Urinary CD80 and Serum suPAR as Biomarkers of Glomerular Disease among Adults in Brazil
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Baseline Patient Data
2.4. Baseline Control Data
2.5. Data Assessed at Six Months after Renal Biopsy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalhoub, R.J. Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 1974, 304, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Cara-Fuentes, G.; Clapp, W.L.; Johnson, R.J.; Garin, E.H. Pathogenesis of proteinuria in idiopathic minimal change disease: Molecular mechanisms. Pediatr. Nephrol. 2016, 31, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Shimada, M.; Araya, C.E.; Huskey, J.; Garin, E.H.; Johnson, R.J. Minimal Change Disease: A CD80 podocytopathy? Semin. Nephrol. 2011, 31, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 Family Revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.H.; Diaz, L.N.; Mu, W.; Wasserfall, C.; Araya, C.; Segal, M.; Johnson, R.J. Urinary CD80 Excretion Increases in Idiopathic Minima-Change Disease. J. Am. Soc. Nephrol. 2009, 20, 260–266. [Google Scholar] [CrossRef]
- Garin, E.H.; Mu, W.; Arthur, J.M.; Rivard, C.J.; Araya, C.E.; Shimada, M.; Johnson, R.J. Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010, 78, 296–302. [Google Scholar] [CrossRef]
- Reiser, J.; von Gersdorff, G.; Loos, M.; Oh, J.; Asanuma, K.; Giardino, L.; Rastaldi, M.P.; Calvaresi, N.; Watanabe, H.; Schwarz, K.; et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J. Clin. Investig. 2004, 113, 1390–1397. [Google Scholar] [CrossRef]
- Hoyer, J.R.; Raij, L.; Vernier, R.L.; Simmons, R.L.; Najarian, J.S.; Michael, A.F. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet 1972, 300, 343–348. [Google Scholar] [CrossRef]
- Vinai, M.; Waber, P.; Seikaly, M.G. Recurrence of focal segmental glomerulosclerosis in renal allograft: An in-depth review. Pediatr. Transplant. 2010, 14, 314–325. [Google Scholar] [CrossRef]
- Gohh, R.Y.; Yango, A.F.; Morrissey, P.E.; Monaco, A.P.; Gautam, A.; Sharma, M.; McCarthy, E.T.; Savin, V.J. Preemptive Plasmapheresis and Recurrence of FSGS in High-Risk Renal Transplant Recipients. Am. J. Transplant. 2005, 5, 2907–2912. [Google Scholar] [CrossRef]
- Kronbichler, A.; Saleem, M.A.; Meijers, B.; Shin, J.I. Soluble Urokinase Receptors in Focal Segmental Glomerulosclerosis: A Review on the Scientific Point of View. J. Immunol. Res. 2016, 2016, 2068691. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; El Hindi, S.; Li, J.; Fornoni, A.; Goes, N.; Sageshima, J.; Maiguel, D.; Karumanchi, S.A.; Yap, H.-K.; Saleem, M.; et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 2011, 17, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Trachtman, H.; Li, J.; Dong, C.; Friedman, A.L.; Gassman, J.J.; McMahan, J.L.; Radeva, M.; Heil, K.M.; Trautmann, A.; et al. Circulating suPAR in two cohorts of primary FSGS. J. Am. Soc. Nephrol. 2012, 23, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cara-Fuentes, G.; Wasserfall, C.H.; Wang, H.; Johnson, R.J.; Garin, E.H. Minimal change disease: A dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr. Nephrol. 2014, 29, 2333–2340. [Google Scholar] [CrossRef][Green Version]
- Liao, J.; Wu, X.-C.; Cheng, Q.; Li, C.-L.; Yi, Z.-W.; Cao, Y.; Shuai, L.-J. Predictability of Urinary CD80 in the Relapse of Primary Nephrotic Syndrome. BioMed Res. Int. 2017, 2017, 9429314. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Kumar, R.; Narayan, G.; Srivastava, P.; Abhinay, A.; Prasad, R.; Singh, A.; Batra, V.V. Toll-like receptor 3 (TLR-3), TLR-4 and CD80 expression in peripheral blood mononuclear cells and urinary CD80 levels in children with idiopathic nephrotic syndrome. Pediatr. Nephrol. 2017, 32, 1355–1361. [Google Scholar] [CrossRef]
- Guerrico, A.M.G.; Lieske, J.; Klee, G.; Kumar, S.; Lopez-Baez, V.; Wright, A.M.; Bobart, S.; Shevell, D.; Maldonado, M.; Troost, J.P.; et al. Urinary CD80 Discriminates Among Glomerular Disease Types and Reflects Disease Activity. Kidney Int. Rep. 2020, 5, 2021–2031. [Google Scholar] [CrossRef]
- Fiorina, P.; Vergani, A.; Bassi, R.; Niewczas, M.A.; Altintas, M.M.; Pezzolesi, M.G.; D’Addio, F.; Chin, M.; Tezza, S.; Ben Nasr, M.; et al. Role of Podocyte B7-1 in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1415–1429. [Google Scholar] [CrossRef]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Costales-Collaguazo, C.; Politei, J.; Stern, A.; Paulero, M.; Rengel, T.; Andrews, J.; Forrester, M.; et al. Increased urinary CD80 excretion and podocyturia in Fabry disease. J. Transl. Med. 2016, 14, 289. [Google Scholar] [CrossRef]
- Novelli, R.; Gagliardini, E.; Ruggiero, B.; Benigni, A.; Remuzzi, G. Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am. J. Physiol. Renal. Physiol. 2016, 310, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, S.; Nozu, K.; Maeta, S.; Yamamura, T.; Nakanishi, K.; Fujimura, J.; Horinouchi, T.; Nagano, C.; Sakakibara, N.; Nagase, H.; et al. The utility of urinary CD80 as a diagnostic marker in patients with renal diseases. Sci. Rep. 2018, 8, 17322. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.; Vinogradov, A.; Cao, V.; Gindis, A.; Berns, A.; Alentov, I.; Sergeeva, N. Serum levels of plasminogen activator urokinase receptor and cardiotrophin-like cytokine factor 1 in patients with nephrotic syndrome. Clin. Nephrol. 2022, 97, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Yu, L.; Huang, J.; Wang, S.; Zou, W.; Yang, L.; Liu, G. Soluble Urokinase Receptor Levels in Secondary Focal Segmental Glomerulosclerosis. Kidney Dis. 2019, 5, 239–246. [Google Scholar] [CrossRef]
- Meijers, B.; Maas, R.J.; Sprangers, B.; Claes, K.; Poesen, R.; Bammens, B.; Naesens, M.; Deegens, J.K.; Dietrich, R.; Storr, M.; et al. The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int. 2014, 85, 636–640. [Google Scholar] [CrossRef]
- Harel, E.; Shoji, J.; Abraham, V.; Miller, L.; Laszik, Z.; Thurison, T.; King, A.; Olshen, A.; Leung, J.; Szabo, G.; et al. Identifying a potential biomarker for primary focal segmental glomerulosclerosis and its association with recurrence after transplantation. Clin. Transplant. 2019, 33, e13487. [Google Scholar] [CrossRef]
- Ling, C.; Liu, X.; Shen, Y.; Chen, Z.; Fan, J.; Jiang, Y.; Meng, Q. Urinary CD80 excretion is a predictor of good outcome in children with primary nephrotic syndrome. Pediatr. Nephrol. 2018, 33, 1183–1187. [Google Scholar] [CrossRef]
- Backes, Y.; Van Der Sluijs, K.F.; Mackie, D.P.; Tacke, F.; Koch, A.; Tenhunen, J.J.; Schultz, M.J. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med. 2012, 38, 1418–1428. [Google Scholar] [CrossRef]
| Characteristic | MCD | FSGS | Membranous Nephropathy | Control | p Value |
|---|---|---|---|---|---|
| (n = 5) | (n = 18) | (n = 14) | (n = 10) | ||
| Age (years), mean ± SD | 37.60 ± 15 | 33.67 ± 13.60 | 42.15 ± 19.64 | 33.60 ± 6.63 | 0.63 |
| Male sex, n (%) | 1 (20.0) | 10 (55.6) | 6 (42.9) | 3 (30.0) | <0.0001 |
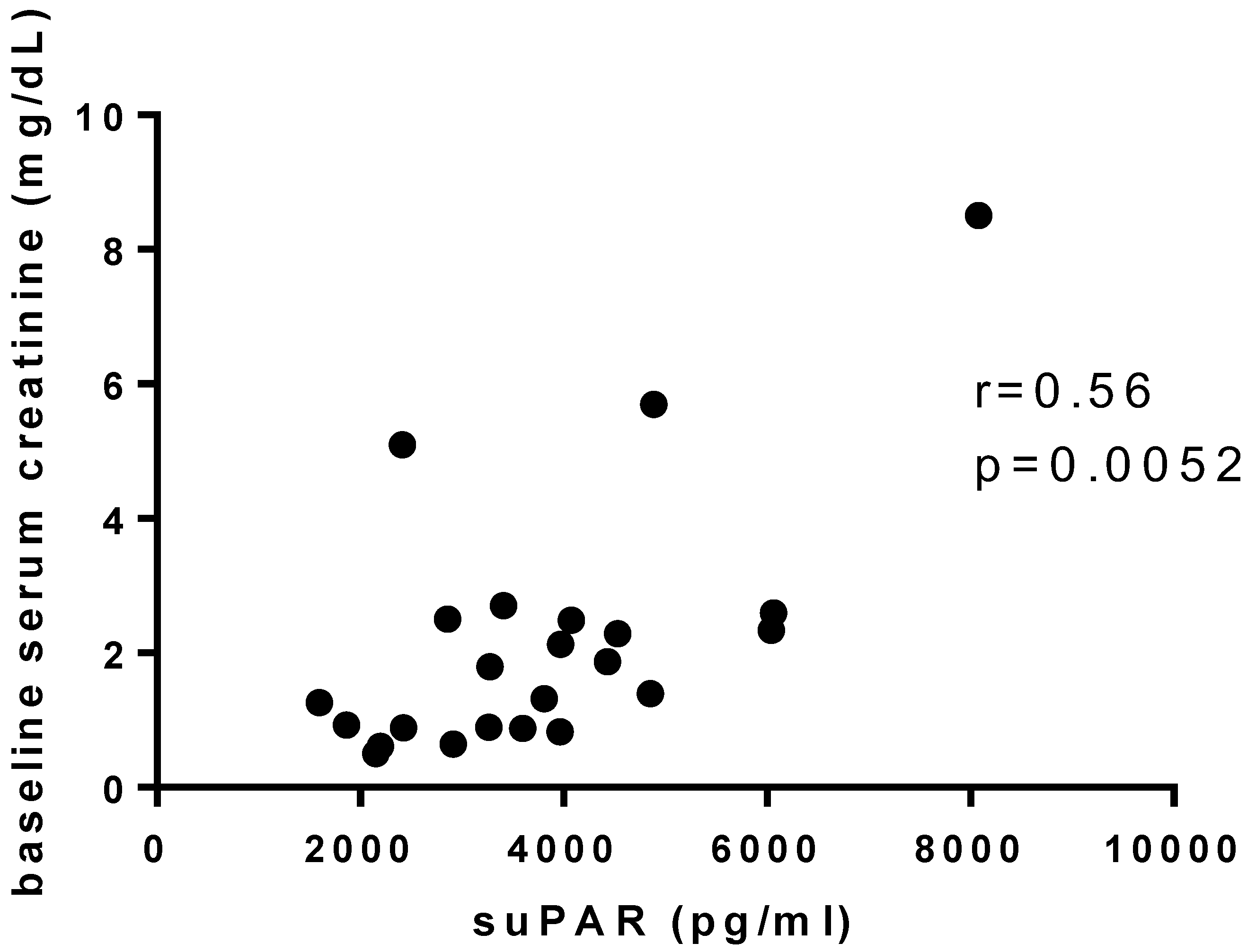
| Serum creatinine (mg/dL), median (IQR) | 0.90 (0.76–1.95) | 2 (0.92–2.62) | 0.65 (0.50–1.17) | 1.11 (0.98–1.18) | 0.086 |
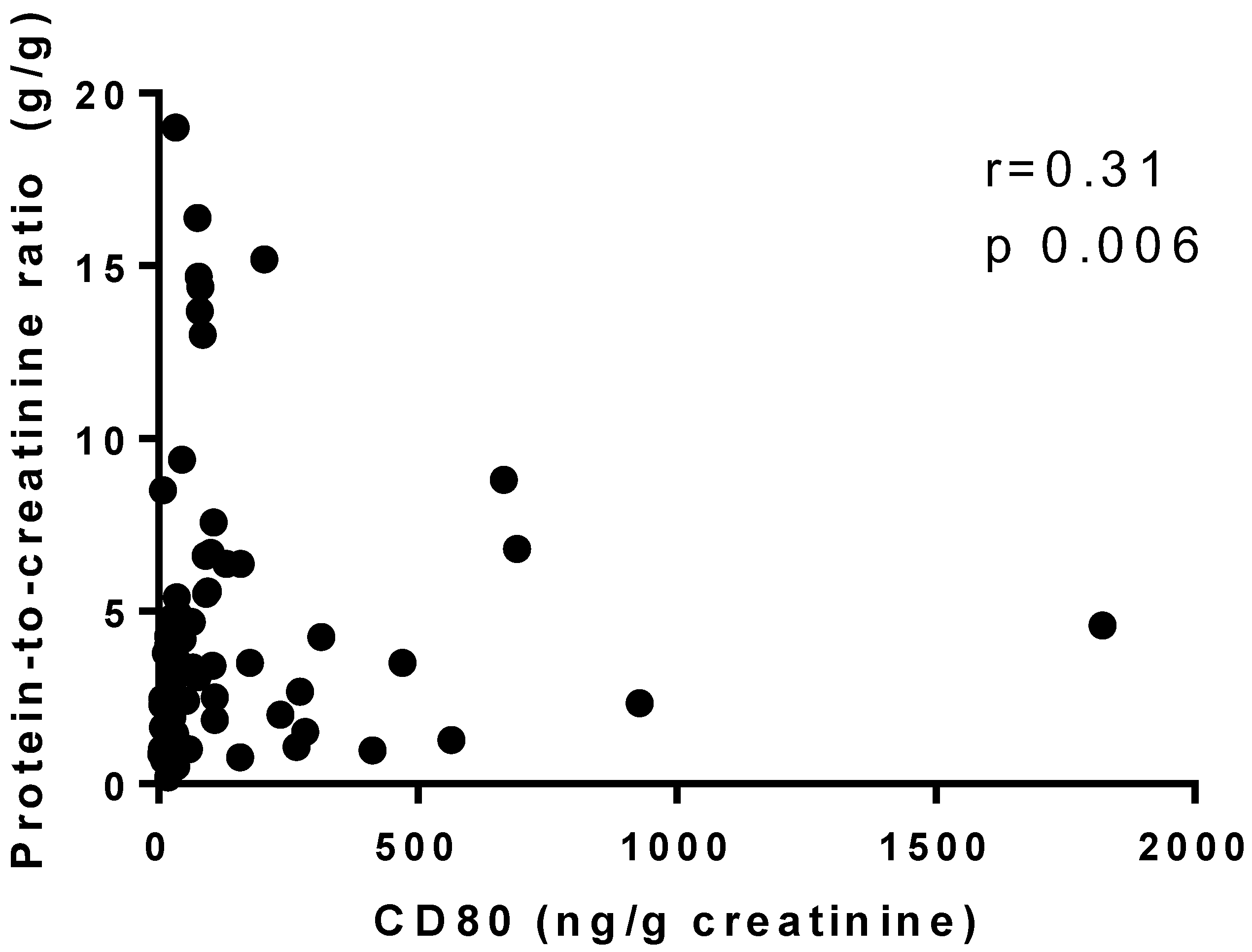
| Protein-to-creatinine ratio (g/g), median (IQR) | 3.10 (1.04–3.46) | 4.05 (1.87–5.44) | 4.42 (2.38–7.14) | 0.003 (0–0.22) | <0.0001 |
| Serum albumin (g/dL), mean ± SD | 2.08 ± 0.99 | 2.06 ± 0.87 | 2.25 ± 0.54 | - | 0.10 |
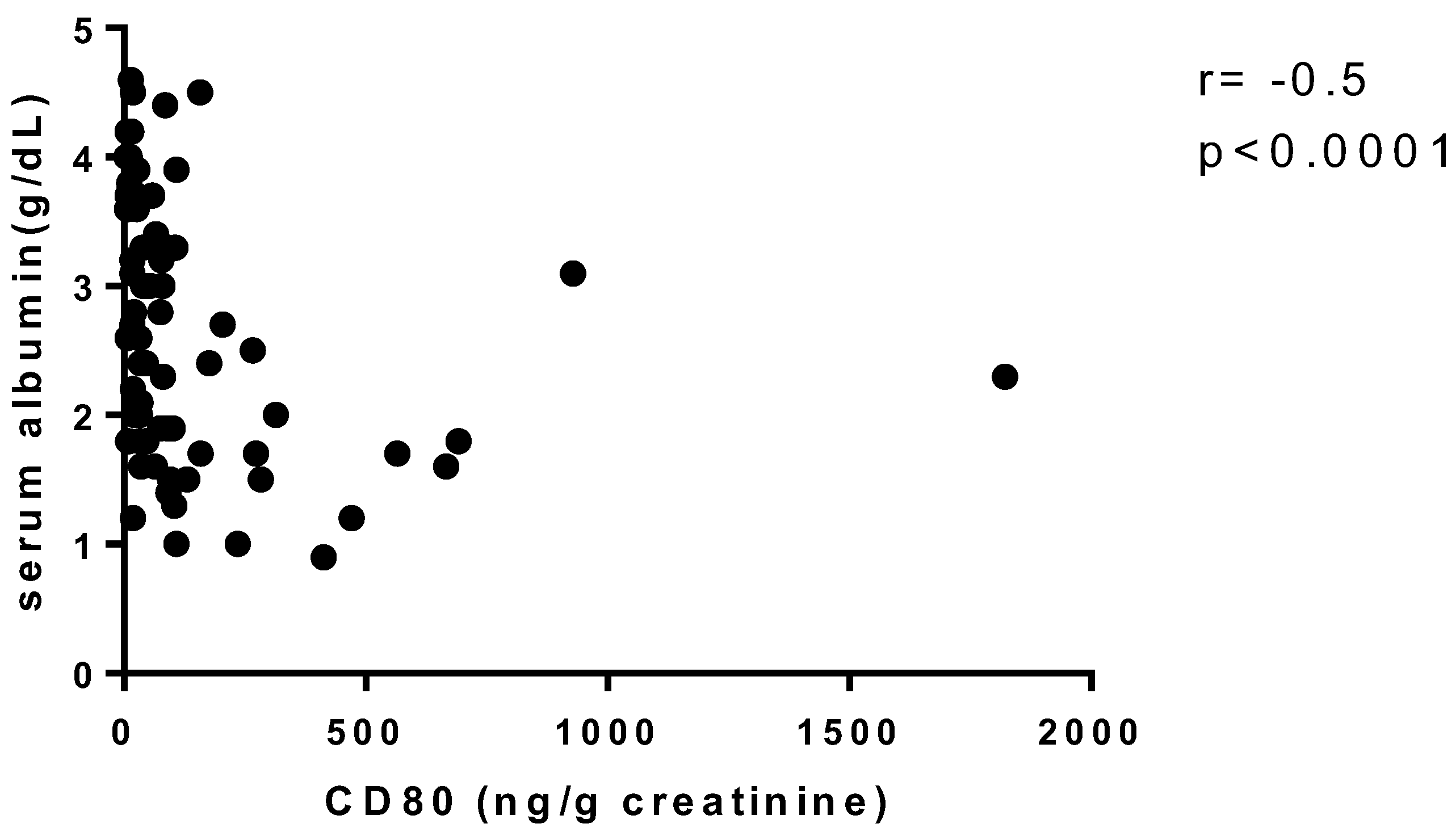
| CD80 (ng/g creatinine), median (IQR) | 104 (19.70–369.60) | 63.15 (30.50–244.60) | 76.80 (31.22–402.20) | 24.70 (15.10–41.40) | 0.048 ‡ |
| suPAR (pg/mL), median (IQR) | 3266 (2887–4225) | 3887 (2359–4620) | 3091 (2018–3711) | 1336 (1033–1586) | 0.0001 * |
| Characteristic | MCD + FSGS | Membrane Nephropathy | Other Glomerulopathies | Control | p Value |
|---|---|---|---|---|---|
| (n = 23) | (n = 14) | (n = 33) | (n = 10) | ||
| Age (years), mean ± SD | 34.52 ± 13.65 | 42.15 ± 19.64 | 41.04 ± 15.68 | 33.60 ± 6.63 | 0.24 |
| Male sex, n (%) | 11 (47.8) | 6 (42.9) | 8 (28.6) | 3 (30.0) | 0.49 |
| Serum creatinine (mg/dL), median (IQR) | 1.80 (0.89–2.50) | 0.65 (0.50–1.17) | 1.39 (0.72–2.10) | 1.11 (0.98–1.18) | 0.16 |
| Protein-to-creatinine ratio (g/g), median (IQR) | 3.50 (1.50–4.90) | 4.42 (2.38–7.14) | 2.11 (1.01–5.32) | 0.003 (0–0.22) | <0.0001 |
| CD80 (ng/g creatinine), median (IQR) | 80 (33–266.6) | 76.80 (31.22–402.20) | 27.15 (14.53–76.55) | 24.70 (15.10–41.40) | 0.005 * |
| suPAR (pg/mL), median (IQR) | 3597 (2424–4531) | 3091 (2018–3711) | 3148 (2431–4015) | 1336 (1033–1586) | <0.0001 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zen, R.d.C.; Dominguez, W.V.; Braga, I.; dos Reis, L.M.; Jorge, L.B.; Yu, L.; Woronik, V.; Dias, C.B. Urinary CD80 and Serum suPAR as Biomarkers of Glomerular Disease among Adults in Brazil. Diagnostics 2023, 13, 203. https://doi.org/10.3390/diagnostics13020203
Zen RdC, Dominguez WV, Braga I, dos Reis LM, Jorge LB, Yu L, Woronik V, Dias CB. Urinary CD80 and Serum suPAR as Biomarkers of Glomerular Disease among Adults in Brazil. Diagnostics. 2023; 13(2):203. https://doi.org/10.3390/diagnostics13020203
Chicago/Turabian StyleZen, Renata de Cássia, Wagner Vasques Dominguez, Ivone Braga, Luciene Machado dos Reis, Lectícia Barbosa Jorge, Luis Yu, Viktoria Woronik, and Cristiane Bitencourt Dias. 2023. "Urinary CD80 and Serum suPAR as Biomarkers of Glomerular Disease among Adults in Brazil" Diagnostics 13, no. 2: 203. https://doi.org/10.3390/diagnostics13020203
APA StyleZen, R. d. C., Dominguez, W. V., Braga, I., dos Reis, L. M., Jorge, L. B., Yu, L., Woronik, V., & Dias, C. B. (2023). Urinary CD80 and Serum suPAR as Biomarkers of Glomerular Disease among Adults in Brazil. Diagnostics, 13(2), 203. https://doi.org/10.3390/diagnostics13020203







