Diagnostic Value of Oral Provocation Tests in Drug Hypersensitivity Reactions Induced by Nonsteroidal Anti-Inflammatory Drugs and Paracetamol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Diagnostic Tests
2.3. Follow-Up
2.4. Identification of Excipients in Generic Drugs of Interest
2.5. Statistical Analysis
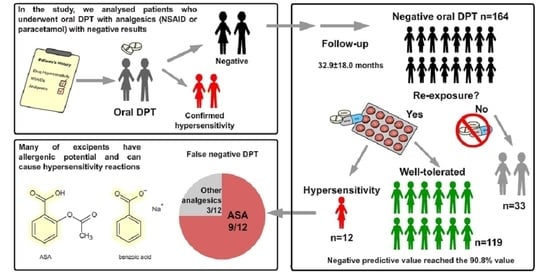
3. Results
3.1. General Characteristic of the Study Group
3.2. Patients with False Negative Drug Provocation Tests (Group A2)
3.3. Patients Who Avoided Reexposure (Group B)
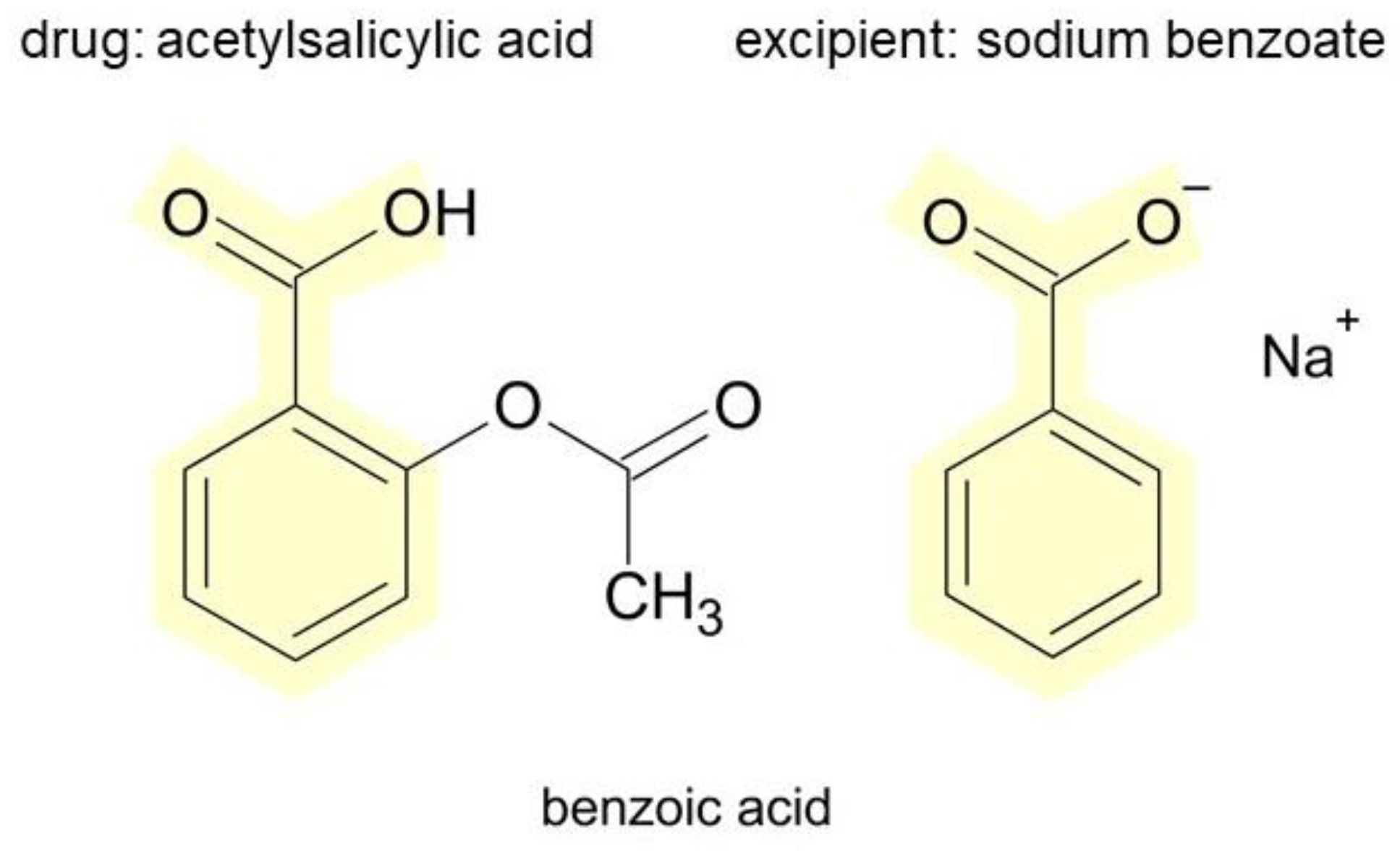
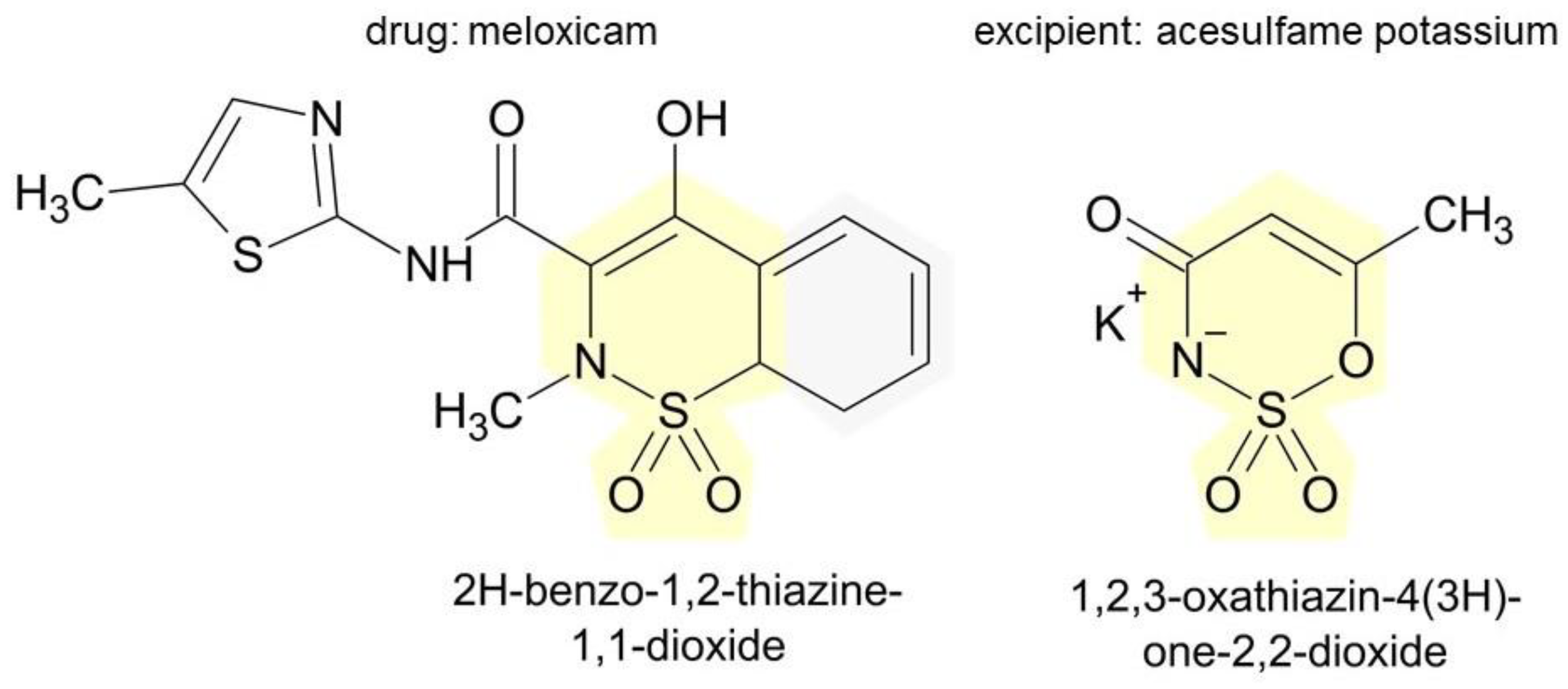
3.4. Excipients in Medicinal Products of Interest and Their Potential for Inducing Hypersensitivity Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.-S.; Pichler, W.; et al. International Consensus on Drug Allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Demoly, P.; Pichler, W.; Pirmohamed, M.; Romano, A. Important Questions in Allergy: 1-Drug Allergy/Hypersensitivity. Allergy 2008, 63, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Bousquet, J. Epidemiology of Drug Allergy. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 305–310. [Google Scholar] [CrossRef]
- Yeung, W.Y.W.; Park, H.S. Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity. Yonsei Med. J. 2020, 61, 4–14. [Google Scholar] [CrossRef]
- McGettigan, P.; Henry, D. Use of Non-Steroidal Anti-Inflammatory Drugs That Elevate Cardiovascular Risk: An Examination of Sales and Essential Medicines Lists in Low-, Middle-, and High-Income Countries. PLoS Med. 2013, 10, e1001388. [Google Scholar] [CrossRef]
- Blanca-Lopez, N.; Soriano, V.; Garcia-Martin, E.; Canto, G.; Blanca, M. NSAID-Induced Reactions: Classification, Prevalence, Impact, and Management Strategies. J. Asthma Allergy 2019, 12, 217–233. [Google Scholar] [CrossRef]
- Doña, I.; Blanca-López, N.; Torres, M.J.; García-Campos, J.; García-Núñez, I.; Gómez, F.; Salas, M.; Rondón, C.; Canto, M.G.; Blanca, M. Drug Hypersensitivity Reactions: Response Patterns, Drug Involved, and Temporal Variations in a Large Series of Patients. J. Investig. Allergol. Clin. Immunol. 2012, 22, 363–371. [Google Scholar]
- Blanca-Lopez, N.; Perez-Alzate, D.; Canto, G.; Blanca, M. Practical Approach to the Treatment of NSAID Hypersensitivity. Expert Rev. Clin. Immunol. 2017, 13, 1017–1027. [Google Scholar] [CrossRef]
- Doña, I.; Blanca-López, N.; Cornejo-García, J.A.; Torres, M.J.; Laguna, J.J.; Fernández, J.; Rosado, A.; Rondón, C.; Campo, P.; Agúndez, J.A.; et al. Characteristics of Subjects Experiencing Hypersensitivity to Non-Steroidal Anti-Inflammatory Drugs: Patterns of Response. Clin. Exp. Allergy 2011, 41, 86–95. [Google Scholar] [CrossRef]
- Messaad, D.; Sahla, H.; Benahmed, S.; Godard, P.; Bousquet, J.; Demoly, P. Drug Provocation Tests in Patients with a History Suggesting an Immediate Drug Hypersensitivity Reaction. Ann. Intern. Med. 2004, 140, 1001–1006. [Google Scholar] [CrossRef]
- Szczeklik, A.; Nizankowska-Mogilnicka, E.; Sanak, M. Chapter 69: Hypersensitivity to Aspirin and Non-Steroidal Antiinflammatory Drugs. In Middleton’s Allergy Principles and Practise; Mosby Elsevier: St. Louis, MO, USA, 2009; Volume 2, pp. 1227–1243. [Google Scholar]
- Neighbour, H. Mechanisms of Aspirin-Intolerant Asthma: Identifying Inflammatory Pathways in the Pathogenesis of Asthma. Int. Arch. Allergy Immunol. 2014, 163, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Asero, R.; Bavbek, S.; Blanca, M.; Blanca-Lopez, N.; Bochenek, G.; Brockow, K.; Campo, P.; Celik, G.; Cernadas, J.; et al. Classification and Practical Approach to the Diagnosis and Management of Hypersensitivity to Nonsteroidal Anti-Inflammatory Drugs. Allergy 2013, 68, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lim, K.-H.; Kim, M.-Y.; Jo, E.-J.; Lee, S.-Y.; Lee, S.-E.; Yang, M.-S.; Song, W.-J.; Kang, H.-R.; Park, H.-W.; et al. Cross-Reactivity to Acetaminophen and Celecoxib According to the Type of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity. Allergy Asthma Immunol. Res. 2014, 6, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Himly, M.; Jahn-Schmid, B.; Pittertschatscher, K.; Bohle, B.; Grubmayr, K.; Ferreira, F.; Ebner, H.; Ebner, C. IgE-Mediated Immediate-Type Hypersensitivity to the Pyrazolone Drug Propyphenazone. J. Allergy Clin. Immunol. 2003, 111, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Rozieres, A.; Vocanson, M.; Saïd, B.B.; Nosbaum, A.; Nicolas, J.-F. Role of T Cells in Nonimmediate Allergic Drug Reactions. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.L.; Makowska, J. Use of Nonsteroidal Anti-Inflammatory Drugs in Patients with Aspirin Hypersensitivity: Safety of Cyclo-Oxygenase-2 Inhibitors. Treat. Respir. Med. 2006, 5, 399–406. [Google Scholar] [CrossRef]
- Doña, I.; Blanca-López, N.; Jagemann, L.R.; Torres, M.J.; Rondón, C.; Campo, P.; Gómez, A.I.; Fernández, J.; Laguna, J.J.; Rosado, A.; et al. Response to a Selective COX-2 Inhibitor in Patients with Urticaria/Angioedema Induced by Nonsteroidal Anti-Inflammatory Drugs. Allergy 2011, 66, 1428–1433. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Agache, I.; Bavbek, S.; Bakirtas, A.; Blanca, M.; Bochenek, G.; Bonini, M.; Heffler, E.; Klimek, L.; Laidlaw, T.M.; et al. Diagnosis and Management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI Position Paper. Allergy 2019, 74, 28–39. [Google Scholar] [CrossRef]
- Aberer, W.; Bircher, A.; Romano, A.; Blanca, M.; Campi, P.; Fernandez, J.; Brockow, K.; Pichler, W.J.; Demoly, P.; European Network for Drug Allergy (ENDA); et al. Drug Provocation Testing in the Diagnosis of Drug Hypersensitivity Reactions: General Considerations. Allergy 2003, 58, 854–863. [Google Scholar] [CrossRef]
- Waton, J.; Pouget-Jasson, C.; Loos-Ayav, C.; Trechot, P.; Bursztejn, A.C.; Schmutz, J.L.; Barbaud, A. Drug Re-Challenges in Cutaneous Adverse Drug Reactions: Information and Effectiveness in the Long-Term Management of Patients. Allergy 2011, 66, 941–947. [Google Scholar] [CrossRef]
- Bommarito, L.; Zisa, G.; Riccobono, F.; Villa, E.; D’Antonio, C.; Calamari, A.M.; Poppa, M.; Moschella, A.; Di Pietrantonj, C.; Galimberti, M. Avoidance of Nonsteroidal Anti-Inflammatory Drugs after Negative Provocation Tests in Urticaria/Angioedema Reactions: Real-World Experience. Allergy Asthma Proc. 2014, 35, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Defrance, C.; Bousquet, P.-J.; Demoly, P. Evaluating the Negative Predictive Value of Provocation Tests with Nonsteroidal Anti-Inflammatory Drugs: NPV of NSAIDs’ Provocation Tests. Allergy 2011, 66, 1410–1414. [Google Scholar] [CrossRef]
- Demoly, P.; Kropf, R.; Bircher, A.; Pichler, W.J. Drug Hypersensitivity: Questionnaire. EAACI Interest Group on Drug Hypersensitivity. Allergy 1999, 54, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General Considerations for Skin Test Procedures in the Diagnosis of Drug Hypersensitivity. Allergy 2002, 57, 45–51. [Google Scholar] [PubMed]
- Brockow, K.; Garvey, L.H.; Aberer, W.; Atanaskovic-Markovic, M.; Barbaud, A.; Bilo, M.B.; Bircher, A.; Blanca, M.; Bonadonna, B.; Campi, P.; et al. Skin Test Concentrations for Systemically Administered Drugs—An ENDA/EAACI Drug Allergy Interest Group Position Paper. Allergy 2013, 68, 702–712. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The Skin Prick Test-European Standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Nizankowska-Mogilnicka, E.; Bochenek, G.; Mastalerz, L.; Swierczyńska, M.; Picado, C.; Scadding, G.; Kowalski, M.L.; Setkowicz, M.; Ring, J.; Brockow, K.; et al. EAACI/GA2LEN Guideline: Aspirin Provocation Tests for Diagnosis of Aspirin Hypersensitivity. Allergy 2007, 62, 1111–1118. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Makowska, J.S.; Blanca, M.; Bavbek, S.; Bochenek, G.; Bousquet, J.; Bousquet, P.; Celik, G.; Demoly, P.; Gomes, E.R.; et al. Hypersensitivity to Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)-Classification, Diagnosis and Management: Review of the EAACI/ENDA (#) and GA2LEN/HANNA*. Allergy 2011, 66, 818–829. [Google Scholar] [CrossRef]
- Rejestr Produktów Leczniczych. Available online: https://rejestrymedyczne.ezdrowie.gov.pl/rpl/search/public (accessed on 12 October 2022).
- Stohs, S.J.; Miller, M.J.S. A Case Study Involving Allergic Reactions to Sulfur-Containing Compounds Including, Sulfite, Taurine, Acesulfame Potassium and Sulfonamides. Food Chem. Toxicol. 2014, 63, 240–243. [Google Scholar] [CrossRef]
- Ramírez Santos, A.; Fernández-Redondo, V.; Pérez Pérez, L.; Concheiro Cao, J.; Toribio, J. Contact Allergy from Vitamins in Cosmetic Products. Dermatitis 2008, 19, 154–156. [Google Scholar] [CrossRef]
- Kulczycki, A. Aspartame-Induced Urticaria. Ann. Intern. Med. 1986, 104, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Takeo, N.; Nakamura, M.; Nakayama, S.; Okamoto, O.; Sugimoto, N.; Sugiura, S.; Sato, N.; Harada, S.; Yamaguchi, M.; Mitsui, N.; et al. Cochineal Dye-Induced Immediate Allergy: Review of Japanese Cases and Proposed New Diagnostic Chart. Allergol. Int. 2018, 67, 496–505. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, T.; Buonomo, A.; Illuminati, I.; D’Alò, S.; Pucci, S. Recurrent Anaphylaxis: A Case of IgE-Mediated Allergy to Carmine Red (E120). J. Investig. Allergol. Clin. Immunol. 2015, 25, 440–441. [Google Scholar]
- Mizowaki, T.; Miyake, S.; Yoshimoto, Y.; Matsuura, Y.; Akiyama, S. Allergy of calcium phosphate cement material following skull reconstruction: A case report. No Shinkei Geka 2013, 41, 323–327. [Google Scholar] [PubMed]
- Kiec-Swierczynska, M.; Krecisz, B.; Swierczynska-Machura, D. Photoallergic and Allergic Reaction to 2-Hydroxy-4-Methoxybenzophenone (Sunscreen) and Allergy to Cetyl Alcohol in Cosmetic Cream. Contact Dermat. 2005, 53, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Sterza, G.; Incorvaia, C.; Riario-Sforza, G.G. Anaphylaxis to the Anticoagulant Acid Citrate Dextrose. Allergy 2007, 62, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, S.; Pellegrini, S.; Contatore, M.; Bignardi, D.; Minale, P. New Risks from Ancient Food Dyes: Cochineal Red Allergy. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 232–233. [Google Scholar]
- Ben Fredj, N.; Ben Fadhel, N.; Chaabane, A.; Chadly, Z.; Ben Romdhane, H.; Boughattas, A.; Aouam, K. Colloidal Silica-Induced Hypersensitivity: Myth or Reality. Int. J. Clin. Pharm. 2016, 38, 7–9. [Google Scholar] [CrossRef]
- Goodacre, R.L.; Clancy, R.L.; Davidson, R.A.; Mullens, J.E. Cell Mediated Immunity to Corn Starch in Starch-Induced Granulomatous Peritonitis. Gut 1976, 17, 202–205. [Google Scholar] [CrossRef]
- Mumoli, N.; Cei, M.; Luschi, R.; Carmignani, G.; Camaiti, A. Allergic Reaction to Croscarmellose Sodium Used as Excipient of a Generic Drug. QJM 2011, 104, 709–710. [Google Scholar] [CrossRef]
- Schianchi, S.; Arcangeli, F.; Calista, D. Compound Allergy to Vea Oil. Contact Dermat. 2003, 49, 222. [Google Scholar] [CrossRef]
- Moskovits, P.E.; Riches, P.; Soni, N. A Red Patient: Immunological Reaction to Glycine? Anaesthesia 1987, 42, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Cinti, B.; Chiarello, M.; Bonifazi, F.; Moneret-Vautrin, D.A. Intraoperative Anaphylaxis: Verba Volant, Scripta Manent! Eur. Ann. Allergy Clin. Immunol. 2005, 37, 339–340. [Google Scholar] [PubMed]
- Shaw, D.W. Allergic Contact Dermatitis from 12-Hydroxystearic Acid and Hydrogenated Castor Oil. Dermatitis 2009, 20, E16–E20. [Google Scholar] [CrossRef]
- Corazza, M.; Virgili, A.; Mantovani, L.; La Malfa, W. Propylene Glycol Allergy from Acyclovir Cream with Cross-Reactivity to Hydroxypropyl Cellulose in a Transdermal Estradiol System? Contact Dermat. 1993, 29, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.; Urwin, R.; Wilkinson, M. Contact Allergy to Thioctic Acid Present in Hypromellose® Eye Drops. Contact Dermat. 2017, 76, 361–362. [Google Scholar] [CrossRef]
- Munk, S.J.; Heegaard, S.; Mosbech, H.; Garvey, L.H. Two Episodes of Anaphylaxis Following Exposure to Hydroxypropyl Methylcellulose during Cataract Surgery. J. Cataract Refract. Surg. 2013, 39, 948–951. [Google Scholar] [CrossRef]
- Hamano, S.; Nishima, D.; Satake, M.; Kudo, K.; Yanagita, K.; Tezuka, J. Recurrent Immediate Type Hypersensitivity Reaction Induced by Macrogol in a 3-Year-Old Boy. J. Investig. Allergol. Clin. Immunol. 2020, 30, 72–73. [Google Scholar] [CrossRef]
- Ekart, R.; Pecovnik-Balon, B.; Dvorsak, B.; Hojs, R. Sterile peritonitis after administration of icodextrin. Acta Med. Croat. 2002, 56, 185–187. [Google Scholar]
- McNeill, I.Y. Hypersensitivity Reaction to Mannitol. Drug Intell. Clin. Pharm. 1985, 19, 552–553. [Google Scholar] [CrossRef]
- Calogiuri, G.F.; Muratore, L.; Nettis, E.; Casto, A.M.; Di Leo, E.; Vacca, A. Immediate-Type Hypersensitivity Reaction to Mannitol as Drug Excipient (E421): A Case Report. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 99–102. [Google Scholar] [PubMed]
- Lightner, D.D.; De Braganca, K.; Gilheeney, S.W.; Khakoo, Y.; Kramer, K.; Balas, M. A Case of Mannitol Hypersensitivity. J Pediatr. Hematol. Oncol. 2013, 35, e274–e275. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.L.; Venkatesh, Y.P. Anaphylaxis to Excipient Mannitol: Evidence for an Immunoglobulin E-Mediated Mechanism. Clin. Exp. Allergy 2004, 34, 1602–1609. [Google Scholar] [CrossRef]
- Badiu, I.; Geuna, M.; Heffler, E.; Rolla, G. Hypersensitivity Reaction to Human Papillomavirus Vaccine Due to Polysorbate 80. BMJ Case Rep. 2012, 2012, bcr0220125797. [Google Scholar] [CrossRef] [PubMed]
- Pantín, C.; Letellez, J.; Calzas, J.; Mohedano, E. Indirect identification of hypersensitivity reaction to etoposide mediated by polysorbate 80. Farm. Hosp. 2018, 42, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Liccioli, G.; Mori, F.; Barni, S.; Pucci, N.; Novembre, E. Anaphylaxis to Polyvinylpyrrolidone in Eye Drops Administered to an Adolescent. J. Investig. Allergol. Clin. Immunol. 2018, 28, 263–265. [Google Scholar] [CrossRef]
- Yoshida, K.; Sakurai, Y.; Kawahara, S.; Takeda, T.; Ishikawa, T.; Murakami, T.; Yoshioka, A. Anaphylaxis to Polyvinylpyrrolidone in Povidone-Iodine for Impetigo Contagiosum in a Boy with Atopic Dermatitis. Int. Arch. Allergy Immunol. 2008, 146, 169–173. [Google Scholar] [CrossRef]
- Moreno-Escobosa, M.C. Aanaphylactic Shock Due to Povidone. J. Paediatr. Child Health 2017, 53, 517. [Google Scholar] [CrossRef]
- Bruusgaard-Mouritsen, M.A.; Mortz, C.; Winther, L.; Garvey, L.H. Repeated Idiopathic Anaphylaxis Caused by Povidone. Ann. Allergy Asthma Immunol. 2021, 126, 598–600. [Google Scholar] [CrossRef]
- Preuss, J.F.; Goddard, C.E.; Clarke, R.C.; Platt, P.R.; Sadleir, P.H. Anaphylaxis to Intravenous Paracetamol Containing Povidone. A Case Report and Narrative Review of Excipient Allergy Related to Anaesthesia. Anaesth. Intensive Care 2020, 48, 404–408. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakamura, M.; Matsunaga, K.; Nakata, J.; Tagami, K.; Sato, N.; Kawabe, T.; Kondo, Y. Anaphylaxis Due to Potato Starch (Possibly Caused by Percutaneous Sensitization). Asia Pac. Allergy 2021, 11, e14. [Google Scholar] [CrossRef] [PubMed]
- Farber, M.K.; Angelo, T.E.; Castells, M.; Tsen, L.C. Anesthetic Management of a Patient with an Allergy to Propylene Glycol and Parabens Anesth. Analg. 2010, 110, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Leleu, C.; Boulitrop, C.; Bel, B.; Jeudy, G.; Vabres, P.; Collet, E. Quinoline Yellow Dye-Induced Fixed Food-and-Drug Eruption. Contact Dermat. 2013, 68, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. Sodium Benzoate-Induced Pruritus. Allergy 2006, 61, 1240–1241. [Google Scholar] [CrossRef]
- Vilaplana, J.; Romaguera, C. Fixed Drug Eruption from Sodium Benzoate. Contact Dermat. 2003, 49, 290–291. [Google Scholar] [CrossRef]
- Jelti, L.; Bauvin, O.; Joly, P.; Tetart, F. Severe immediate hypersensitivity reaction with generalized contact urticaria after cutaneous application of topical permethrin. Ann. Dermatol. Vénéréologie 2019, 146, 720–724. [Google Scholar] [CrossRef]
- Saussy, K.; Couvillion, M.; Holcomb, K. Allergic Contact Dermatitis from Sorbitans in Beer and Bread. Cutis 2019, 104, 184–186. [Google Scholar]
- Ebo, D.G.; Schuerwegh, A.; Stevens, W.J. Anaphylaxis to Starch. Allergy 2000, 55, 1098–1099. [Google Scholar] [CrossRef]
- De Groot, A.C.; van der Meeren, H.L.; Weyland, J.W. Cosmetic Allergy from Stearic Acid and Glyceryl Stearate. Contact Dermat. 1988, 19, 77–78. [Google Scholar] [CrossRef]
- Sweatman, M.C.; Tasker, R.; Warner, J.O.; Ferguson, M.M.; Mitchell, D.N. Oro-Facial Granulomatosis. Response to Elemental Diet and Provocation by Food Additives. Clin. Allergy 1986, 16, 331–338. [Google Scholar] [CrossRef]
- Sornin de Leysat, C.; Boone, M.; Blondeel, A.; Song, M. Two Cases of Cross-Sensitivity in Subjects Allergic to Paraphenylenediamine Following Ingestion of Polaronil. Dermatology 2003, 206, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic Contact Dermatitis Caused by Titanium Screws and Dental Implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Czarnobilska, E.; Bosak, M. Cytotoxic-based Assays in Delayed Drug Hypersensitivity Reactions Induced by Antiepileptic Drugs. Pol. Arch. Intern. Med. 2015, 125, 823–834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mangodt, E.A.; Van Gasse, A.L.; Decuyper, I.; Uyttebroek, A.; Faber, M.A.; Sabato, V.; Bridts, C.H.; Hagendorens, M.M.; Ebo, D.G. In Vitro Diagnosis of Immediate Drug Hypersensitivity: Should We Go with the Flow? Int. Arch. Allergy Immunol. 2015, 168, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Romano, A.; Botelho, C.; Bousquet-Rouanet, L.; Gaeta, F.; Silva, R.; Rumi, G.; Rodrigues Cernadas, J.; Bousquet, P.J. Determining the Negative Predictive Value of Provocation Tests with Beta-Lactams. Allergy 2010, 65, 327–332. [Google Scholar] [CrossRef]
- Kulhas Celik, I.; Guvenir, H.; Hurmuzlu, S.; Toyran, M.; Civelek, E.; Kocabas, C.N.; Dibek Misirlioglu, E. The Negative Predictive Value of 5-Day Drug Provocation Test in Nonimmediate Beta-Lactam Allergy in Children. Ann. Allergy Asthma Immunol. 2020, 124, 494–499. [Google Scholar] [CrossRef]
- Topal, O.Y.; Ilknur, K.C.; Irem, Y.T.; Muge, T.; Ersoy, C.; Betul, K.; Emine, D.M. Negative Predictive Value of Provocation Tests for Nonsteroidal Anti-Inflammatory Drugs in Children. Allergy Asthma Proc. 2020, 41, 285–289. [Google Scholar] [CrossRef]
- Jakič, M.; Jager, M.; Košnik, M. Predictive Value of a Negative Oral Provocation Test in Patients with Hypersensitivity to Analgesics. Acta Derm. APA 2016, 25, 39–41. [Google Scholar] [CrossRef]
- Cox, F.; Khalib, K.; Conlon, N. PEG That Reaction: A Case Series of Allergy to Polyethylene Glycol. J. Clin. Pharmacol. 2021, 61, 832–835. [Google Scholar] [CrossRef]
- Misirlioglu, E.D.; Toyran, M.; Capanoglu, M.; Kaya, A.; Civelek, E.; Kocabas, C.N. Negative Predictive Value of Drug Provocation Tests in Children. Pediatr. Allergy Immunol. 2014, 25, 685–690. [Google Scholar] [CrossRef]
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008, 63, 1418–1427. [Google Scholar] [CrossRef] [PubMed]

| Patient’s Code | Suspected Drug | Phenotype of Initial Reaction | Tested Drug | Reaction Developed after Reexposure |
|---|---|---|---|---|
| 18 | NSAID | NIUA | ASA | angioedema (face, larynx) |
| 124 | NSAID | NIUA | urticaria | |
| 96 | ASA | SNIUAA | urticaria | |
| 106 | ASA | SNIUAA | urticaria and erythema | |
| 78 | IBU | SNIUAA | angioedema (lips and eyelids) | |
| 9 | PRC | SNIUAA | urticaria | |
| 47 | NAP | SNIUAA | urticaria | |
| 68 | KET | SNIUAA | urticaria | |
| 104 | KET | NERD | dyspnoea | |
| 128 | PRC | NERD | PRC | erythema |
| 22 | NSAID | NIUA | MEL | rush |
| 70 | DIC | SNIUAA | DIC | rush |
| Group A2 (Positive Reexposure) | Group A1 (Negative Reexposure) | p-Value | |
|---|---|---|---|
| age (y) | 55.8 ± 11.1 | 52.2 ± 1.6 | ns |
| time span to interview (m) | 36.5 ± 19.7 | 32.8 ± 18.0 | ns |
| sex: F/M (n) | 5/7 | 98/21 | 0.001 |
| NIUA (%) | 25% | 58% | ns |
| SNIUAA (%) | 58% | 40% | ns |
| NERD (%) | 17% | 2% | ns |
| any positive SPT for seasonal allergens (%) | 14% | 25% | ns |
| any positive SPT for perennial allergens (%) | 43% | 27% | ns |
| Group A (n = 131) The Reexposure Took Place | Group B (n = 33) No Reexposure Took Place | p-Value | |
|---|---|---|---|
| age (y) | 52.5 ± 16.2 | 54.2 ± 15.8 | ns |
| time span to interview (m) | 33.1 ± 18.1 | 30.9 ± 17.9 | ns |
| sex: F/M (n) | 103/28 | 27/6 | ns |
| NIUA (%) | 54% | 52% | ns |
| SNIUAA (%) | 42% | 45% | ns |
| NERD (%) | 4% | 3% | ns |
| any positive SPT for seasonal allergens (%) | 24% | 9% | ns |
| any positive SPT for perennial allergens (%) | 29% | 25% | ns |
| Substance | Number of Records | Hypersensitivity Reaction or Immune-Mediate Response to a Given Substance | References |
|---|---|---|---|
| Acesulfame potassium | 2 | hives and discomfort in the throat, swelling of the lips and face, sinus congestion, and difficulty breathing | [31] |
| Alpha-tocopherol | 5 | contact dermatitis | [32] |
| Aspartame | 5 | urticaria | [33] |
| Carmine | 30 | anaphylaxis, dye-induced immediate allergy | [34,35] |
| Calcium phosphate | 4 | positive patch test, contact dermatitis | [36] |
| Cetyl alcohol | 5 | contact dermatitis | [37] |
| Citric acid | 7 | anaphylaxis | [38] |
| Cochineal red | 2 | anaphylaxis | [39] |
| Colloidal silica | 1 | skin hypersensitivity | [40] |
| Corn starch | 1 | cell-mediated immunity | [41] |
| Croscarmellose sodium | 1 | erythematous skin rash with diffuse itching | [42] |
| Dimethicone | 1 | contact dermatitis | [43] |
| Glycine | 18 | anaphylaxis | [44] |
| Gelatine | 6 | anaphylaxis | [45] |
| Hydrogenated castor oil | 1 | contact dermatitis | [46] |
| Hydroxypropyl cellulose | 1 | cross-reactivity to propylene glycol, contact dermatitis | [47] |
| Hypromellose | 3 | contact dermatitis, anaphylaxis | [48,49] |
| Macrogol | 14 | anaphylaxis | [50] |
| Maltodextrin | 1 | sterile peritonitis, delayed reaction | [51] |
| Mannitol | 19 | anaphylaxis | [52,53,54,55] |
| Polysorbate 80 | 19 | anaphylaxis, urticaria | [56,57] |
| Polyvinylpyrrolidone | 6 | anaphylaxis | [58,59] |
| Povidone | 41 | anaphylaxis | [60,61,62] |
| Potato starch | 1 | anaphylaxis | [63] |
| Propylene glycol | 20 | immediate drug hypersensitivity reactions | [64] |
| Quinoline yellow | 3 | fixed food and drug-induced eruption | [65] |
| Sodium benzoate | 2 | pruritus, fixed drug eruption | [66,67] |
| Sorbic acid | 2 | generalized contact urticaria | [68] |
| Sorbitol | 7 | allergic contact dermatitis | [69] |
| Starch | 28 | anaphylaxis | [70] |
| Stearic acid | 5 | cosmetic allergy from stearic acid and glyceryl stearate | [71] |
| Sunset yellow | 3 | oro-facial granulomatosis, eczema | [72,73] |
| Titanium dioxide | 7 | allergic contact dermatitis | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiolek, I.; Blasiak, M.; Kozak, A.; Pietak, E.; Bulanda, M.; Porebski, G. Diagnostic Value of Oral Provocation Tests in Drug Hypersensitivity Reactions Induced by Nonsteroidal Anti-Inflammatory Drugs and Paracetamol. Diagnostics 2022, 12, 3074. https://doi.org/10.3390/diagnostics12123074
Popiolek I, Blasiak M, Kozak A, Pietak E, Bulanda M, Porebski G. Diagnostic Value of Oral Provocation Tests in Drug Hypersensitivity Reactions Induced by Nonsteroidal Anti-Inflammatory Drugs and Paracetamol. Diagnostics. 2022; 12(12):3074. https://doi.org/10.3390/diagnostics12123074
Chicago/Turabian StylePopiolek, Iwona, Magdalena Blasiak, Aleksandra Kozak, Ewelina Pietak, Malgorzata Bulanda, and Grzegorz Porebski. 2022. "Diagnostic Value of Oral Provocation Tests in Drug Hypersensitivity Reactions Induced by Nonsteroidal Anti-Inflammatory Drugs and Paracetamol" Diagnostics 12, no. 12: 3074. https://doi.org/10.3390/diagnostics12123074
APA StylePopiolek, I., Blasiak, M., Kozak, A., Pietak, E., Bulanda, M., & Porebski, G. (2022). Diagnostic Value of Oral Provocation Tests in Drug Hypersensitivity Reactions Induced by Nonsteroidal Anti-Inflammatory Drugs and Paracetamol. Diagnostics, 12(12), 3074. https://doi.org/10.3390/diagnostics12123074