Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors
Abstract
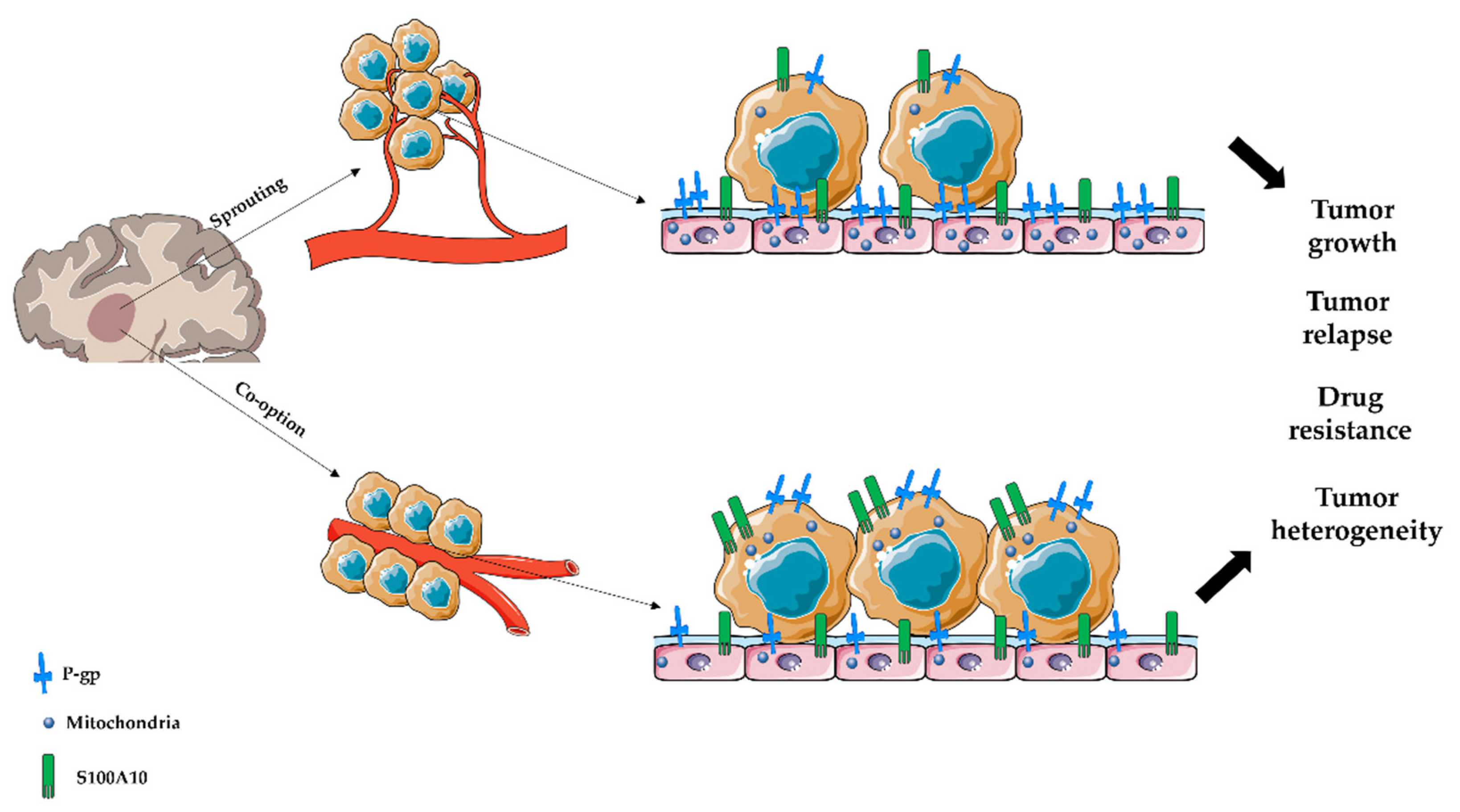
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethical Approval
2.2. Double-Labeling Immunohistochemistry
2.3. Identification and Quantification of Vasculatures
2.4. Statistical Analysis
3. Results
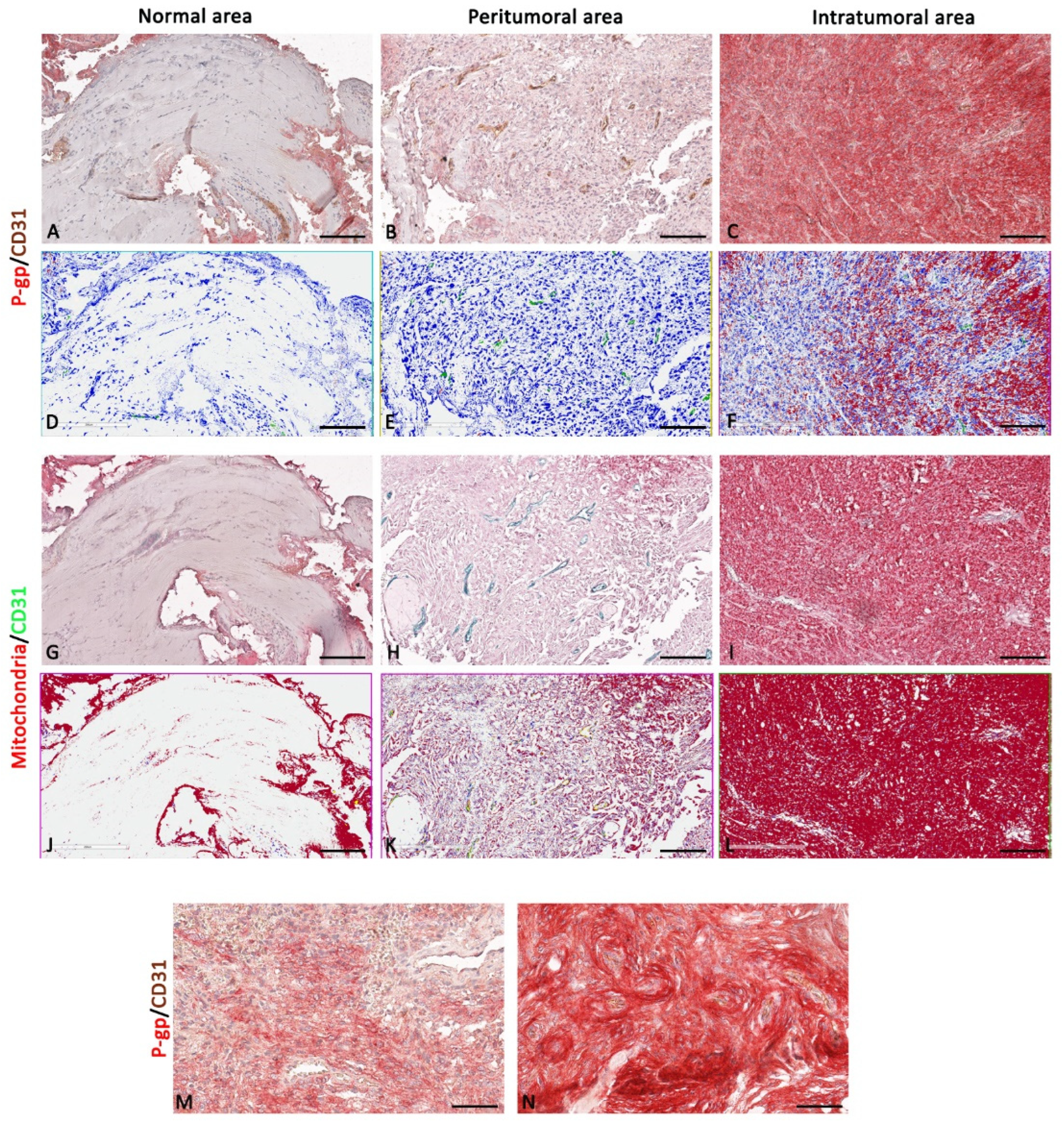
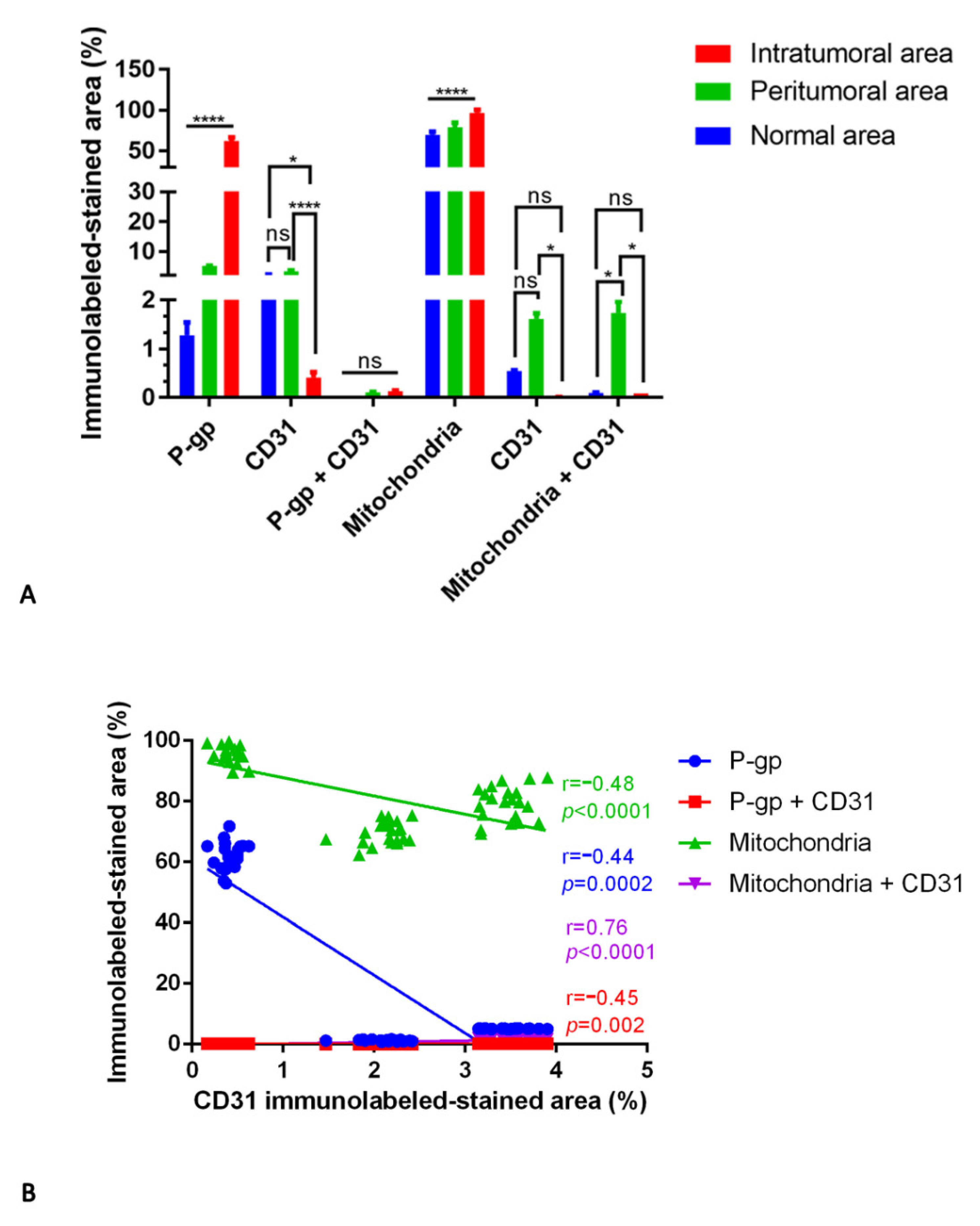
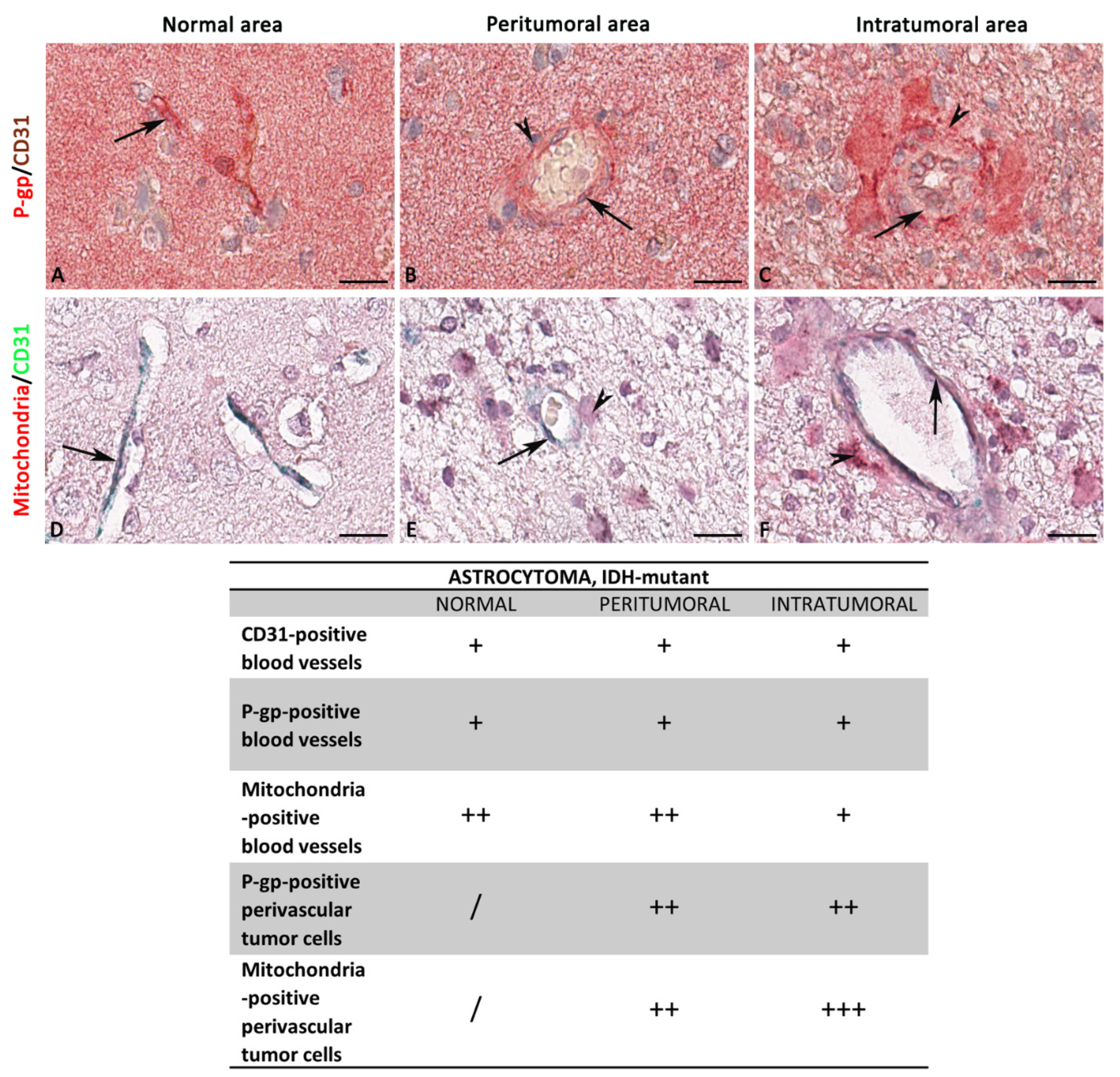
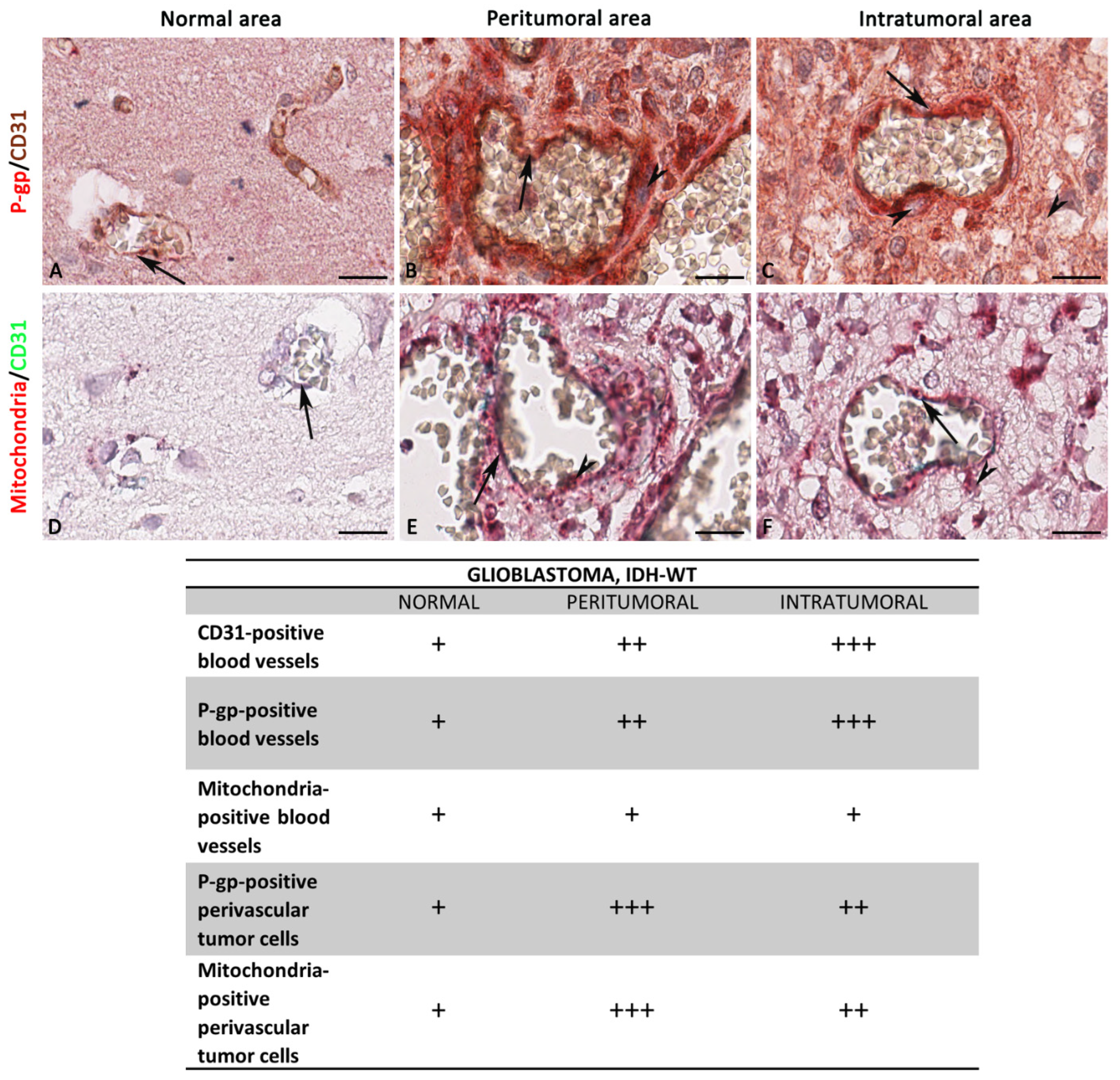
3.1. P-gp/CD31 and Mitochondria/CD31 Expression and Colocalization in Central Nervous System Tumors
Summary Results
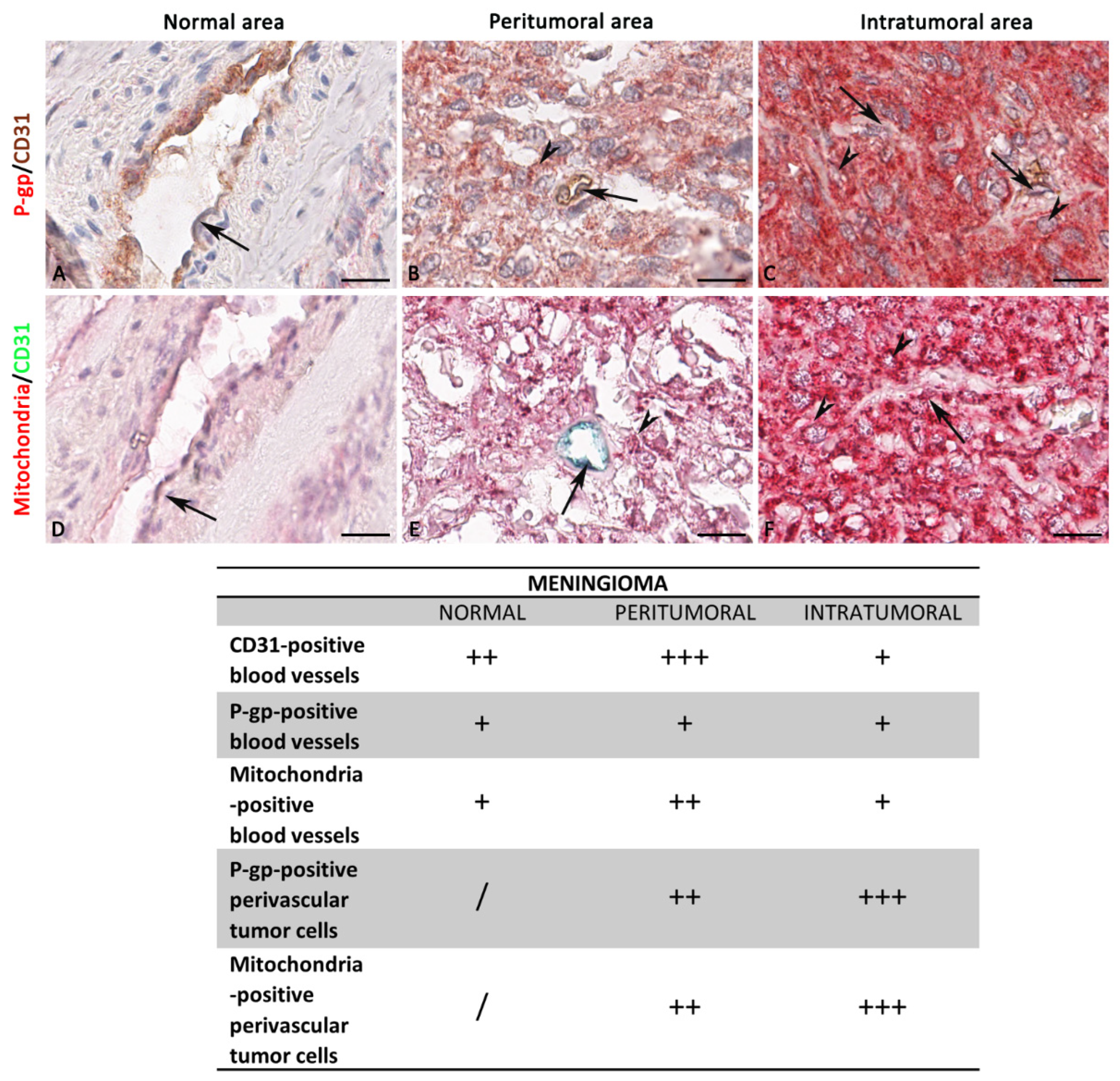
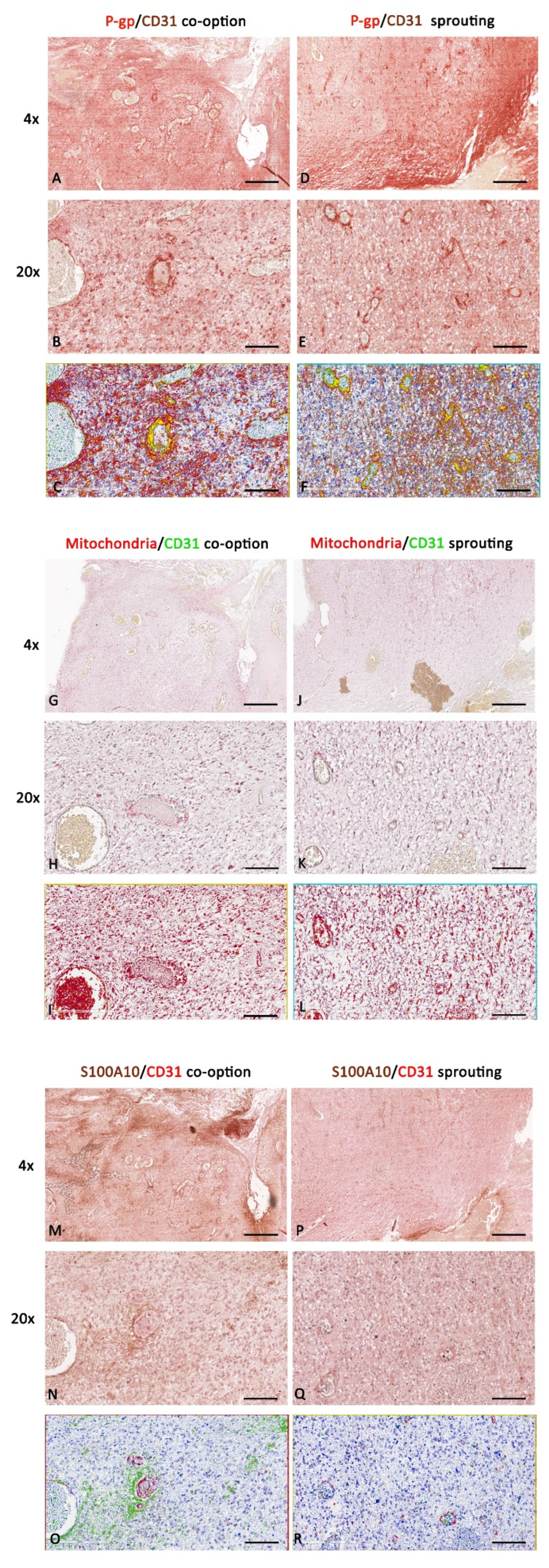
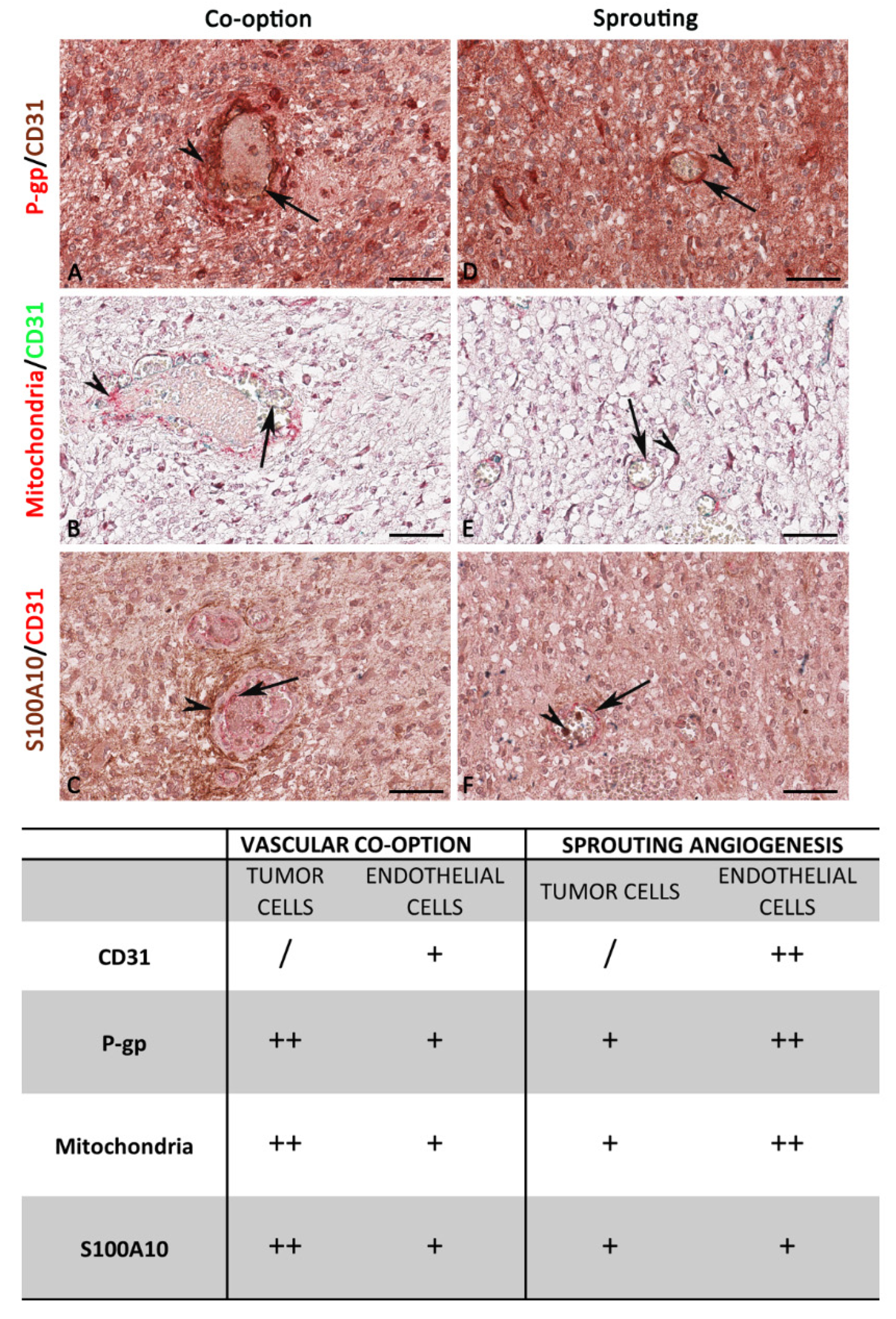
3.2. P-gp/CD31, Mitochondria/CD31, and S100A10/CD31 Expression and Colocalization in Glioblastoma
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grochans, S.; Cybulska, A.M.; Siminska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Angara, K.; Rashid, M.H.; Shankar, A.; Ara, R.; Iskander, A.; Borin, T.F.; Jain, M.; Achyut, B.R.; Arbab, A.S. Vascular mimicry in glioblastoma following anti-angiogenic and anti-20-HETE therapies. Histol. Histopathol. 2017, 32, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, L.M.; Bruns, C.J.; Schmidt, T. Resistance Mechanisms of the Metastatic Tumor Microenvironment to Anti-Angiogenic Therapy. Front. Oncol. 2022, 12, 897927. [Google Scholar] [CrossRef]
- Ribatti, D.; Pezzella, F. Vascular Co-Option and Other Alternative Modalities of Growth of Tumor Vasculature in Glioblastoma. Front. Oncol. 2022, 12, 1221. [Google Scholar] [CrossRef]
- Hardee, M.E.; Zagzag, D. Mechanisms of glioma-associated neovascularization. Am. J. Pathol. 2012, 181, 1126–1141. [Google Scholar] [CrossRef] [Green Version]
- Seano, G.; Jain, R.K. Vessel co-option in glioblastoma: Emerging insights and opportunities. Angiogenesis 2020, 23, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Zagzag, D.; Amirnovin, R.; Greco, M.A.; Yee, H.; Holash, J.; Wiegand, S.J.; Zabski, S.; Yancopoulos, G.D.; Grumet, M. Vascular apoptosis and involution in gliomas precede neovascularization: A novel concept for glioma growth and angiogenesis. Lab. Investig. 2000, 80, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Raman, V.S.; Sharma, A.; Agarwala, S.; Bakshi, S.; Bhatnagar, V. Intussusception following treatment for glioblastoma multiforme: A rare association. J. Indian Assoc. Pediatr. Surg. 2014, 19, 246. [Google Scholar] [CrossRef]
- Huizer, K.; Sacchetti, A.; Dik, W.A.; Mustafa, D.A.; Kros, J.M. Circulating Proangiogenic Cells and Proteins in Patients with Glioma and Acute Myocardial Infarction: Differences in Neovascularization between Neoplasia and Tissue Regeneration. J. Oncol. 2019, 2019, 3560830. [Google Scholar] [CrossRef]
- Moschetta, M.; Mishima, Y.; Sahin, I.; Manier, S.; Glavey, S.; Vacca, A.; Roccaro, A.M.; Ghobrial, I.M. Role of endothelial progenitor cells in cancer progression. Biochim. Biophys. Acta 2014, 1846, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Hata, N.; Mizoguchi, M.; Yokokawa, R.; Kawamura, Y.; Hatae, R.; Sangatsuda, Y.; Kuga, D.; Fujioka, Y.; Takigawa, K.; et al. Mesenchymal glioblastoma-induced mature de-novo vessel formation of vascular endothelial cells in a microfluidic device. Mol. Biol. Rep. 2021, 48, 395–403. [Google Scholar] [CrossRef]
- Francescone, R.; Scully, S.; Bentley, B.; Yan, W.; Taylor, S.L.; Oh, D.; Moral, L.; Shao, R. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J. Biol. Chem. 2012, 287, 24821–24831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Chen, Y.S.; Chen, F.R.; Xi, S.Y.; Chen, Z.P. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 2017, 19, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Pezzella, F.; Ribatti, D. Vascular co-option and vasculogenic mimicry mediate resistance to anti-angiogenic strategies. Cancer Rep. 2020, 9, e1318. [Google Scholar] [CrossRef]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med. 2013, 2, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gomez, P.; Valiente, M. Vascular co-option in brain metastasis. Angiogenesis 2020, 23, 3–8. [Google Scholar] [CrossRef]
- Winkler, F.; Kienast, Y.; Fuhrmann, M.; Von Baumgarten, L.; Burgold, S.; Mitteregger, G.; Kretzschmar, H.; Herms, J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 2009, 57, 1306–1315. [Google Scholar] [CrossRef]
- Passalidou, E.; Trivella, M.; Singh, N.; Ferguson, M.; Hu, J.; Cesario, A.; Granone, P.; Nicholson, A.G.; Goldstraw, P.; Ratcliffe, C.; et al. Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br. J. Cancer 2002, 86, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronca, R.; Benkheil, M.; Mitola, S.; Struyf, S.; Liekens, S. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev. 2017, 37, 1231–1274. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Cardo, C.; O’Brien, J.P.; Boccia, J.; Casals, D.; Bertino, J.R.; Melamed, M.R. Expression of the multi-drug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1990, 38, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- van Helvoort, A.; Smith, A.J.; Sprong, H.; Fritzsche, I.; Schinkel, A.H.; Borst, P.; van Meer, G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 1996, 87, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Chen, C.J.; Kriegler, M.; Roninson, I.B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell 1988, 53, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Duran, G.E.; Steger, K.A.; Lacayo, N.J.; Jaffrezou, J.P.; Dumontet, C.; Sikic, B.I. Multidrug-resistant human sarcoma cells with a mutant P-glycoprotein, altered phenotype, and resistance to cyclosporins. J. Biol. Chem. 1997, 272, 5974–5982. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as Phospholipid Flippases-Structure, Function, and Enigmas. Front. Physiol. 2016, 7, 275. [Google Scholar] [CrossRef] [Green Version]
- de Trizio, I.; Errede, M.; d’Amati, A.; Girolamo, F.; Virgintino, D. Expression of P-gp in Glioblastoma: What we can Learn from Brain Development. Curr. Pharm. Des. 2020, 26, 1428–1437. [Google Scholar] [CrossRef]
- Karssen, A.M.; Meijer, O.; Pons, D.; De Kloet, E.R. Localization of mRNA expression of P-glycoprotein at the blood-brain barrier and in the hippocampus. Ann. N. Y. Acad. Sci. 2004, 1032, 308–311. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Sauna, Z.E.; Gottesman, M.M.; Szakacs, G. A novel way to spread drug resistance in tumor cells: Functional intercellular transfer of P-glycoprotein (ABCB1). Trends Pharmacol. Sci. 2005, 26, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Levchenko, A.; Mehta, B.M.; Niu, X.; Kang, G.; Villafania, L.; Way, D.; Polycarpe, D.; Sadelain, M.; Larson, S.M. Intercellular transfer of P-glycoprotein mediates acquired multi-drug resistance in tumor cells. Proc. Natl. Acad. Sci. USA 2005, 102, 1933–1938. [Google Scholar] [CrossRef]
- Virgintino, D.; Errede, M.; Girolamo, F.; Capobianco, C.; Robertson, D.; Vimercati, A.; Serio, G.; Di Benedetto, A.; Yonekawa, Y.; Frei, K.; et al. Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J. Neuropathol. Exp. Neurol. 2008, 67, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Song, J.; Wang, Z.; Kan, J.; Ge, Y.; Wang, D.; Zhang, R.; Zhang, W.; Liu, Y. A Novel S100 Family-Based Signature Associated with Prognosis and Immune Microenvironment in Glioma. J. Oncol. 2021, 2021, 3586589. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Haeich, J.; Aulestia, F.J.; Kilhoffer, M.C.; Miller, A.L.; Neant, I.; Webb, S.E.; Schaeffer, E.; Junier, M.P.; Chneiweiss, H.; et al. Calcium signaling orchestrates glioblastoma development: Facts and conjunctures. Biochim. Biophys. Acta 2016, 1863, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Myrvang, H.K.; Guo, X.; Li, C.; Dekker, L.V. Protein interactions between surface annexin A2 and S100A10 mediate adhesion of breast cancer cells to microvascular endothelial cells. FEBS Lett. 2013, 587, 3210–3215. [Google Scholar] [CrossRef] [Green Version]
- Chedeville, A.L.; Lourdusamy, A.; Monteiro, A.R.; Hill, R.; Madureira, P.A. Investigating Glioblastoma Response to Hypoxia. Biomedicines 2020, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Madureira, P.A.; O’Connell, P.A.; Surette, A.P.; Miller, V.A.; Waisman, D.M. The biochemistry and regulation of S100A10: A multifunctional plasminogen receptor involved in oncogenesis. J. Biomed. Biotechnol. 2012, 2012, 353687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annese, T.; Errede, M.; De Giorgis, M.; Lorusso, L.; Tamma, R.; Ribatti, D. Double Immunohistochemical Staining on Formalin-Fixed Paraffin-Embedded Tissue Samples to Study Vascular Co-option. Methods Mol. Biol. 2023, 2572, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Tumours, W.C.o. WHO Classification of Tumours Editorial Board. Central Nervous System Tumours. Lyon (France): International Agency for Research on Cancer. In WHO Classification of Tu-Mours Series, 5th ed.; 2021; Volume 6, Available online: https://publications.iarc.fr/601 (accessed on 30 May 2021).
- Barresi, V. Angiogenesis in meningiomas. Brain Tumor Pathol. 2011, 28, 99–106. [Google Scholar] [CrossRef]
- Dijk, S.N.; Protasoni, M.; Elpidorou, M.; Kroon, A.M.; Taanman, J.W. Mitochondria as target to inhibit proliferation and induce apoptosis of cancer cells: The effects of doxycycline and gemcitabine. Sci. Rep. 2020, 10, 4363. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, F.; Notopoulos, A. S100 protein family and its application in clinical practice. Hippokratia 2008, 12, 198–204. [Google Scholar] [PubMed]
- Musumeci, G.; Castorina, A.; Magro, G.; Cardile, V.; Castorina, S.; Ribatti, D. Enhanced expression of CD31/platelet endothelial cell adhesion molecule 1 (PECAM1) correlates with hypoxia inducible factor-1 alpha (HIF-1alpha) in human glioblastoma multiforme. Exp. Cell Res. 2015, 339, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Lazaris, A.; Kapelanski-Lamoureux, A.; Mayer, T.Z.; Metrakos, P. Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: A theoretical model. Semin. Cancer Biol. 2021, 71, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel co-option, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosinska, S.; Gavard, J. Tumor Vessels Fuel the Fire in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6514. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [Green Version]
- Nico, B.; Mangieri, D.; Tamma, R.; Longo, V.; Annese, T.; Crivellato, E.; Pollo, B.; Maderna, E.; Ribatti, D.; Salmaggi, A. Aquaporin-4 contributes to the resolution of peritumoural brain oedema in human glioblastoma multiforme after combined chemotherapy and radiotherapy. Eur. J. Cancer 2009, 45, 3315–3325. [Google Scholar] [CrossRef]
- Ruggieri, S.; De Giorgis, M.; Annese, T.; Tamma, R.; Notarangelo, A.; Marzullo, A.; Senetta, R.; Cassoni, P.; Notarangelo, M.; Ribatti, D.; et al. Dp71 Expression in Human Glioblastoma. Int. J. Mol. Sci. 2019, 20, 5429. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K.; Tong, R.T.; Munn, L.L. Effect of vascular normalization by anti-angiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007, 67, 2729–2735. [Google Scholar] [CrossRef] [Green Version]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 2008, 4, 241–246. [Google Scholar] [CrossRef]
- Djonov, V.; Baum, O.; Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003, 314, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Gonzalez-Gomez, M.; Garcia, M.D.; Diaz-Flores, L., Jr.; Gonzalez-Marrero, I.; Avila, J.; Martin-Vasallo, P. Disproportion in Pericyte/Endothelial Cell Proliferation and Mechanisms of Intussusceptive Angiogenesis Participate in Bizarre Vessel Formation in Glioblastoma. Cells 2021, 10, 2625. [Google Scholar] [CrossRef] [PubMed]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ciarrocchi, A.; Mellick, A.S.; Jaggi, J.S.; Bambino, K.; Gupta, S.; Heikamp, E.; McDevitt, M.R.; Scheinberg, D.A.; Benezra, R.; et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007, 21, 1546–1558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.H.; Xiang, P.; Wang, H.Y.; Lu, Y.Y.; Luo, Y.L.; Jiang, H. The characteristics of bone marrow-derived endothelial progenitor cells and their effect on glioma. Cancer Cell Int. 2012, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, L.; Wang, Q.; Wang, L.; Wang, H.; Shen, Y.; Li, X.; Fu, Y.; Shen, Y.; Yu, Y. Circulating endothelial progenitor cells are involved in VEGFR-2-related endothelial differentiation in glioma. Oncol. Rep. 2014, 32, 2007–2014. [Google Scholar] [CrossRef] [Green Version]
- De Palma, M.; Murdoch, C.; Venneri, M.A.; Naldini, L.; Lewis, C.E. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007, 28, 519–524. [Google Scholar] [CrossRef]
- Dunleavey, J.M.; Dudley, A.C. Vascular Mimicry: Concepts and Implications for Anti-Angiogenic Therapy. Curr. Angiogenes. 2012, 1, 133–138. [Google Scholar] [CrossRef]
- El Hallani, S.; Boisselier, B.; Peglion, F.; Rousseau, A.; Colin, C.; Idbaih, A.; Marie, Y.; Mokhtari, K.; Thomas, J.L.; Eichmann, A.; et al. A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain 2010, 133, 973–982. [Google Scholar] [CrossRef]
- Mao, J.-m.; Liu, J.; Guo, G.; Mao, X.-g.; Li, C.-x. Glioblastoma vasculogenic mimicry: Signaling pathways progression and potential anti-angiogenesis targets. Biomark. Res. 2015, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, W.Y.; Chen, Z.P. Does vasculogenic mimicry exist in astrocytoma? J. Histochem. Cytochem. Off. J. Histochem. Soc. 2005, 53, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Guelfi, S.; Duffau, H.; Bauchet, L.; Rothhut, B.; Hugnot, J.-P. Vascular Transdifferentiation in the CNS: A Focus on Neural and Glioblastoma Stem-Like Cells. Stem Cells Int. 2016, 2016, 2759403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.; Kim, Y.J.; Lee, S.H.; Kim, T.M.; Choi, S.H.; Kim, D.W.; Park, C.K.; Kim, I.H.; Kim, J.H.; Kim, E.; et al. Post-bevacizumab Clinical Outcomes and the Impact of Early Discontinuation of Bevacizumab in Patients with Recurrent Malignant Glioma. Cancer Res. Treat. 2017, 49, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, J.F.; Fuller, G.; Kumar, A.J.; Piao, Y.; Eterovic, K.; Ji, Y.; Conrad, C.A. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010, 12, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Du, X.; Wu, H.; Liu, H.; Xie, T.; Tong, H.; Chen, X.; Guo, Y.; Zhang, W. Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment. Sci. Rep. 2017, 7, 13894. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. Anti-angiogenic Cancer Therapy: Development of Resistance. In Tumor Angiogenesis: A Key Target for Cancer Therapy; Springer: Berlin, Germany, 2019; pp. 313–323. [Google Scholar]
- Huang, M.; Lin, Y.; Wang, C.; Deng, L.; Chen, M.; Assaraf, Y.G.; Chen, Z.-S.; Ye, W.; Zhang, D. New insights into anti-angiogenic therapy resistance in cancer: Mechanisms and therapeutic aspects. Drug Resist. Updat. 2022, 64, 100849. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, L.A.; De Rooij, L.; Cuypers, A.; Rohlenova, K.; Dumas, S.J.; Garcia-Caballero, M.; Meta, E.; Amersfoort, J.; Taverna, F.; Becker, L.M.; et al. Tumor vessel co-option probed by single-cell analysis. Cell Rep. 2021, 35, 109253. [Google Scholar] [CrossRef]
- Rodriguez-Enriquez, S.; Vital-Gonzalez, P.A.; Flores-Rodriguez, F.L.; Marin-Hernandez, A.; Ruiz-Azuara, L.; Moreno-Sanchez, R. Control of cellular proliferation by modulation of oxidative phosphorylation in human and rodent fast-growing tumor cells. Toxicol. Appl. Pharmacol. 2006, 215, 208–217. [Google Scholar] [CrossRef]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013, 9, 712. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | IDH-WT Glioblastoma (n = 30) | IDH-Mutant Astrocytoma (n = 25) | Meningioma (n = 22) |
|---|---|---|---|
| Gender | |||
| Male | 18 (60%) | 15 (60%) | 6 (27.3%) |
| Female | 12 (40%) | 10 (40%) | 16 (72.7%) |
| Age group | |||
| <50 | 0 | 22 (88%) | 5 (22.7%) |
| 50–70 | 16 (53.3%) | 3 (12%) | 14 (63.7%) |
| >70 | 14 (46.7%) | 0 | 3 (13.6%) |
| Tumor location | |||
| Frontal lobe | 13 (43.4%) | 9 (36%) | 5 (22.7%) |
| Parietal lobe | 10 (33.3%) | 11 (44%) | 7 (31.8%) |
| Temporal lobe | 6 (20%) | 4 (16%) | 4 (18.2%) |
| Occipital lobe | 1 (3.3%) | 1 (4%) | 0 |
| Cerebellum | 0 | 0 | 0 |
| Brainstem | 0 | 0 | 0 |
| Spinal cord | 0 | 0 | 6 (27.3%) |
| Grading | |||
| 1 | / | 0 | 22 (100%) |
| 2 | / | 21 (84%) | 0 |
| 3 | / | 4 (16%) | 0 |
| 4 | 30 (100%) | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annese, T.; Errede, M.; d’Amati, A.; De Giorgis, M.; Lorusso, L.; Tamma, R.; Ribatti, D. Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors. Diagnostics 2022, 12, 3120. https://doi.org/10.3390/diagnostics12123120
Annese T, Errede M, d’Amati A, De Giorgis M, Lorusso L, Tamma R, Ribatti D. Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors. Diagnostics. 2022; 12(12):3120. https://doi.org/10.3390/diagnostics12123120
Chicago/Turabian StyleAnnese, Tiziana, Mariella Errede, Antonio d’Amati, Michelina De Giorgis, Loredana Lorusso, Roberto Tamma, and Domenico Ribatti. 2022. "Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors" Diagnostics 12, no. 12: 3120. https://doi.org/10.3390/diagnostics12123120
APA StyleAnnese, T., Errede, M., d’Amati, A., De Giorgis, M., Lorusso, L., Tamma, R., & Ribatti, D. (2022). Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors. Diagnostics, 12(12), 3120. https://doi.org/10.3390/diagnostics12123120









