Impact of an Electronic Alert in Combination with a Care Bundle on the Outcomes of Acute Kidney Injury
Abstract
:1. Introduction
2. Materials and Methods
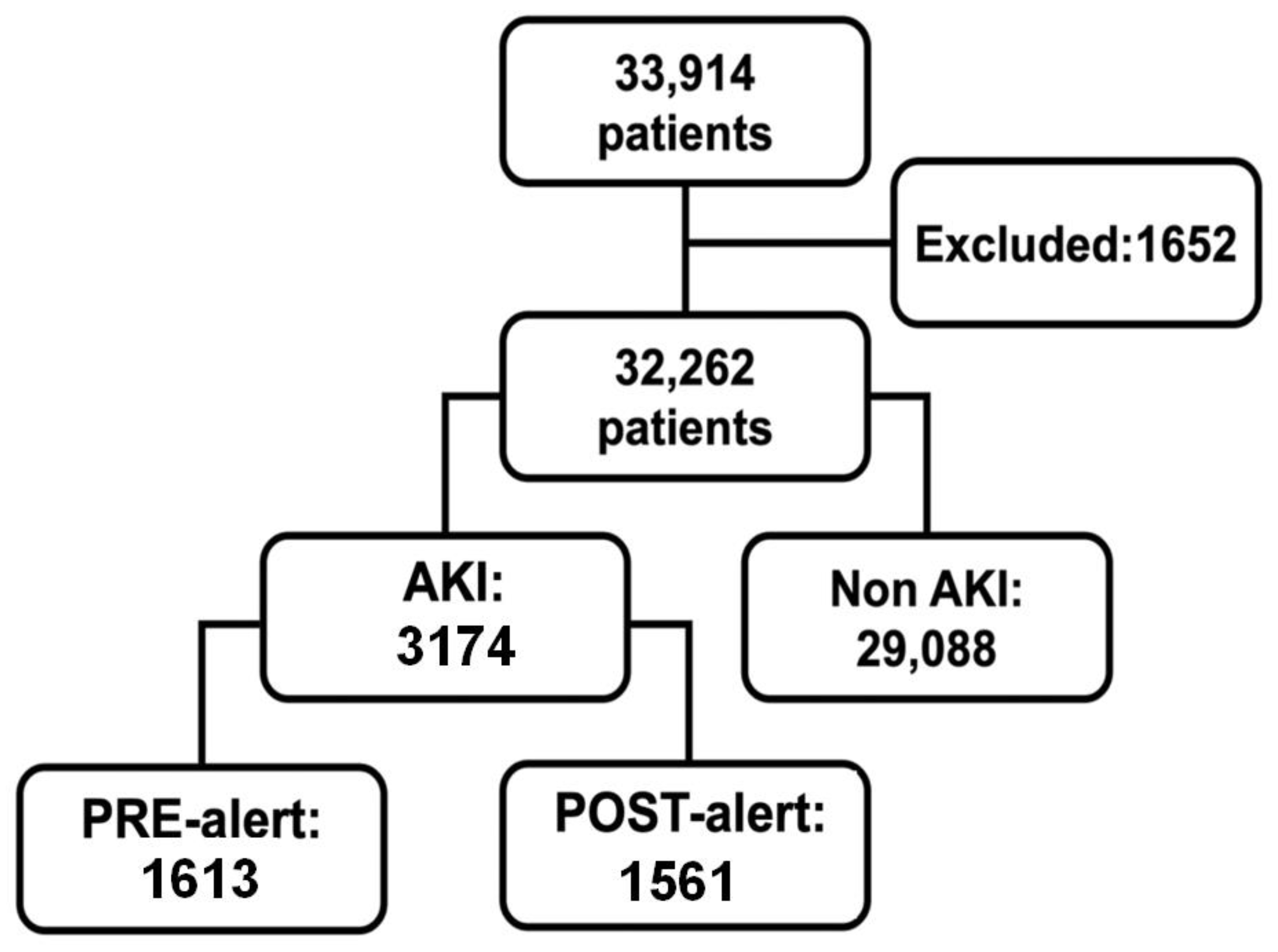
2.1. Setting and Participants
2.2. Electronic Alert and Care Bundle
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. AKI Severity
3.3. Nephrology Consultation and Renal Replacement Therapy
3.4. Care Bundle Adherence
3.5. Mortality
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L.; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerda, J.; Bagga, A.; Kher, V.; Chakravarthi, R.M. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat. Clin. Pract. Nephrol. 2008, 4, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Muniraju, T.M.; Lillicrap, M.H.; Horrocks, J.L.; Fisher, J.M.; Clark, R.M.; Kanagasundaram, N.S. Diagnosis and management of acute kidney injury: Deficiencies in the knowledge base of non-specialist, trainee medical staff. Clin. Med. Lond. 2012, 12, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Stevens, P.E.; Tamimi, N.A.; Al-Hasani, M.K.; Mikhail, A.I.; Kearney, E.; Lapworth, R.; Prosser, D.I.; Carmichael, P. Non-specialist management of acute renal failure. QJM 2001, 94, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.B.; National Confidential Enquiry into Perioperative, D.; Stewart, J.; National Confidential Enquiry into Patient Outcome and Death. Adding insult to Injury: A Review of the Care of Patients Who Died in Hospital with a Primary Diagnosis of Acute Kidney Injury (Actute Renal Failure): A Report by the National Confidential Enquiry into Patient Outcome and Death; National Confidential Enquiry into Patient Outcome and Death: London, UK, 2009; p. 98, Illustrations. Online Access; Available online: http://www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf (accessed on 17 October 2022).
- Mehta, R.L.; Cerda, J.; Burdmann, E.A.; Tonelli, M.; Garcia-Garcia, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Selby, N.M.; Kolhe, N.V. Care Bundles for Acute Kidney Injury: Do They Work? Nephron 2016, 134, 195–199. [Google Scholar] [CrossRef]
- Palevsky, P.M. Electronic Alerts for Acute Kidney Injury. Am. J. Kidney Dis. 2018, 71, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.P.; Greenberg, J.H. Acute Kidney Injury in Real Time: Prediction, Alerts, and Clinical Decision Support. Nephron 2018, 140, 116–119. [Google Scholar] [CrossRef]
- Kashani, K.B. Automated acute kidney injury alerts. Kidney Int. 2018, 94, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2012, 2, 19–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Section 3: Prevention and Treatment of AKI. Kidney Int. Suppl. (2011) 2012, 2, 37–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicent Harding–UCL Medical Illustration Services. London AKI. Available online: https://apps.apple.com/gb/app/london-aki/id503139013 (accessed on 17 October 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Haase, M.; Lewington, A.J.P.; O’Donoghue, D.J.; Wilson, F.P.; Nadim, M.K.; Silver, S.A.; Zarbock, A.; Ostermann, M.; et al. Quality Improvement Goals for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2019, 14, 941–953. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.; Kellum, J.A.; Chawla, L.S.; Cruz, D.; Ince, C.; Okusa, M.D.; et al. Potential use of biomarkers in acute kidney injury: Report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014, 85, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.; Kashani, K.; Gibney, N.; Wilson, F.P.; Ronco, C.; Goldstein, S.L.; Kellum, J.A.; Bagshaw, S.M.; Group, A.C. Impact of electronic-alerting of acute kidney injury: Workgroup statements from the 15(th) ADQI Consensus Conference. Can. J. Kidney Health Dis. 2016, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Kashani, K.; Ronco, C. Acute Kidney Injury Electronic Alert for Nephrologist: Reactive versus Proactive? Blood Purif. 2016, 42, 323–328. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Kirkendall, E.; Nguyen, H.; Schaffzin, J.K.; Bucuvalas, J.; Bracke, T.; Seid, M.; Ashby, M.; Foertmeyer, N.; Brunner, L.; et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 2013, 132, e756–e767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, S.L.; Mottes, T.; Simpson, K.; Barclay, C.; Muething, S.; Haslam, D.B.; Kirkendall, E.S. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. 2016, 90, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baek, S.H.; Ahn, S.; Lee, K.H.; Hwang, H.; Ryu, J.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.W.; et al. Impact of Electronic Acute Kidney Injury (AKI) Alerts With Automated Nephrologist Consultation on Detection and Severity of AKI: A Quality Improvement Study. Am. J. Kidney Dis. 2018, 71, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, G.; Al-Aly, Z.; Moiz, A.; Rauchman, M.; Zhang, Z.; Gopalakrishnan, R.; Balasubramanian, S.; El-Achkar, T.M. Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am. J. Kidney Dis. 2011, 57, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.L.; McDonald, B.; Gabbai, F.; Pahl, M.; Farkas, A.; Pascual, M.T.; Zhuang, S.; Kaplan, R.M.; Chertow, G.M. Nephrology consultation in acute renal failure: Does timing matter? Am. J. Med. 2002, 113, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ponce, D.; Zorzenon Cde, P.; dos Santos, N.Y.; Balbi, A.L. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol. Dial. Transplant. 2011, 26, 3202–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolhe, N.V.; Reilly, T.; Leung, J.; Fluck, R.J.; Swinscoe, K.E.; Selby, N.M.; Taal, M.W. A simple care bundle for use in acute kidney injury: A propensity score-matched cohort study. Nephrol. Dial. Transplant. 2016, 31, 1846–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, J.; Smith-Byrne, K.; Sayers, O.; Joseph, K.; Sleeman, M.; Lasserson, D.; Vaux, E. Electronic alerts for acute kidney injury across primary and secondary care. BMJ Open Qual. 2021, 10, e000956. [Google Scholar] [CrossRef]
- Joslin, J.; Wilson, H.; Zubli, D.; Gauge, N.; Kinirons, M.; Hopper, A.; Pile, T.; Ostermann, M. Recognition and management of acute kidney injury in hospitalized patients can be partially improved with the use of a care bundle. Clin. Med. Lond. 2015, 15, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Colpaert, K.; Hoste, E.A.; Steurbaut, K.; Benoit, D.; Van Hoecke, S.; De Turck, F.; Decruyenaere, J. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit. Care Med. 2012, 40, 1164–1170. [Google Scholar] [CrossRef]
- Tomson, C.; Tomlinson, L.A. Stopping RAS Inhibitors to Minimize AKI: More Harm than Good? Clin. J. Am. Soc. Nephrol. 2019, 14, 617–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leisman, D.E.; Fernandes, T.D.; Bijol, V.; Abraham, M.N.; Lehman, J.R.; Taylor, M.D.; Capone, C.; Yaipan, O.; Bellomo, R.; Deutschman, C.S. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachance, P.; Villeneuve, P.M.; Rewa, O.G.; Wilson, F.P.; Selby, N.M.; Featherstone, R.M.; Bagshaw, S.M. Association between e-alert implementation for detection of acute kidney injury and outcomes: A systematic review. Nephrol. Dial. Transpl. 2017, 32, 265–272. [Google Scholar] [CrossRef]
- Sykes, L.; Nipah, R.; Kalra, P.; Green, D. A narrative review of the impact of interventions in acute kidney injury. J. Nephrol. 2018, 31, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.P.; Shashaty, M.; Testani, J.; Aqeel, I.; Borovskiy, Y.; Ellenberg, S.S.; Feldman, H.I.; Fernandez, H.; Gitelman, Y.; Lin, J.; et al. Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 2015, 385, 1966–1974. [Google Scholar] [CrossRef] [Green Version]
- Ebah, L.; Hanumapura, P.; Waring, D.; Challiner, R.; Hayden, K.; Alexander, J.; Henney, R.; Royston, R.; Butterworth, C.; Vincent, M.; et al. A Multifaceted Quality Improvement Programme to Improve Acute Kidney Injury Care and Outcomes in a Large Teaching Hospital. BMJ Qual. Improv. Rep. 2017, 6. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Sharma, A.; Tennent, L.; Wong, C.; Chamberlain, P.; Abraham, K.A. A whole system approach to improving mortality associated with acute kidney injury. QJM 2017, 110, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Sykes, L.; Sinha, S.; Hegarty, J.; Flanagan, E.; Doyle, L.; Hoolickin, C.; Edwards, L.; Ferris, P.; Lamerton, E.; Poulikakos, D.; et al. Reducing acute kidney injury incidence and progression in a large teaching hospital. BMJ Open Qual. 2018, 7, e000308. [Google Scholar] [CrossRef]
- Wilson, F.P.; Martin, M.; Yamamoto, Y.; Partridge, C.; Moreira, E.; Arora, T.; Biswas, A.; Feldman, H.; Garg, A.X.; Greenberg, J.H.; et al. Electronic health record alerts for acute kidney injury: Multicenter, randomized clinical trial. BMJ 2021, 372, m4786. [Google Scholar] [CrossRef]


| Overall | Pre-Alert | Post-Alert | p | |
|---|---|---|---|---|
| N = 3174 (100%) | N = 1613 (50.8%) | N = 1561 (49.2%) | ||
| Male | 1839 (57.9) | 960 (59.5) | 879 (56.3) | 0.344 |
| White | 2808 (88.5) | 1431 (88.7) | 1377 (88.2) | 0.728 |
| Age (years) | 66 (54–76) | 66 (54–76) | 66 (54–76) | 0.728 |
| 18 to <40 | 297 (9.4) | 155 (9.6) | 142 (9.1) | 0.728 |
| 40 to <65 | 1173 (37.0) | 598 (37.1) | 575 (36.8) | 0.889 |
| 65 to <75 | 784 (24.7) | 385 (23.9) | 399 (25.6) | 0.587 |
| ≥75 | 920 (29.0) | 475 (29.4) | 445 (28.5) | 0.728 |
| Smoking | 673 (21.2) | 355 (22.0) | 318 (20.4) | 0.587 |
| Alcoholism | 395 (12.4%) | 193 (12.0) | 202 (12.9) | 0.718 |
| Body mass index (BMI) | 25 (22–28) | 25 (22–28) | 25 (23–29) | 0.168 |
| Cardiovascular disease | 1907 (60.1) | 960 (59.5) | 947 (60.7) | 0.728 |
| Pulmonary disease | 243 (7.7) | 128 (7.9) | 115 (7.4) | 0.728 |
| Gastrointestinal disease | 289 (9.1) | 172 (10.7) | 117 (7.5) | 0.024 |
| Neurologic disease | 476 (15.0) | 246 (15.3) | 230 (14.7) | 0.728 |
| Rheumatologic disease | 81 (2.6) | 47 (2.9) | 34 (2.2) | 0.576 |
| Malignancy | 519 (16.4) | 282 (17.5) | 237 (15.2) | 0.344 |
| Immunosuppressive conditions | 135 (4.3) | 90 (5.6) | 45 (2.9) | 0.022 |
| Endocrine disease | 1087 (34.2) | 541 (33.5) | 546 (35.0) | 0.718 |
| ICU admission | 2082 (65.6) | 1081 (67.0) | 1001 (64.1) | 0.344 |
| Baseline creatinine (mg/dL) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 0.587 |
| eGFR CKD-EPI (mL/min/1.73 m2) | 65 (41–92) | 64 (41–91) | 66 (41–92) | 0.728 |
| KDIGO criteria AKI diagnosis | ||||
| sCr ≥ 0.3 mg/dL in 48 h | 2182 (68.7) | 1099 (68.1) | 1083 (69.4) | 0.718 |
| sCr ≥ 50% in 7 days | 992 (31.3) | 514 (31.9) | 478 (30.6) | 0.718 |
| Overall | Pre-Alert | Post-Alert | p | |
|---|---|---|---|---|
| N = 3174 | N = 1613 | N = 1561 | ||
| KDIGO at diagnosis | ||||
| KDIGO 1 | 2357 (74.3) | 1185 (73.5) | 1172 (75.1) | 0.430 |
| KDIGO 2 | 399 (12.6) | 204 (12.6) | 195 (12.5) | 0.929 |
| KDIGO 3 | 418 (13.2) | 224 (13.9) | 194 (12.4) | 0.364 |
| Maximum KDIGO | ||||
| KDIGO 1 | 1697 (53.5) | 822 (51.0) | 875 (56.1) | 0.026 |
| KDIGO 2 | 472 (14.9) | 254 (15.7) | 218 (14.0) | 0.306 |
| KDIGO 3 | 1005 (31.7) | 537 (33.3) | 468 (30.0) | 0.117 |
| AKI progression | 813 (25.6) | 439 (27.2) | 374 (24.0) | 0.117 |
| Nephrology consultation | 832 (26.2) | 440 (27.3) | 392 (25.1) | 0.306 |
| Time from diagnosis to nephrology consultation (days) | 1 (−1 a 2) | 1 (−1 a 3) | 0 (−1 a 2) | 0.117 |
| RRT | 476 (15.0) | 241 (14.9) | 235 (15.1) | 0.929 |
| Length of stay (days) | 13 (7–23) | 13 (7–23) | 13 (7–22) | 0.675 |
| 30-day hospital readmission | 350 (18.1) | 168 (18.2) | 182 (18.0) | 0.929 |
| 30-day mortality | 1068 (33.6) | 592 (36.7) | 476 (30.5) | 0.012 |
| 24 h before Alert | 24 h after Alert | p | |
|---|---|---|---|
| Complete fluid chart | 1042 (66.8) | 1005 (64.4) | 0.073 |
| Creatinine request | 1366 (87.5) | 672 (43.0) | 0.002 |
| Capillary glycemia test | 1054 (67.5) | 1097 (70.3) | 0.039 |
| Glycemia >180 mg/dL | 440 (28.2) | 492 (31.5) | 0.024 |
| Hypotension | 473 (30.3) | 416 (26.6) | 0.029 |
| NSAIDs | 62 (4.0) | 41 (2.6) | 0.002 |
| Furosemide | 833 (53.4) | 852 (54.6) | 0.132 |
| ACEis/ARBs | 538 (34.5) | 444 (28.4) | 0.002 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Male gender | 1.02 | 0.90–1.15 | 0.847 | |||
| Age | ||||||
| 18–<40 years | Reference | Reference | Reference | |||
| 40–<65 years | 1.51 | 1.15–1.99 | 0.007 | 1.33 | 1.0–1.75 | 0.047 |
| 65–<75 years | 2.06 | 1.56–2.71 | 0.004 | 1.60 | 1.19–2.14 | 0.002 |
| ≥75 years | 2.85 | 2.18–3.74 | 0.004 | 2.14 | 1.60–2.87 | <0.001 |
| ICU admission | 1.25 | 1.09–1.45 | 0.005 | 1.24 | 1.07–1.43 | 0.004 |
| Baseline eGFR CKD-EPI (each 10 mL/min/1.73 m2) | 0.92 | 0.91–0.94 | 0.004 | 0.95 | 0.93–0.97 | <0.001 |
| Cardiovascular disease | 1.06 | 0.94–1.20 | 0.449 | |||
| Pulmonary disease | 1.42 | 1.17–1.73 | 0.004 | 1.25 | 1.03–1.53 | 0.026 |
| Gastrointestinal disease | 0.86 | 0.69–1.07 | 0.253 | |||
| Neurologic disease | 1.27 | 1.09–1.48 | 0.005 | 1.14 | 0.98–1.34 | 0.094 |
| Rheumatologic disease | 0.87 | 0.59–1.28 | 0.597 | |||
| Malignancy | 1.16 | 0.99–1.36 | 0.098 | 1.29 | 1.10–1.51 | 0.002 |
| Immunosuppressive | 0.63 | 0.45–0.89 | 0.016 | 0.77 | 0.54–1.09 | 0.137 |
| Endocrine disease | 1.02 | 0.90–1.16 | 0.847 | |||
| Electronic AKI alert | 0.84 | 0.75–0.95 | 0.012 | 0.87 | 0.77–0.98 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tome, A.C.N.; Ramalho, R.J.; dos Santos, K.F.; Ponte, B.; Agostinho, H.; Machado, M.N.; Lopes, M.B.; Abbud-Filho, M.; de Lima, E.Q. Impact of an Electronic Alert in Combination with a Care Bundle on the Outcomes of Acute Kidney Injury. Diagnostics 2022, 12, 3121. https://doi.org/10.3390/diagnostics12123121
Tome ACN, Ramalho RJ, dos Santos KF, Ponte B, Agostinho H, Machado MN, Lopes MB, Abbud-Filho M, de Lima EQ. Impact of an Electronic Alert in Combination with a Care Bundle on the Outcomes of Acute Kidney Injury. Diagnostics. 2022; 12(12):3121. https://doi.org/10.3390/diagnostics12123121
Chicago/Turabian StyleTome, Ana Carolina Nakamura, Rodrigo José Ramalho, Karise Fernandes dos Santos, Bianca Ponte, Helga Agostinho, Mauricio Nassau Machado, Marcelo Barreto Lopes, Mario Abbud-Filho, and Emerson Quintino de Lima. 2022. "Impact of an Electronic Alert in Combination with a Care Bundle on the Outcomes of Acute Kidney Injury" Diagnostics 12, no. 12: 3121. https://doi.org/10.3390/diagnostics12123121
APA StyleTome, A. C. N., Ramalho, R. J., dos Santos, K. F., Ponte, B., Agostinho, H., Machado, M. N., Lopes, M. B., Abbud-Filho, M., & de Lima, E. Q. (2022). Impact of an Electronic Alert in Combination with a Care Bundle on the Outcomes of Acute Kidney Injury. Diagnostics, 12(12), 3121. https://doi.org/10.3390/diagnostics12123121







