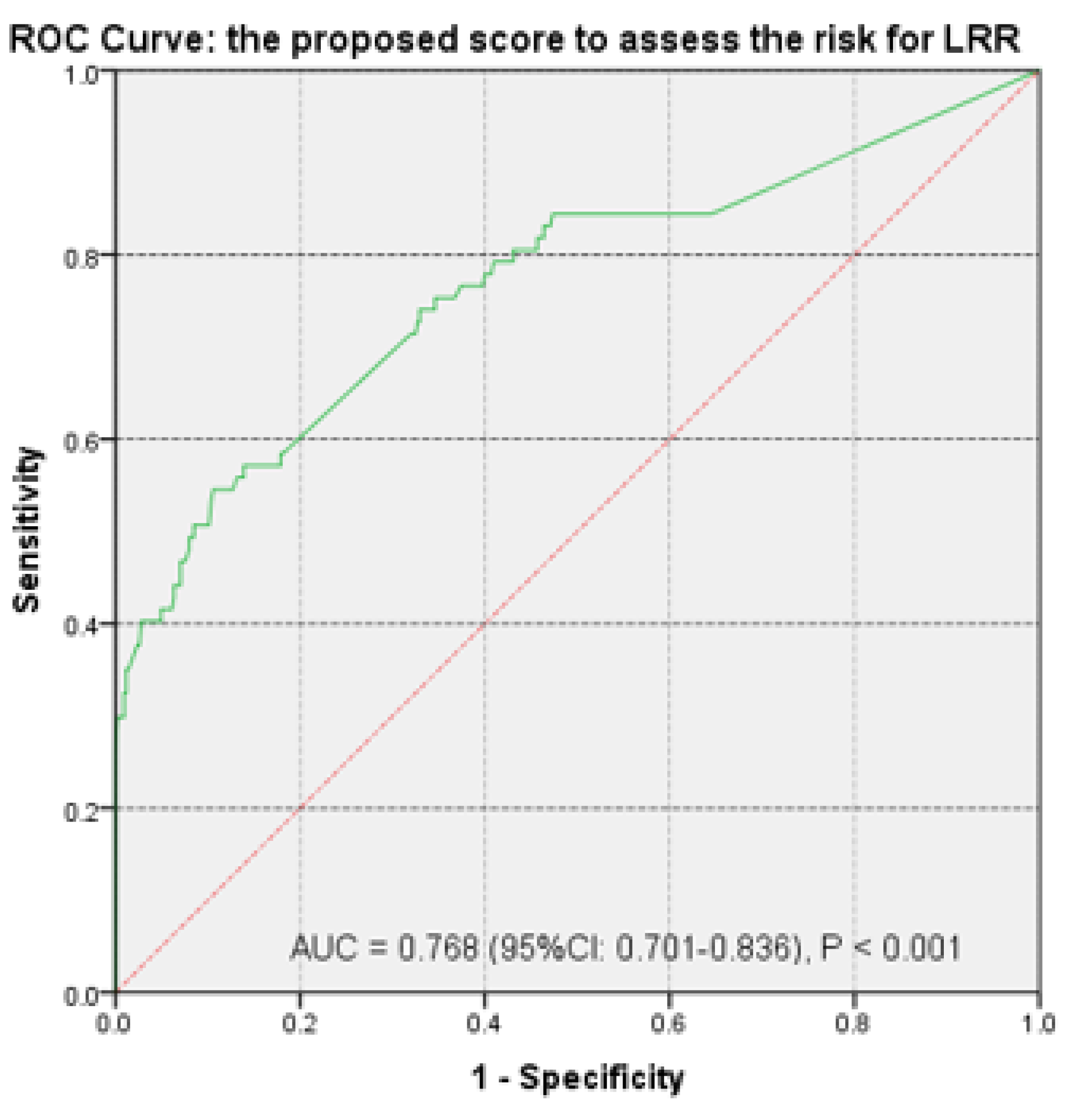
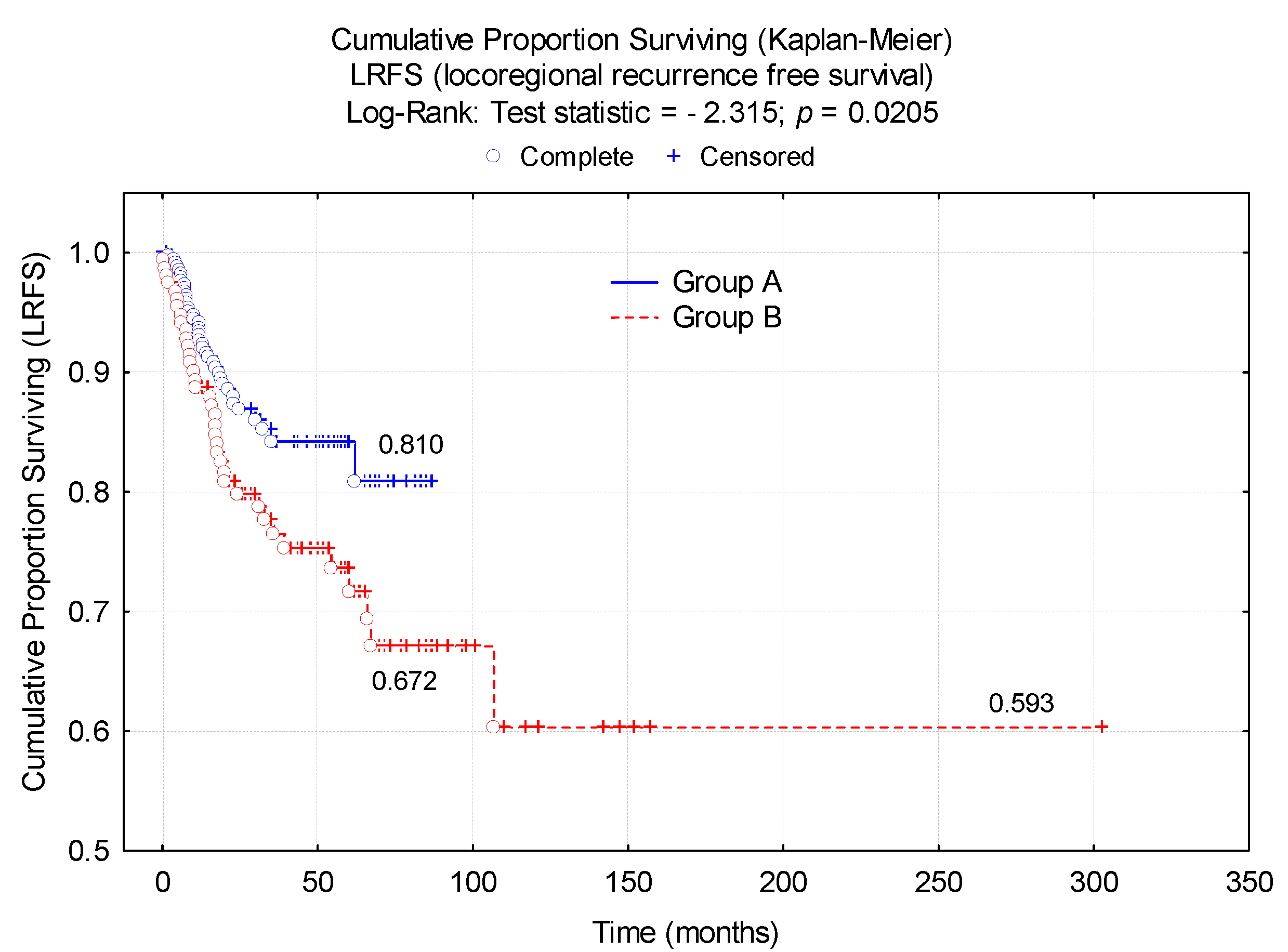

| Characteristics | Group A | Group B | p-Value |
|---|---|---|---|
| n = 502 | n = 348 | n = 154 | |
| Age at surgery, in years Mean ± SD | 64.2 ± 10.2 | 59.5 ± 10.3 | <0.001 |
| Median (Q25; Q75) | 66 (58.5; 71) | 60 (54; 67) | |
| Sex | 190/158 | 89/65 | 0.506 |
| Male/female, n (%) | (54.6%/45.4%) | (57.7%/42.2%) | |
| Type of colectomy, n (%) | |||
| Right hemicolectomy | 139 (39.9%) | 44 (28.6%) | 0.092 |
| Extended right hemicolectomy | 10 (2.9%) | 5 (3.2%) | |
| Left sided colectomies | 199 (57.2%) | 105 (68.2%) | |
| Left hemicolectomy | 40 (11.5%) | 14 (9.1%) | |
| Extended left hemicolectomy | 2 (0.6%) | 2 (1.3%) | |
| Sigmoidectomy | 135 (38.8%) | 80 (51.9%) | |
| Segmentectomy for left colon flexure | 22 (6.3%) | 9 (5.8%) | |
| pT stage, n (%) | |||
| pTx | 1 (0.3%) | 0 (0%) | 0.018 |
| pTis | 1 0.3%) | 1 (0.6%) | |
| pT1 | 10 (2.9%) | 0 (0%) | |
| pT2 | 27 (7.8%) | 11 (7.1%) | |
| pT3 | 236 (67.8%) | 94 (61%) | |
| pT4a | 73 (21%) | 48 (31.2%) | |
| pN stage, n (%) | |||
| pNx | 0 (0%) | 4 | 0.008 |
| pN0 | 166 (47.7%) | 66 | |
| pN1 | 119 (34.2%) | 48 (31.2%) | |
| pN1apN1b/pN1c | 63 (18.1%)/49 (14.1%)/7 (2%) | 15 (9.7%)/31 (20.1%)/2(1.3%) | |
| pN2 | 63 (18.1%) | 36 (23.4%) | |
| pN2a/pN2b | 40 (11.5%)/23 (6.6%) | 14 (9.1%)/22 (14.3%) | |
| M (metastasized) stage M0/M1, n | 348/0 | 154/0 | |
| LRR | 40 (11.5%) | 37 (24.03%) | |
| Time (Op-LRR), months | 0.198 | ||
| Mean ± SD | 13.1 ± 8.2 | 23.1 ± 25 | |
| Median (Q25; Q75) | 12 (7–16.8) | 16 (8–29) | |
| Time (Op-CT), months Mean ± SD Median (Q25; Q75) | 12.7 ± 7.92 8.25 (6; 15) | 26.2 ± 17.08 12 (6; 37.5) | <0.001 |
| Histology | |||
| Adenocarcinoma only | 265 (76.2%) | 119 (77.3%) | 0.816 |
| Adenocarcinoma with mucinous cell carcinoma component | 77 (22.1%) | 31 (20.1%) | |
| Adenocarcinoma with signet cell carcinoma component | 4 (1.2%) | 2 (1.3%) | |
| Adenocarcinoma with both mucinous and signet cell carcinoma components | 2 (0.6%) | 2 (1.3%) | |
| ASL in mm | <0.001 | ||
| Mean ± SD | 40.04 ± 25.34 | 60.72 ± 28.87 | |
| Median (Q25; Q75) | 33.25 (23.25; 51.6) | 58 (37.6; 80) | |
| D2PLL in mm | <0.001 | ||
| Mean ± SD | 26.2 ± 14.4 | 31.2 ± 16.0 | |
| Median (Q25; Q75) | 23 (13.5; 38.4) | 34.9 (14; 42.8) | |
| LN(s) < 12, n (%) | 24 (6.9%) | 53 (34.4%) | <0.001 |
| TNM Stage | Group A | Group B | p-Value |
|---|---|---|---|
| n (%) | n = 348 | n = 154 | |
| 0 | 1 (0.29%) | 1 (0.65%) | 0.028 |
| I | 23 (6.61%) | 7 (4.55%) | |
| II (II A/II B) | 141 (122/19) 35.06%/5.46% | 58 (47/11) 30.52%/7.14% | |
| III (III A/III B/III C) | 184 (12/130/40) 52.87% (3.45%/37.36%/11.49%) | 84 (1/54/29) 54.54% (0.65%/35.06%/18.83%) | |
| N/A (pTx or pNx) | 1 (0.28%) | 4 (2.60%) |
| LRR | Cases, n | ASL in mm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (95%CI) | SD | SE | Min | Max | Q25 | Median | Q75 | ||
| No | 425 | 45.5 (42.9–48.2) | 28.0 | 1.3 | 0 | 176.0 | 26 | 39 | 59.4 |
| Yes | 77 | 50.8 (44.3–57.2) | 28.5 | 3.2 | 1 | 131.0 | 26 | 48 | 70.0 |
| All groups | 502 | 46.3 (43.9–48.8) | 28.1 | 1.2 | 0 | 176.0 | 26 | 40 | 61.0 |
| LRR | LN(s) | Cases, n | ASL in mm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (95%CI) | SD | SE | Min | Max | Median | Q25; Q75 | |||
| No | <12 | 53 | 58.9 (51.6–66.2) | 26.5 | 3.6 | 20 | 137 | 57 | 37; 76.6 |
| >12 | 372 | 43.7 (40.9–46.5) | 27.7 | 1.4 | 0 | 176 | 37 | 25; 55 | |
| Yes | <12 | 24 | 62.2 (51.4–72.9) | 25.5 | 5.2 | 20 | 130 | 61.6 | 50; 75 |
| >12 | 53 | 45.6 (37.7–53.5) | 28.5 | 3.9 | 1 | 131 | 40 | 23.6; 63 | |
| All groups | 502 | 46.4 (43.9–48.9) | 28.1 | 1.3 | 0 | 176 | 40 | 26; 61 | |
| Logistic Regression | Odds Ratio (95%CI) | SE | p-Value |
|---|---|---|---|
| Univariate Analysis | |||
| Age in years | 1.2 (1.1–1.5) | 0.011 | 0.003 |
| Study group B, ref. Group A | 2.4 (1.5–3.9) | 0.253 | <0.001 |
| Type of colectomy, ref. left sided colectomies Right hemicolectomy | 1.1 (0.7–1.8) | 0.252 | 0.680 |
| Time (Op-CT) in months | 0.99 (0.98–1.01) | 0.06 | 0.852 |
| Degree of differentiation | 1.2 (0.7–1.9) | 0.240 | 0.442 |
| ASL | 1.7 (1.1–5.1) | 0.004 | 0.014 |
| LNI | 7.8 (3.9–9.5) | 0.087 | <0.001 |
| Total LN(s) | 0.97 (0.95–0.99) | 0.009 | 0.002 |
| LN(s) < 12 | 3.2 (1.8–5.6) | 0.287 | <0.001 |
| Positive LN(s) | 1.08 (1.03–1.14) | 0.028 | 0.005 |
| pT | 1.7 (1.1–2.6) | 0.211 | 0.011 |
| pN | 1.09 (0.81–1.48) | 0.157 | 0.566 |
| Histology, ref. adenocarcinoma only Adenocarcinoma with mucinous cell carcinoma component Adenocarcinoma with signet cell carcinoma component Adenocarcinoma with both mucinous and signet cell carcinoma components | 1.5 (0.8–2.6) 3.1 (0.5–17.4) 2.1 (0.2–20.4) | 0.285 0.879 1.164 | 0.149 0.195 0.529 |
| Perineural invasion | 1.8 (1.1–3.2) | 0.280 | 0.025 |
| Lymphatic invasion | 0.7 (0.4–1.2) | 0.263 | 0.180 |
| Vascular invasion | 0.9 (0.5–1.7) | 0.270 | 0.960 |
| Tumor deposits, pN1c | 1.3 (0.5–3.4) | 0.472 | 0.531 |
| D2IP | 1.01 (0.99–1.07) | 0.005 | 0.187 |
| D3IP | 1.01 (0.99–1.01) | 0.004 | 0.199 |
| Multivariate analysis, Method: Backward Stepwise Step 1 | |||
| Study group B, ref. Group A | 1.81 (1.02–3.2) | 0.291 | 0.041 |
| ASL | 1.18 (1.01–3.6) | 0.005 | 0.040 |
| LNI | 6.03 (1.6–12.6) | 0.673 | 0.008 |
| pT | 1.3 (0.8–2) | 0.220 | 0.225 |
| Perineural invasion | 1.5 (0.8–2.7) | 0.303 | 0.189 |
| Step 4 | |||
| Study group B, ref. Group A | 1.82 (1.06–3.1) | 0.274 | 0.029 |
| ASL | 1.1 (1.01–1.3) | 0.005 | 0.043 |
| LNI | 8.8 (2.4−12.1) | 0.644 | 0.001 |
| Score | p-Value | |||
|---|---|---|---|---|
| Step 4 | Variables | pT | 2.184 | 0.139 |
| Perineural invasion | 2.565 | 0.109 | ||
| Overall statistics | 4.095 | 0.251 | ||
| Variables in the Equation | β | SE | |
|---|---|---|---|
| Step 4 | Study group B, ref. group A | 0.596 | 0.274 |
| ASL | 0.101 | 0.005 | |
| LNI | 2.176 | 0.664 | |
| Intercept | 0.215 | 0.180 |
| Variables in the Equation Bootstrap § | β | SE | |
|---|---|---|---|
| Step 4 | Study group B, ref. group A | 0.596 | 0.241 |
| ASL | 0.101 | 0.002 | |
| LNI | 2.176 | 0.017 | |
| Intercept | 0.215 | 0.205 |
| Low Risk | Moderate Risk | High Risk | Total | |
|---|---|---|---|---|
| Without LRR | 373 (87.76%) | 43 (10.12%) | 9 (2.12%) | 425 |
| With LRR | 4 (5.19%) | 14 (18.18%) | 59 (76.62%) | 77 |
| Total | 377 | 57 | 68 | 502 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livadaru, C.; Moscalu, M.; Ghitun, F.A.; Huluta, A.R.; Terinte, C.; Ferariu, D.; Lunca, S.; Dimofte, G.M. Postoperative Quality Assessment Score Can Select Patients with High Risk for Locoregional Recurrence in Colon Cancer. Diagnostics 2022, 12, 363. https://doi.org/10.3390/diagnostics12020363
Livadaru C, Moscalu M, Ghitun FA, Huluta AR, Terinte C, Ferariu D, Lunca S, Dimofte GM. Postoperative Quality Assessment Score Can Select Patients with High Risk for Locoregional Recurrence in Colon Cancer. Diagnostics. 2022; 12(2):363. https://doi.org/10.3390/diagnostics12020363
Chicago/Turabian StyleLivadaru, Cristian, Mihaela Moscalu, Florina Adriana Ghitun, Alexandra Ramona Huluta, Cristina Terinte, Dan Ferariu, Sorinel Lunca, and Gabriel Mihail Dimofte. 2022. "Postoperative Quality Assessment Score Can Select Patients with High Risk for Locoregional Recurrence in Colon Cancer" Diagnostics 12, no. 2: 363. https://doi.org/10.3390/diagnostics12020363
APA StyleLivadaru, C., Moscalu, M., Ghitun, F. A., Huluta, A. R., Terinte, C., Ferariu, D., Lunca, S., & Dimofte, G. M. (2022). Postoperative Quality Assessment Score Can Select Patients with High Risk for Locoregional Recurrence in Colon Cancer. Diagnostics, 12(2), 363. https://doi.org/10.3390/diagnostics12020363






