Dual-Energy Lung Perfusion in Portal Venous Phase CT—A Comparison with the Pulmonary Arterial Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Dual Energy CT
2.3. Image Reconstruction
2.4. Objective Image Evaluation
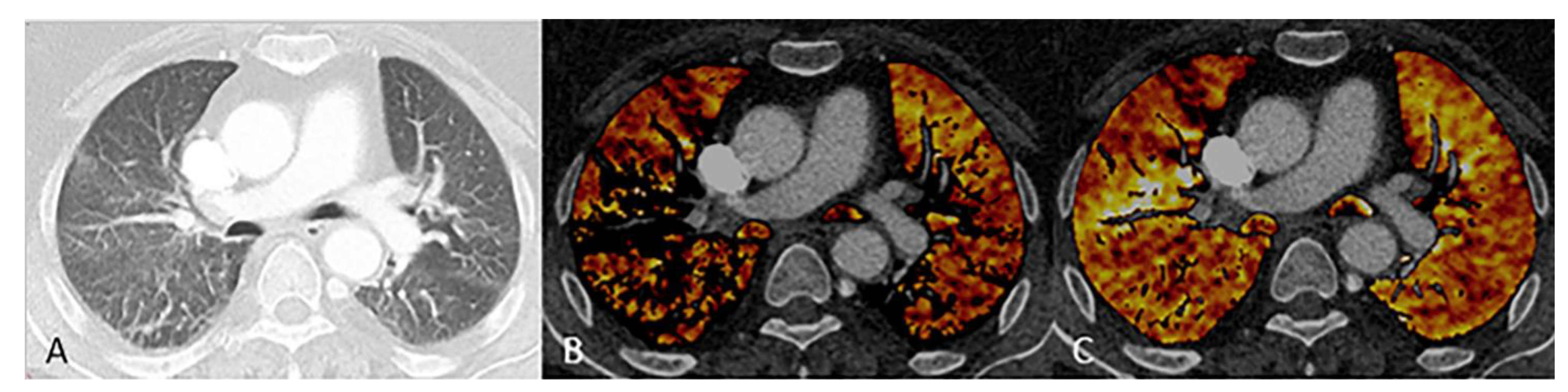
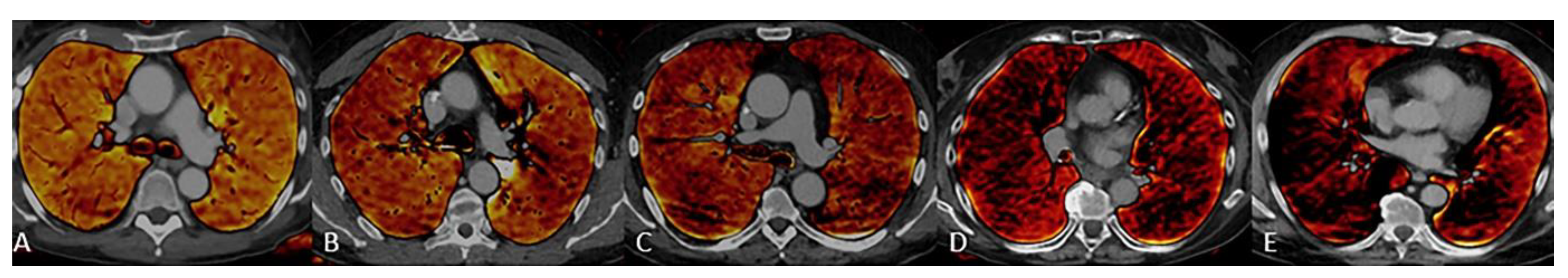
2.5. Subjective Image Evaluation
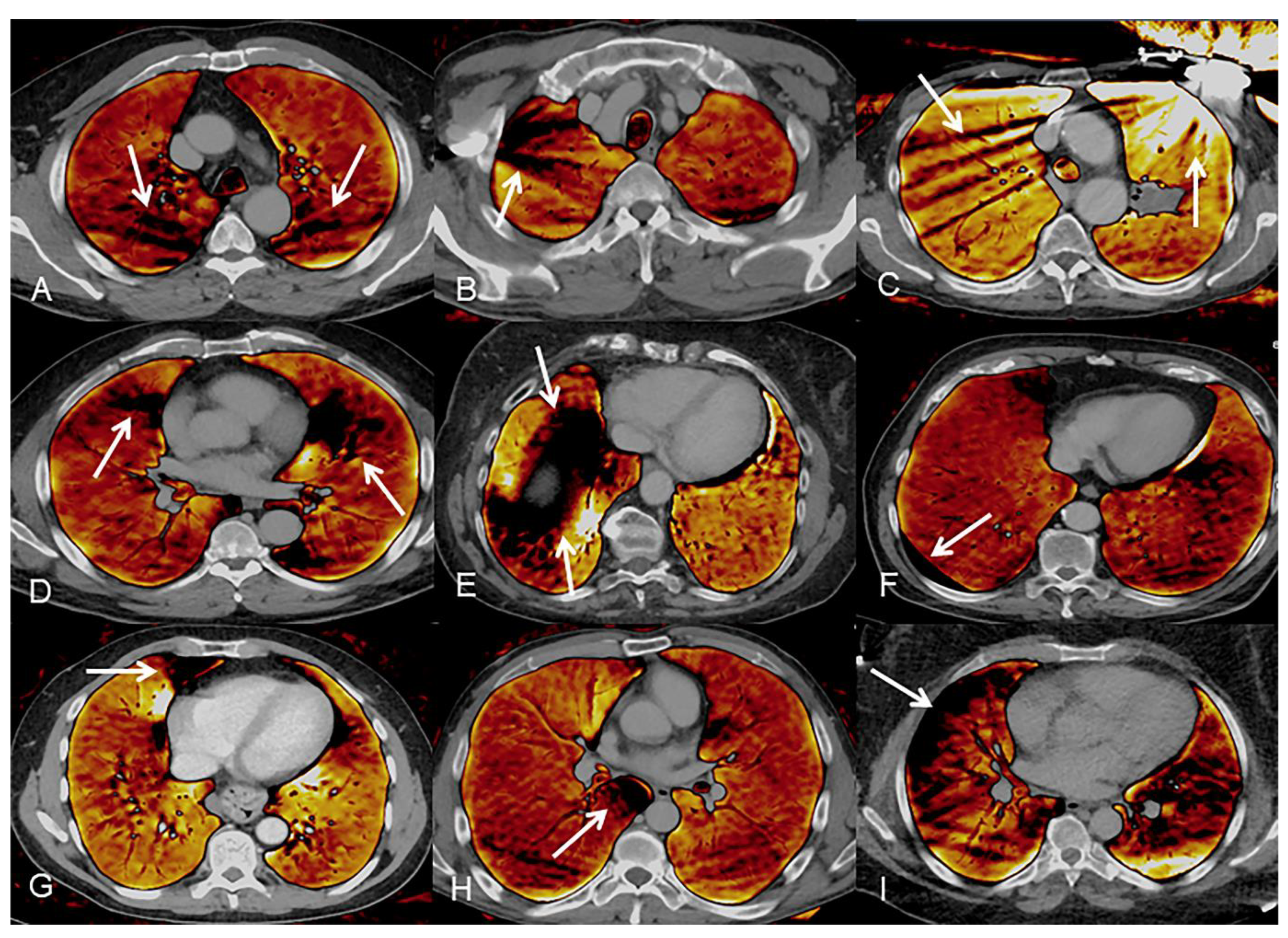
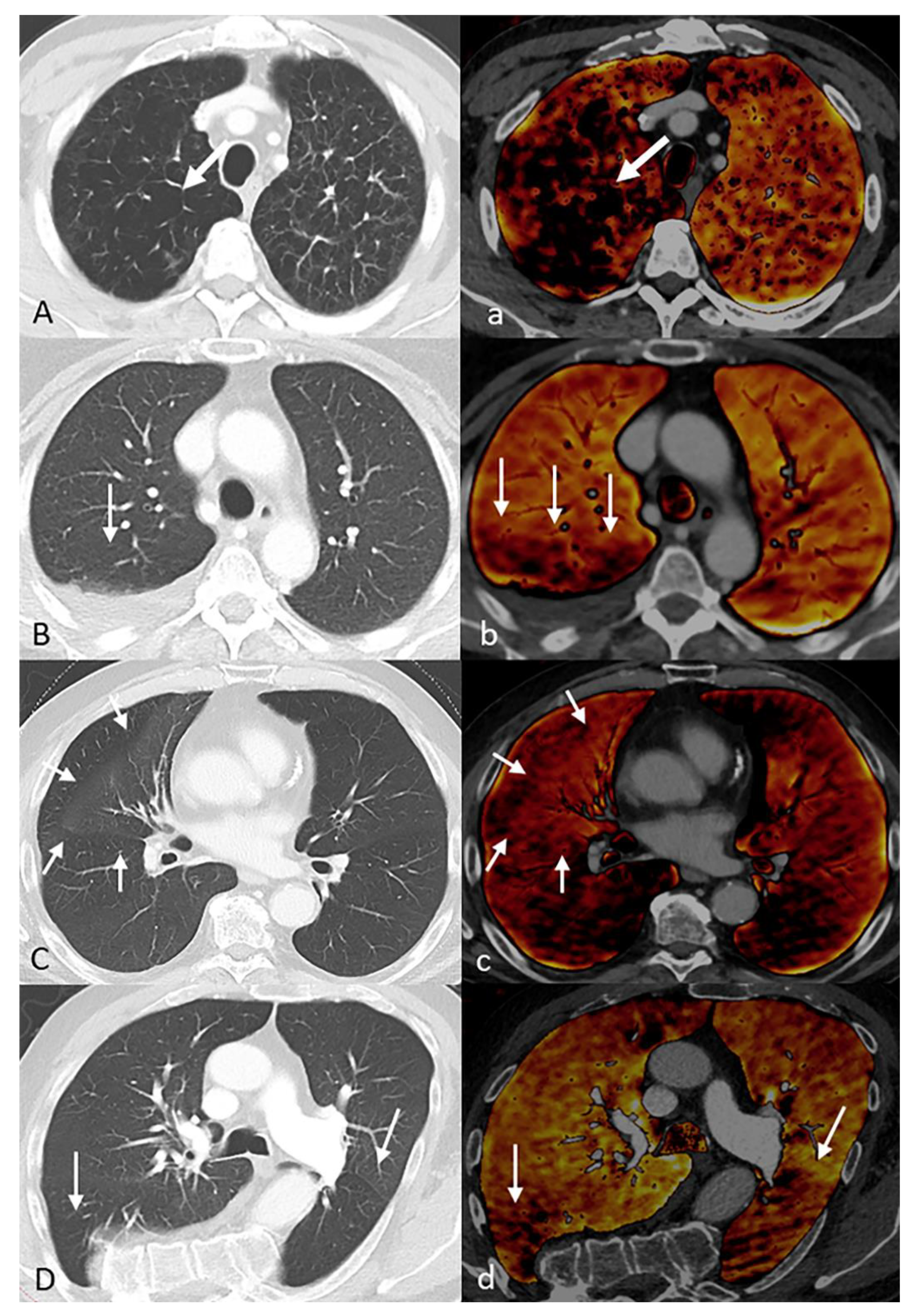
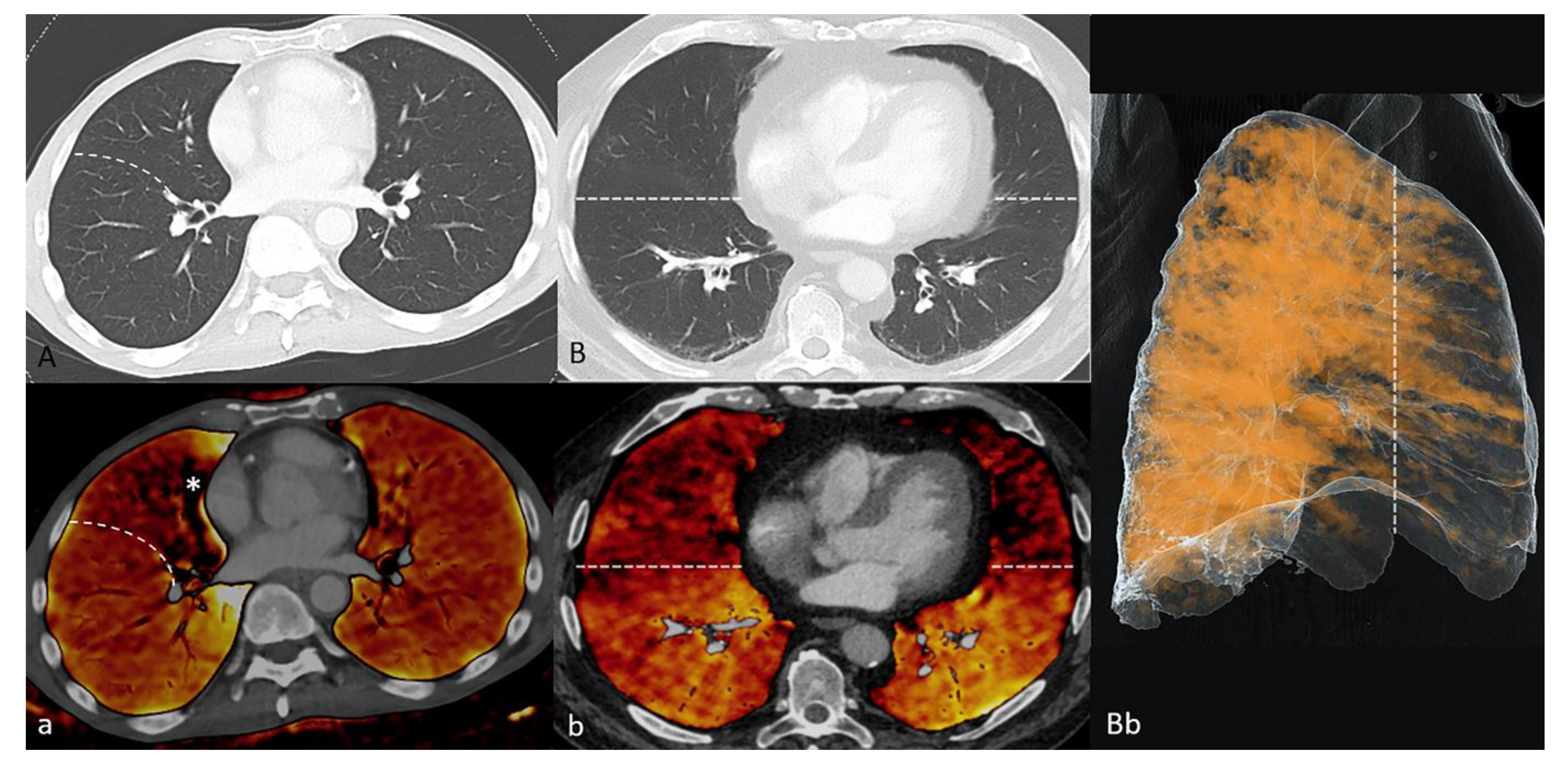
2.6. Artifacts
2.7. Pathologic Findings
2.8. Statistics
3. Results
3.1. Patients
3.2. Objective Image Quality
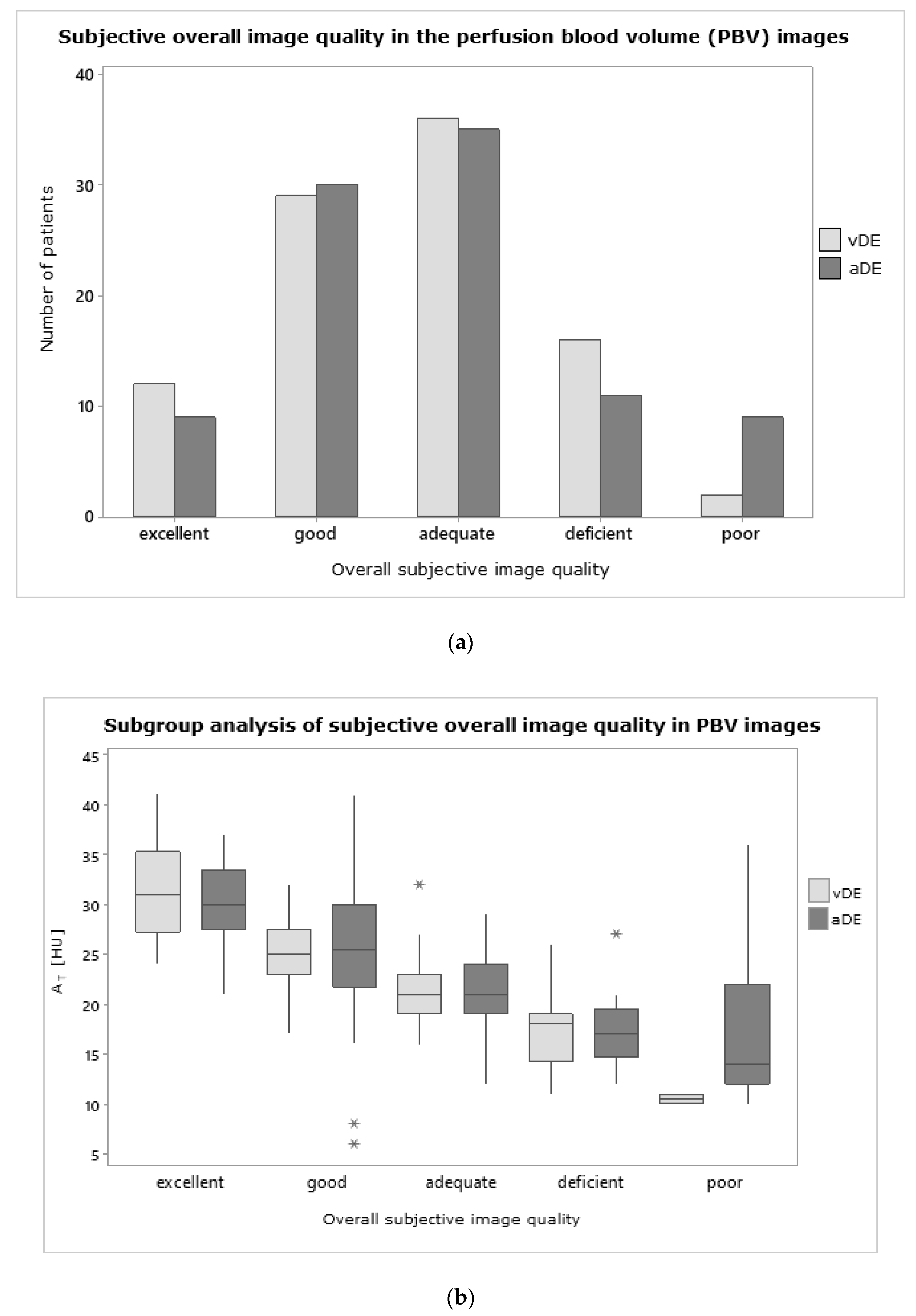
3.3. Subjective Image Quality
3.4. Artifacts
3.5. Pathologic Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Johnson, T.R.C.; Krauss, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2006, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.X.; Zhou, C.S.; Zhao, Y.E.; Schoepf, U.J.; Mangold, S.; Ball, B.D.; Han, Z.H.; Qi, L.; Zhang, L.J.; Lu, G.M. Detection of pulmonary fat embolism with dual-energy CT: An experimental study in rab-bits. Eur. Radiol. 2017, 27, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Hur, J.; Han, K.H.; Lee, H.-J.; Kwon, W.; Choi, B.W.; Kim, Y.J. Acute Pulmonary Embolism: Retrospective Cohort Study of the Predictive Value of Perfusion Defect Volume Measured with Dual-Energy CT. Am. J. Roentgenol. 2017, 209, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dournes, G.; Verdier, D.; Montaudon, M.; Bullier, E.; Rivière, A.; Dromer, C.; Picard, F.; Billes, M.A.; Corneloup, O.; Laurent, F.; et al. Dual-energy CT perfusion and angiography in chronic thromboembolic pulmo-nary hypertension: Diagnostic accuracy and concordance with radionuclide scintigraphy. Eur. Radiol. 2014, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Haneder, S.; Siedek, F.; Doerner, J.; Pahn, G.; Hokamp, N.G.; Maintz, D.; Wybranski, C. Thoracic-abdominal imaging with a novel dual-layer spectral detector CT: Intra-individual comparison of image quality and radiation dose with 128-row single-energy acquisition. Acta Radiol. 2018, 59, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Albrecht, M.H.; D’Angelo, T.; Leithner, D.; Lenga, L.; Booz, C.; Scholtz, J.-E.; Bodelle, B.; et al. Dual-energy computed tomography in patients with cutaneous malignant melanoma: Comparison of noise-optimized and traditional virtual monoenergetic imaging. Eur. J. Radiol. 2017, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, J.Y.; Choe, K.O.; Hur, J.; Lee, H.-J.; Choi, B.W.; Kim, Y.J. Different Perfusion Pattern Between Acute and Chronic Pulmonary Thromboembolism: Evaluation With Two-Phase Dual-Energy Perfusion CT. Am. J. Roentgenol. 2013, 200, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Sueyoshi, E.; Sakamoto, I.; Uetani, M. Clinical Significance of Late Phase of Lung Perfusion Blood Volume (Lung Perfusion Blood Volume) Quantified by Dual-Energy Computed Tomography in Patients with Pulmonary Thromboembolism. J. Thorac. Imaging 2017, 32, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Park, C.M.; Lee, C.H.; Goo, J.M.; Lee, H.J. Focal Iodine Defects on Color-Coded Iodine Perfusion Maps of Du-al-Energy Pulmonary CT Angiography Images: A Potential Diagnostic Pitfall. AJR Am. J. Roentgenol. 2010, 195, W325–W330. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, K.T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.F.; Keat, N. Artifacts in CT: Recognition and Avoidance. RadioGraphics 2004, 24, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Khanduri, S.; Goyal, A.; Singh, B.; Chaudhary, M.; Sabharwal, T.; Jain, S.; Sharma, H. The Utility of Dual Energy Computed Tomography in Musculoskele-tal Imaging. J. Clin. Imaging Sci. 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boas, F.; Fleischmann, D. CT artifacts: Causes and reduction techniques. Imaging Med. 2012, 4, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Kerl, J.M.; Bauer, R.W.; Renker, M.; Weber, E.; Weisser, P.; Korkusuz, H.; Schell, B.; Larson, M.C.; Kromen, W.; Jacobi, V.; et al. Triphasic contrast injection improves evaluation of dual energy lung perfusion in pulmonary CT angiography. Eur. J. Radiol. 2011, 80, e483–e487. [Google Scholar] [CrossRef] [PubMed]
- Saltybaeva, N.; Schmidt, B.; Wimmer, A.; Flohr, T.; Alkadhi, H. Precise and Automatic Patient Positioning in Computed Tomography: Avatar Modeling of the Patient Surface Using a 3-Dimensional Camera. Investig. Radiol. 2018, 53, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felloni, P.; Duhamel, A.; Faivre, J.B.; Giordano, J.; Khung, S.; Deken, V.; Remy, J.; Remy-Jardin, M. Regional Distribution of Pulmonary Blood Volume with Dual-Energy Computed Tomography: Results in 42 Subjects. Acad Radiol. 2017, 24, 1412–1421. [Google Scholar] [CrossRef] [PubMed]

| Scan Parameters | vDE Study Group (n = 95) | aDE Reference Group (n = 94) | ||
|---|---|---|---|---|
| Tube | A | B | A | B |
| Tube voltage | 70 kV | Sn 150 kV | 80 kV | Sn 150 kV |
| Ref. mAs | 560 mAs | 140 mAs | 100 mAs | 77 mAs |
| Rotation Time | 0.5 s | 0.25 s | ||
| Delay | 70 s | Bolus tracking + 8 s | ||
| CTDI | 8.23 ± 2.0 mGy | 5.4 ± 2.4 mGy | ||
| DLP | 588 ± 151 mGy*cm | 208 ± 95 mGy*cm | ||
| Pitch | 0.6 | 0.6 | ||
| Collimation | 128 × 0.6 mm | 192 × 0.6 mm | ||
| vDE (n = 95) | aDE (n = 94) | p-Value | aDEex (n = 62) | p-Value | |
|---|---|---|---|---|---|
| VT in [ml] | 4795 ± 1195 | 3885 ± 1307 | <0.01 | 3760 ± 1267 | <0.01 |
| AT in [HU] | 23 ± 6 | 23 ± 7 | 0.87 | 23 ± 7 | 0.84 |
| AA in [HU] | 224 ± 39 | 252 ± 78 | <0.01 | 247 ± 79 | 0.03 |
| AP in [HU] | 224 ± 39 | 304 ± 107 | <0.01 | 301 ± 102 | <0.01 |
| DL in % | 10 ± 2 | 8 ± 2 | <0.01 | 9 ± 2 | <0.01 |
| Artifact Group | vDE n = 95 | aDE n = 94 | p-Value | ||
|---|---|---|---|---|---|
| and subgroup | total cases (n) | relative cases (%) | total cases (n) | relative cases (%) | vDE vs aDE |
| Streak artifacts | 117 | 123 | 109 | 116 | 0.560 |
| Spine | 82 | 86 | 35 | 37 | <0.001 |
| Contrast agent filled vessels | 27 | 28 | 70 | 75 | <0.001 |
| Metal | 8 | 8 | 4 | 4 | 0.243 |
| Rotation artifacts | 121 | 127 | 125 | 133 | 0.568 |
| Heart | 87 | 92 | 80 | 85 | 0.167 |
| Diaphragm | 34 | 35 | 45 | 48 | 0.093 |
| Dual Source artifacts | 5 | 5 | 7 | 7 | 0.541 |
| Recess artifacts | 62 | 65 | 23 | 24 | <0.001 |
| Anterior (costomediastinal) | 45 | 47 | 21 | 22 | <0.001 |
| Posterior (azygoesophageal) | 17 | 18 | 2 | 2 | <0.001 |
| Subpleural artifacts | 26 | 27 | 7 | 7 | <0.001 |
| Pathological Finding | vDE (n = 95) | aDE (n = 94) | p-Value | ||
|---|---|---|---|---|---|
| Type | Total | % | Total | % | vDE vs aDE |
| With anatomical correlate | 11 | 12 | 39 | 41 | 0.001 |
| Decreased vascularization | 1 | 1 | 25 | 27 | 0.002 |
| Increased vascularization | 0 | 0 | 3 | 3 | 0.757 |
| Decreased parenchyma density | 4 | 4 | 3 | 3 | 0.609 |
| Increased parenchyma density | 4 | 4 | 6 | 6 | 0.306 |
| Architectural distortion | 2 | 2 | 2 | 2 | 0.999 |
| Without anatomical correlate | 7 | 7 | 12 | 13 | 0.049 |
| Lobar | 7 | 7 | 7 | 7 | 0.999 |
| Anteroposterior gravity gradient | 0 | 0 | 5 | 5 | 0.606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praël, I.; Wuest, W.; Heiss, R.; Wiesmueller, M.; Kopp, M.; Uder, M.; May, M.S. Dual-Energy Lung Perfusion in Portal Venous Phase CT—A Comparison with the Pulmonary Arterial Phase. Diagnostics 2021, 11, 1989. https://doi.org/10.3390/diagnostics11111989
Praël I, Wuest W, Heiss R, Wiesmueller M, Kopp M, Uder M, May MS. Dual-Energy Lung Perfusion in Portal Venous Phase CT—A Comparison with the Pulmonary Arterial Phase. Diagnostics. 2021; 11(11):1989. https://doi.org/10.3390/diagnostics11111989
Chicago/Turabian StylePraël, Isabelle, Wolfgang Wuest, Rafael Heiss, Marco Wiesmueller, Markus Kopp, Michael Uder, and Matthias S. May. 2021. "Dual-Energy Lung Perfusion in Portal Venous Phase CT—A Comparison with the Pulmonary Arterial Phase" Diagnostics 11, no. 11: 1989. https://doi.org/10.3390/diagnostics11111989
APA StylePraël, I., Wuest, W., Heiss, R., Wiesmueller, M., Kopp, M., Uder, M., & May, M. S. (2021). Dual-Energy Lung Perfusion in Portal Venous Phase CT—A Comparison with the Pulmonary Arterial Phase. Diagnostics, 11(11), 1989. https://doi.org/10.3390/diagnostics11111989







